Droplet-Based Screening for the Investigation of Microbial Nonlinear Dose–Response Characteristics System, Background and Examples
Abstract
:1. Introduction
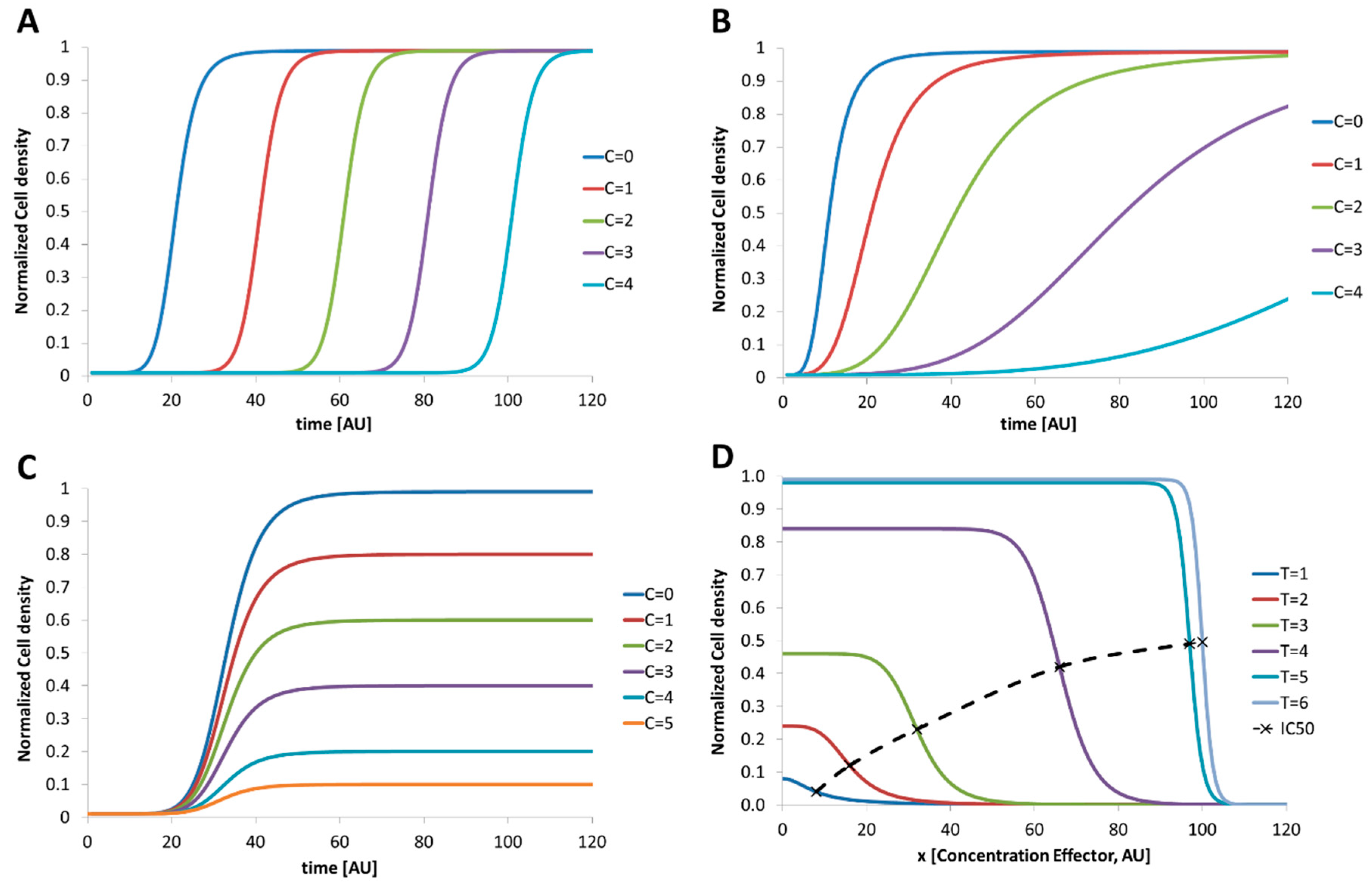
2. Dose–Response Screening Background
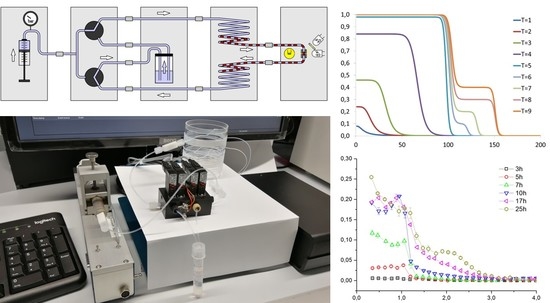
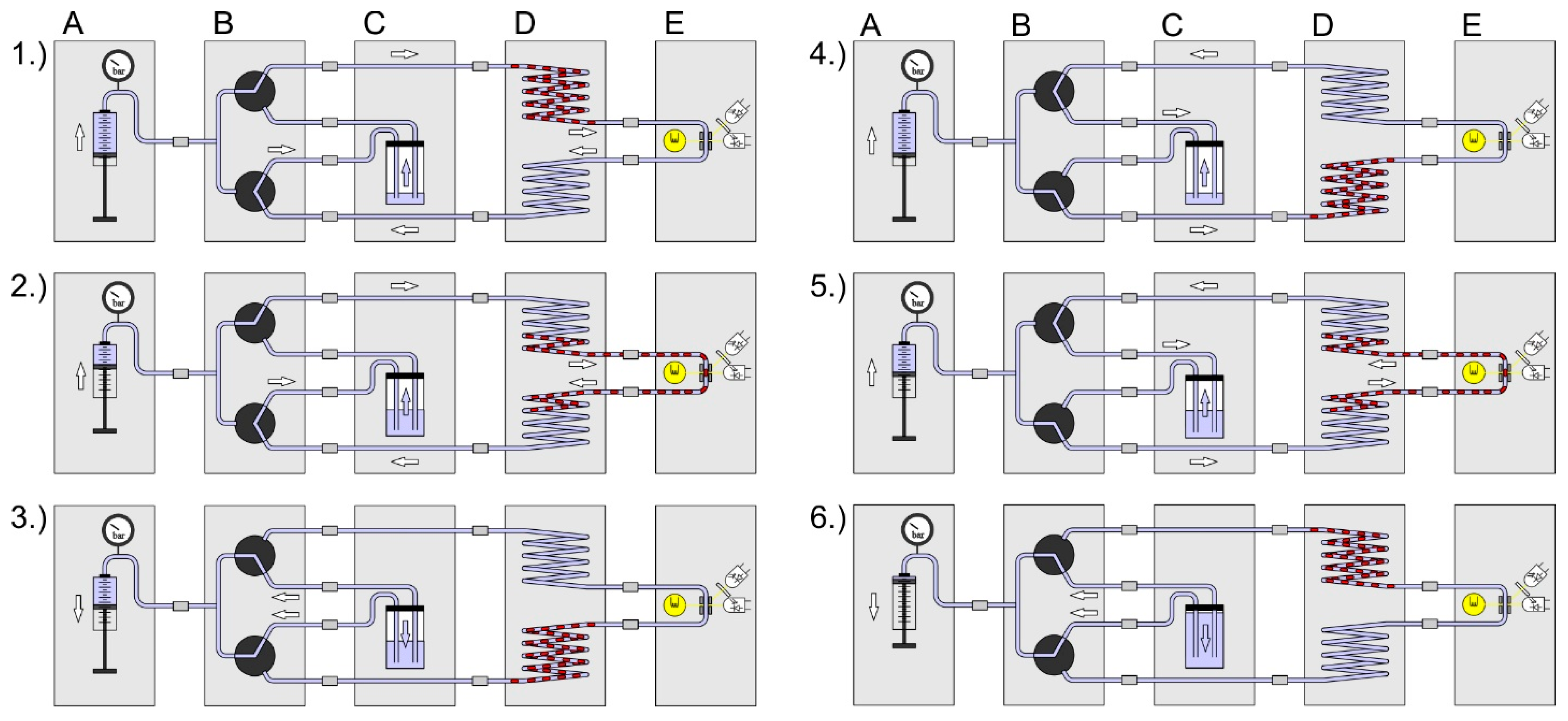
