Seawater Desalination by Interfacial Solar Vapor Generation Method Using Plasmonic Heating Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cu2-xS Nanorods (NRs)
2.3. Preparation of Polyvinyl Alcohol (PVA) gel/Cu2-xS NRs-PVA gel
2.4. Characterizations
2.5. Solar Vapor Generation
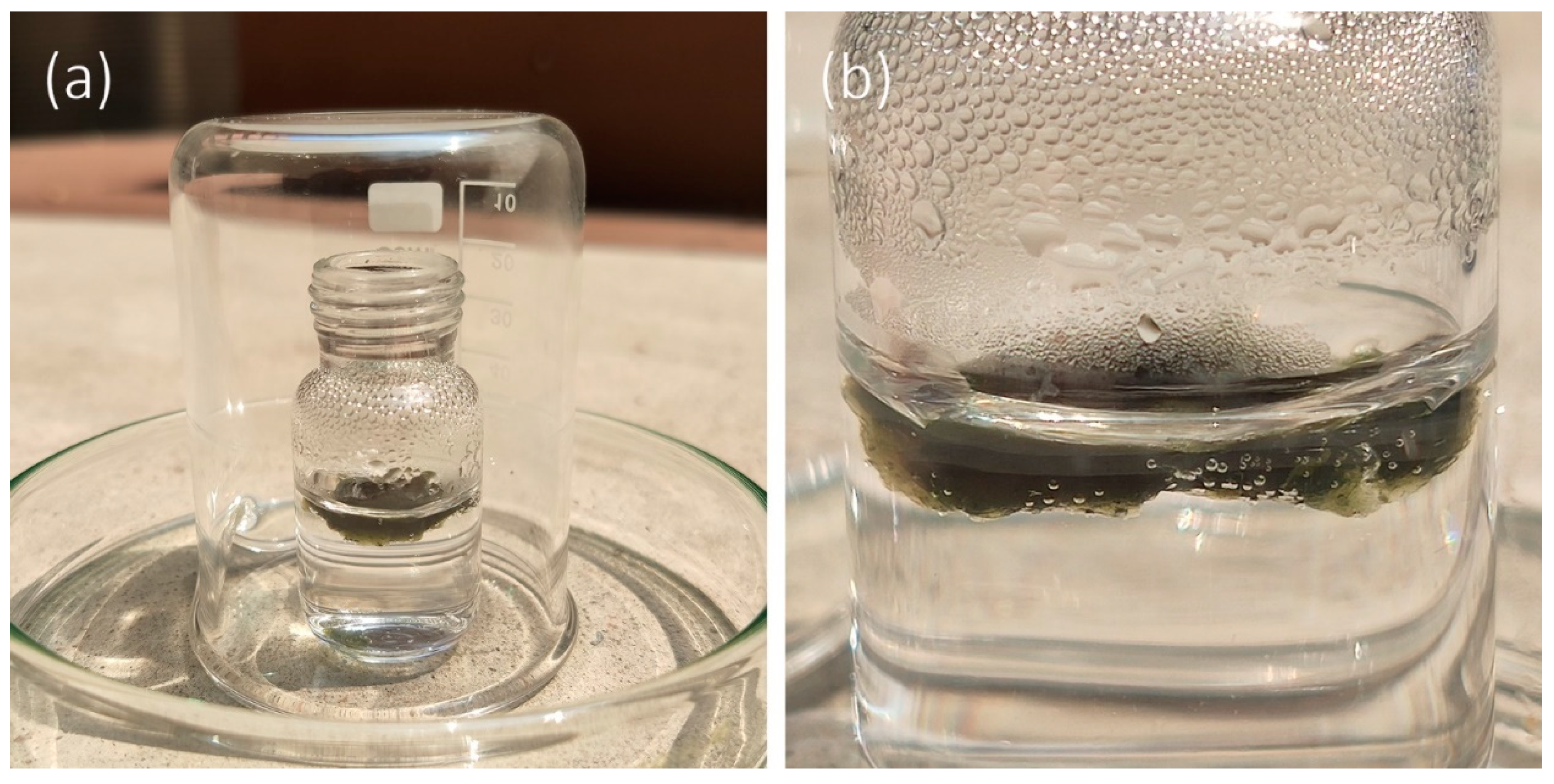
2.6. Water Purity Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McDonald, R.I.; Green, P.; Balk, D.; Fekete, B.M.; Revenga, C.; Todd, M.; Montgomery, M. Urban growth, climate change, and freshwater availability. Proc. Natl. Acad. Sci. USA 2011, 108, 6312–6317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politano, A.; Argurio, P.; Di Profio, G.; Sanna, V.; Cupolillo, A.; Chakraborty, S.; Arafat, H.A.; Curcio, E. Photothermal Membrane Distillation for Seawater Desalination. Adv. Mater. 2017, 29, 1603504. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Law, W.C.; Chan, K.C. Floating, highly efficient, and scalable graphene membranes for seawater desalination using solar energy. Green Chem. 2018, 20, 3689–3695. [Google Scholar] [CrossRef]
- Li, W.D.; Su, X.; Palazzolo, A.; Ahmed, S.; Thomas, E. Reverse osmosis membrane, seawater desalination with vibration assisted reduced inorganic fouling. Desalination 2017, 417, 102–114. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Zhu, M.W.; Li, Y.J.; Chen, F.J.; Zhu, X.Y.; Dai, J.Q.; Li, Y.F.; Yang, Z.; Yan, X.J.; Song, J.W.; Wang, Y.B.; et al. Plasmonic Wood for High-Efficiency Solar Steam Generation. Adv. Energy Mater. 2018, 8, 1701028. [Google Scholar] [CrossRef]
- Wang, Z.; Horseman, T.; Straub, A.P.; Yip, N.Y.; Li, D.; Elimelech, M.; Lin, S. Pathways and challenges for efficient solar-thermal desalination. Sci. Adv. 2019, 5, eaax0763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Gu, Y.; Liu, P.; Wang, P.; Miao, L.; Liu, J.; Wei, A.; Mu, X.; Li, J.; Zhu, J. Development and Evolution of the System Structure for Highly Efficient Solar Steam Generation from Zero to Three Dimensions. Adv. Funct. Mater. 2019, 29, 1903255. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Liu, K.; Hu, X.; Zhou, J. Interfacial Solar-to-Heat Conversion for Desalination. Adv. Energy Mater. 2019, 9, 1900310. [Google Scholar] [CrossRef]