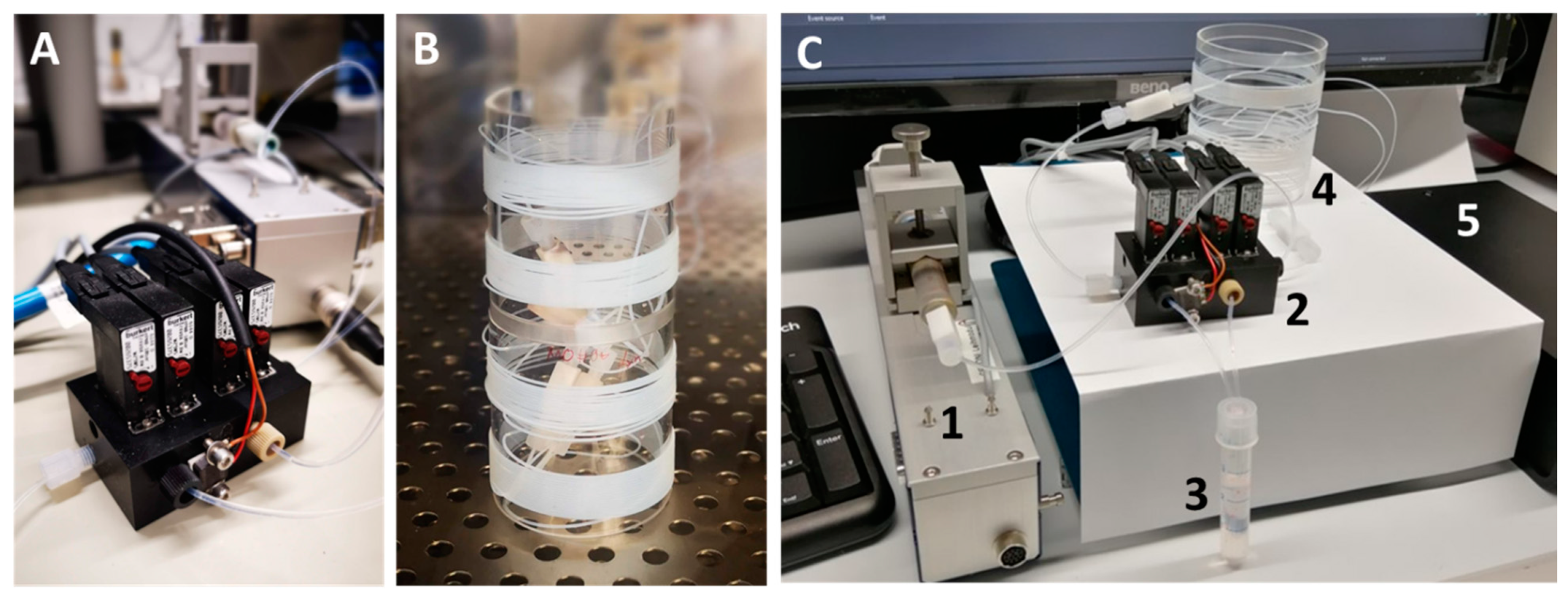
3. System Setup and New Developed Modules
4. Materials and Methods
4.1. Microorganisms and Chemicals