- Dao, V.-D.; Choi, H.-S. Carbon-Based Sunlight Absorbers in Solar-Driven Steam Generation Devices. Glob. Chall. 2018, 2, 1700094. [Google Scholar] [CrossRef]
- Liu, K.K.; Jiang, Q.; Tadepallifit, S.; Raliya, R.; Biswas, P.; Naik, R.R.; Singamaneni, S. Wood Graphene Oxide Composite for Highly Efficient Solar Steam Generation and Desalination. ACS Appl. Mater. Interfaces 2017, 9, 7675–7681. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.B.; Lau, K.T.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, P. Self-Floating Carbon Nanotube Membrane on Macroporous Silica Substrate for Highly Efficient Solar-Driven Interfacial Water Evaporation. ACS Sustain. Chem. Eng. 2016, 4, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Liu, J.; Gu, J.J.; Liu, Q.L.; Zhang, W.; Su, H.L.; Zhang, D. Hierarchical Porous Carbonized Lotus Seedpods for Highly Efficient Solar Steam Generation. Chem. Mater. 2018, 30, 6217–6221. [Google Scholar] [CrossRef]
- Tao, F.J.; Zhang, Y.L.; Yin, K.; Cao, S.J.; Chang, X.T.; Lei, Y.H.; Wang, D.S.; Fan, R.H.; Dong, L.H.; Yin, Y.S.; et al. A plasmonic interfacial evaporator for high-efficiency solar vapor generation. Sustain. Energy Fuels 2018, 2, 2762–2769. [Google Scholar] [CrossRef]
- Anjaneyulu, O.; Ishii, S.; Imai, T.; Tanabe, T.; Ueda, S.; Nagao, T.; Abe, H. Plasmon-mediated photothermal conversion by TiN nanocubes toward CO oxidation under solar light illumination. RSC Adv. 2016, 6, 110566–110570. [Google Scholar] [CrossRef]
- Zielinski, M.S.; Choi, J.W.; La Grange, T.; Modestino, M.; Hashemi, S.M.H.; Pu, Y.; Birkhold, S.; Hubbell, J.A.; Psaltis, D. Hollow Mesoporous Plasmonic Nanoshells for Enhanced Solar Vapor Generation. Nano Lett. 2016, 16, 2159–2167. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, J.; Han, Y.; Zhang, J.; Cui, F.; Zhao, Y.; Li, X.; Wang, W. Multifunctional CuO Nanowire Mesh for Highly Efficient Solar Evaporation and Water Purification. ACS Sustain. Chem. Eng. 2019, 7, 5476–5485. [Google Scholar] [CrossRef]
- Guo, Z.Z.; Wang, G.; Ming, X.; Mei, T.; Wang, J.Y.; Li, J.H.; Qian, J.W.; Wang, X.B. PEGylated Self-Growth MoS2 on a Cotton Cloth Substrate for High-Efficiency Solar Energy Utilization. ACS Appl. Mater. Interfaces 2018, 10, 24583–24589. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.Y.; Deng, L.; Wei, N.N.; Weng, Y.K.; Dong, S.; Qi, D.P.; Qiu, J.; Chen, X.D.; Wu, T. High-Performance Photothermal Conversion of Narrow-Bandgap Ti2O3 Nanoparticles. Adv. Mater. 2017, 29, 1603730. [Google Scholar] [CrossRef]
- Gao, S.; Dong, X.; Huang, J.; Dong, J.; Maggio, F.D.; Wang, S.; Guo, F.; Zhu, T.; Chen, Z.; Lai, Y. Bioinspired Soot-Deposited Janus Fabrics for Sustainable Solar Steam Generation with Salt-Rejection. Glob. Chall. 2019, 3, 1800117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhou, X.Y.; Shi, Y.; Qian, X.; Alexander, M.; Zhao, X.P.; Mendez, S.; Yang, R.G.; Qu, L.T.; Yu, G.H. Highly efficient solar vapour generation via hierarchically nanostructured gels. Nat. Nanotechnol. 2018, 13, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Rao, N.X.; Tang, C.Y.; Cheng, C.H.; Law, W.C. Aqueous Phase Synthesis of Cu2-xS Nanostructures and Their Photothermal Generation Study. ACS Omega 2019, 4, 14655–14662. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Wu, P. Biomimetic MXene-Polyvinyl Alcohol Composite Hydrogel with Vertically Aligned Channels for Highly Efficient Solar Steam Generation. Adv. Mater. Technol. 2020, 5, 2000065. [Google Scholar] [CrossRef]
- Kriegel, I.; Rodriguez-Fernandez, J.; Wisnet, A.; Zhang, H.; Waurisch, C.; Eychmuller, A.; Dubavik, A.; Govorov, A.O.; Feldmann, J. Shedding Light on Vacancy-Doped Copper Chalcogenides: Shape-Controlled Synthesis, Optical Properties, and Modeling of Copper Telluride Nanocrystals with Near-Infrared Plasmon Resonances. ACS Nano 2013, 7, 4367–4377. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.J.; Hu, N.; Wang, X.Y.; Zhang, Y.S.; Sun, R.C. Copper Sulfide Nanoparticle/Cellulose Composite Paper: Room-Temperature Green Fabrication for NIR Laser-Inducible Ablation of Pathogenic Microorganisms. ACS Sustain. Chem. Eng. 2017, 5, 2648–2655. [Google Scholar] [CrossRef]
- Zhang, C.B.; Yan, C.; Xue, Z.J.; Yu, W.; Xie, Y.D.; Wang, T. Shape-Controlled Synthesis of High-Quality Cu7S4 Nanocrystals for Efficient Light-Induced Water Evaporation. Small 2016, 12, 5320–5328. [Google Scholar] [CrossRef] [PubMed]
- Amalathas, A.P.; Alkaisi, M.M. Nanostructures for Light Trapping in Thin Film Solar Cells. Micromachines 2019, 10, 619. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.W.; Jiang, F.R.; Zou, R.J.; Liu, Q.; Chen, Z.G.; Zhu, M.F.; Yang, S.P.; Wang, J.L.; Wang, J.H.; Hu, J.Q. Hydrophilic Cu9S5 Nanocrystals: A Photothermal Agent with a 25.7% Heat Conversion Efficiency for Photothermal Ablation of Cancer Cells in Vivo. ACS Nano 2011, 5, 9761–9771. [Google Scholar] [CrossRef]
- Mou, J.; Li, P.; Liu, C.B.; Xu, H.X.; Song, L.; Wang, J.; Zhang, K.; Chen, Y.; Shi, J.L.; Chen, H.R. Ultrasmall Cu2-xS Nanodots for Highly Efficient Photoacoustic Imaging-Guided Photothermal Therapy. Small 2015, 11, 2275–2283. [Google Scholar] [CrossRef]
- Tao, F.J.; Zhang, Y.L.; Cao, S.J.; Yin, K.; Chang, X.T.; Lei, Y.H.; Fan, R.H.; Dong, L.H.; Yin, Y.S.; Chen, X.B. CuS nanoflowers/semipermeable collodion membrane composite for high-efficiency solar vapor generation. Mater. Today Energy 2018, 9, 285–294. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Swihart, M.T. Plasmonic Copper Sulfide-Based Materials: A Brief Introduction to Their Synthesis, Doping, Alloying, and Applications. J. Phys. Chem. C 2017, 121, 13435–13447. [Google Scholar] [CrossRef]
- Liu, M.X.; Liu, Y.; Gu, B.B.; Wei, X.B.; Xu, G.X.; Wang, X.M.; Swihart, M.T.; Yong, K.T. Recent advances in copper sulphide-based nanoheterostructures. Chem. Soc. Rev. 2019, 48, 4950–4965. [Google Scholar] [CrossRef]
- Barandiaran, I.; Gutierrez, J.; Etxeberria, H.; Tercjak, A.; Kortaberria, G. Tuning photoresponsive and dielectric properties of PVA/CdSe films by capping agent change. Compos. Part A Appl. Sci. 2019, 118, 194–201. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; He, S.; Hitz, E.M.; Wang, Y.; Gan, W.; Mi, R.; Hu, L. A High-Performance Self-Regenerating Solar Evaporator for Continuous Water Desalination. Adv. Mater. 2019, 31, 1900498. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tanabe, Y.; Han, J.; Fujita, T.; Tanigaki, K.; Chen, M. Multifunctional Porous Graphene for High-Efficiency Steam Generation by Heat Localization. Adv. Mater. 2015, 27, 4302–4307. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Ni, G.; Zandavi, S.H.; Javid, S.M.; Boriskina, S.V.; Cooper, T.A.; Chen, G. A salt-rejecting floating solar still for low-cost desalination. Energy Environ. Sci. 2018, 11, 1510–1519. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Zhao, L.; Li, B.; Bhatia, B.; Wang, C.; Wilke, K.L.; Song, Y.; Labban, O.; Lienhard, J.H.; et al. Ultrahigh-efficiency desalination via a thermally-localized multistage solar still. Energy Environ. Sci. 2020, 13, 830–839. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, A.; Pikulin, A.; Sapogova, N.; Bityurin, N. Femtosecond Laser Irradiation of Plasmonic Nanoparticles in Polymer Matrix: Implications for Photothermal and Photochemical Material Alteration. Micromachines 2014, 5, 1202–1218. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]


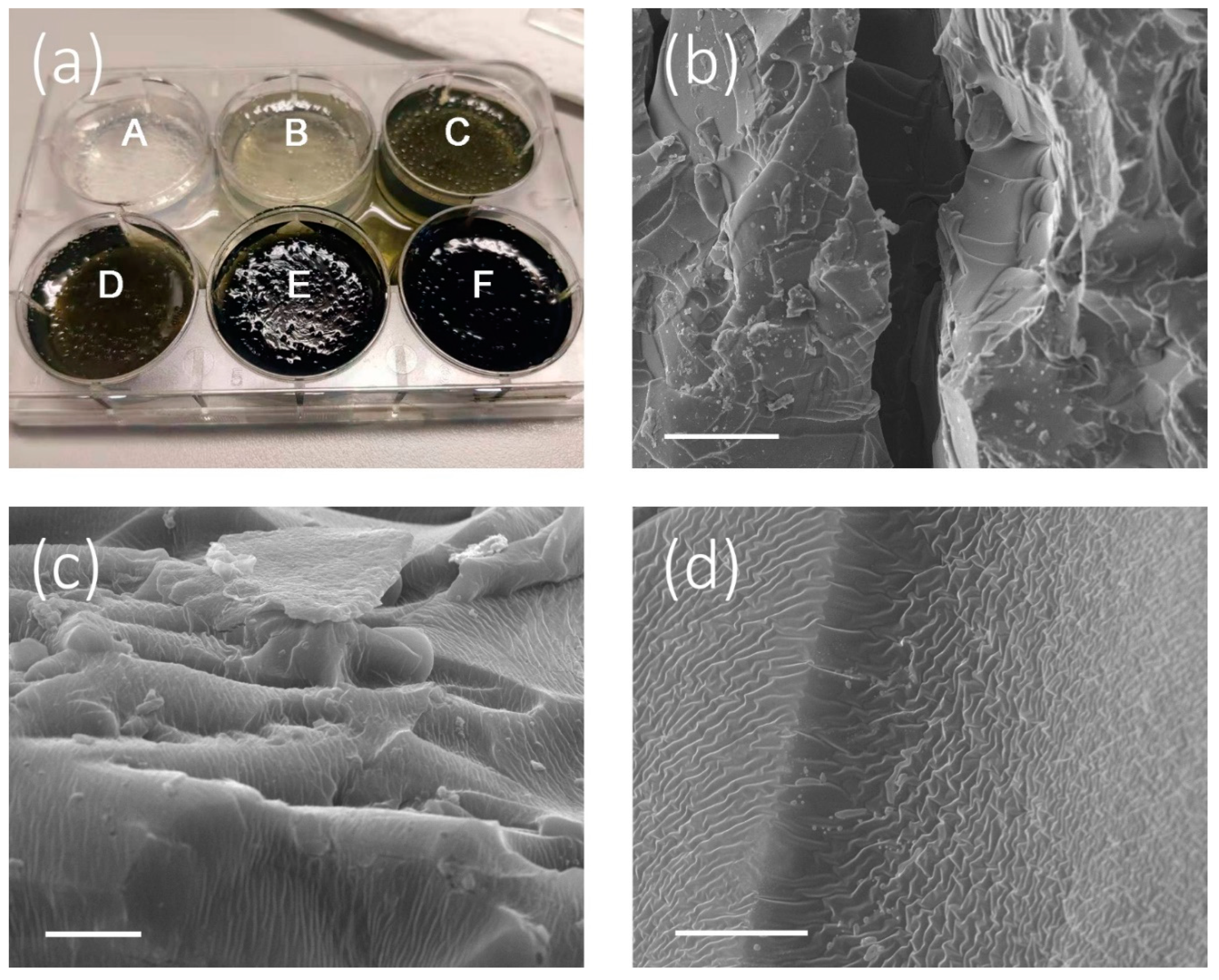



| Sample | Cu2-xS Loading Amount (mg) | υ (kg m−2 h−1) | ΔT (°C) | η (%) |
|---|---|---|---|---|
| A | 0 | 0.484 ± 0.010 | 9 ± 0.3 | 33 ± 0.4 |
| B | 1 | 0.652 ± 0.009 | 11 ± 0.2 | 44 ± 0.5 |
| C | 2 | 0.906 ± 0.010 | 13 ± 0.2 | 62 ± 0.6 |
| D | 4 | 1.102 ± 0.011 | 16 ± 0.3 | 75 ± 0.7 |
| E | 8 | 1.270 ± 0.010 | 19 ± 0.2 | 87 ± 0.4 |
| F | 12 | 1.214 ± 0.007 | 18 ± 0.4 | 83 ± 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Rao, N.; Tang, C.-Y.; Law, W.-C. Seawater Desalination by Interfacial Solar Vapor Generation Method Using Plasmonic Heating Nanocomposites. Micromachines 2020, 11, 867. https://doi.org/10.3390/mi11090867
Xu Z, Rao N, Tang C-Y, Law W-C. Seawater Desalination by Interfacial Solar Vapor Generation Method Using Plasmonic Heating Nanocomposites. Micromachines. 2020; 11(9):867. https://doi.org/10.3390/mi11090867
Chicago/Turabian StyleXu, Zhourui, Nanxi Rao, Chak-Yin Tang, and Wing-Cheung Law. 2020. "Seawater Desalination by Interfacial Solar Vapor Generation Method Using Plasmonic Heating Nanocomposites" Micromachines 11, no. 9: 867. https://doi.org/10.3390/mi11090867