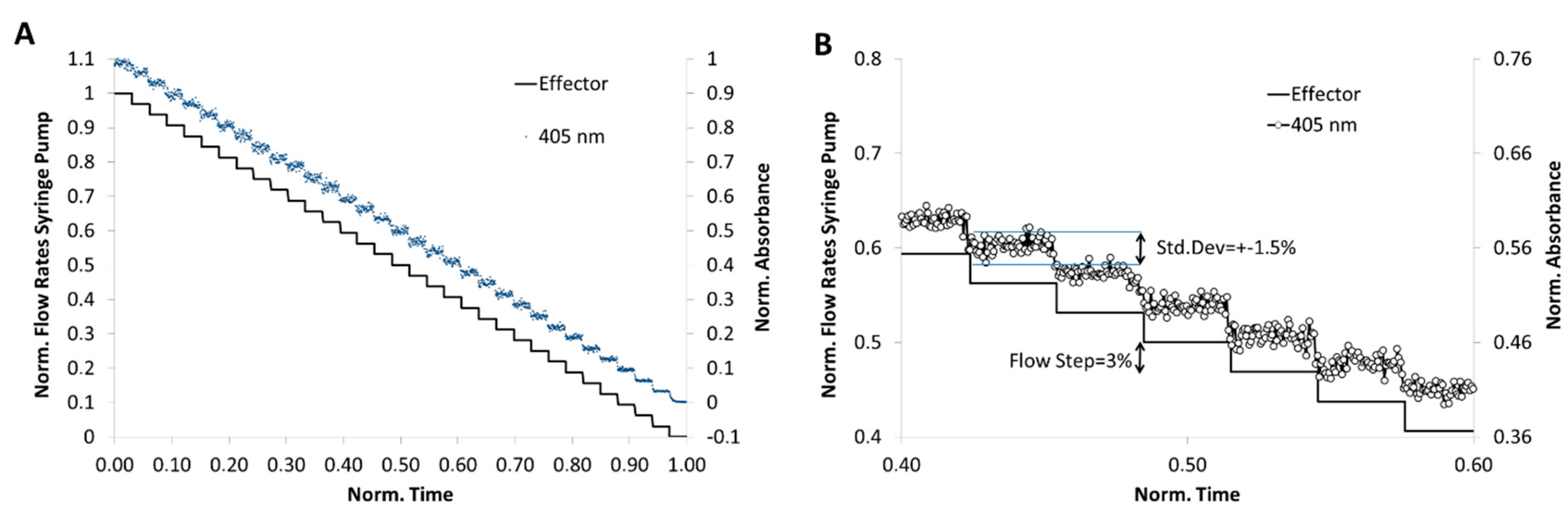
4.2. Fluidic System Characterization
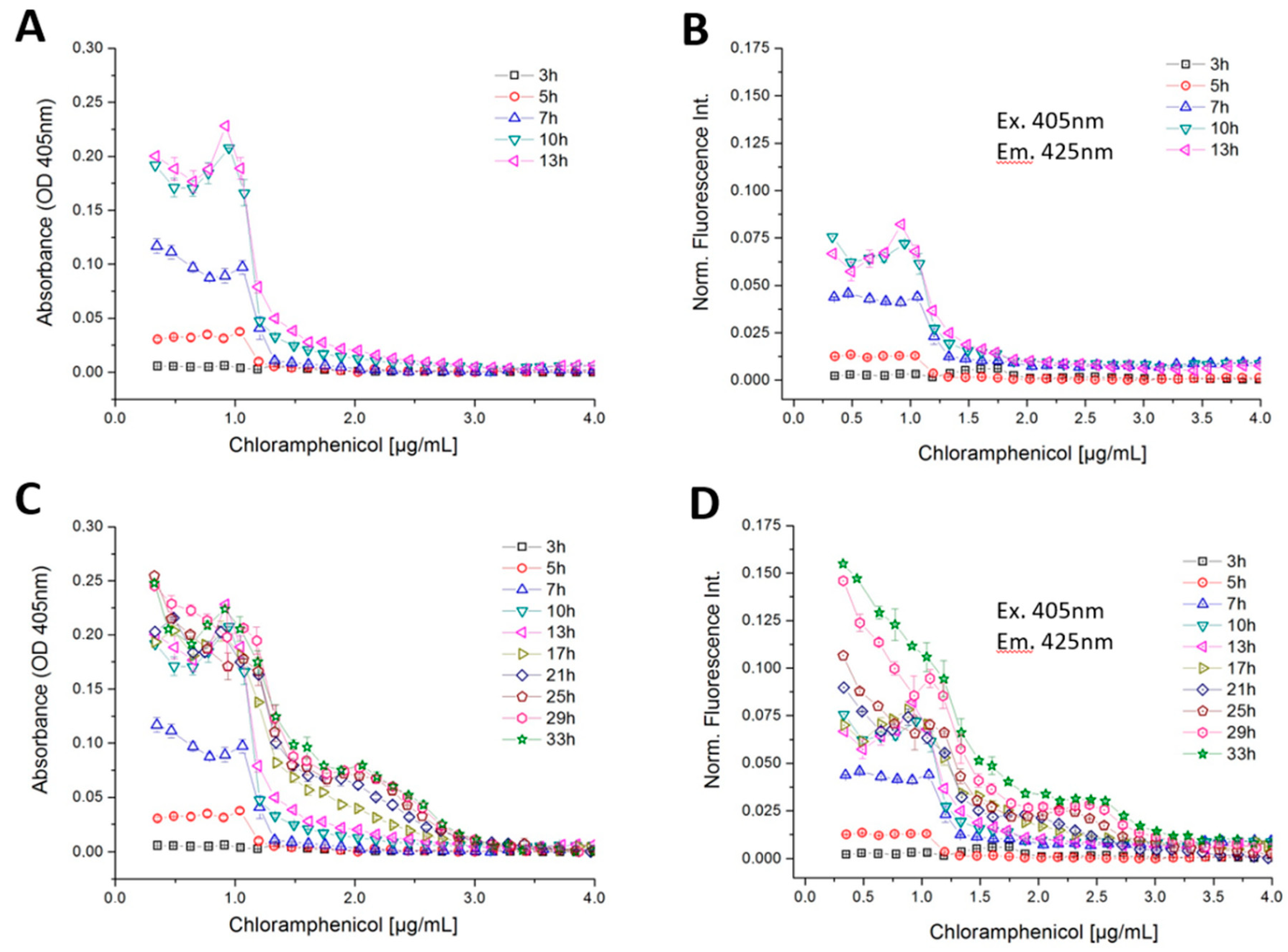
4.3. 1D-Dose Response Screening
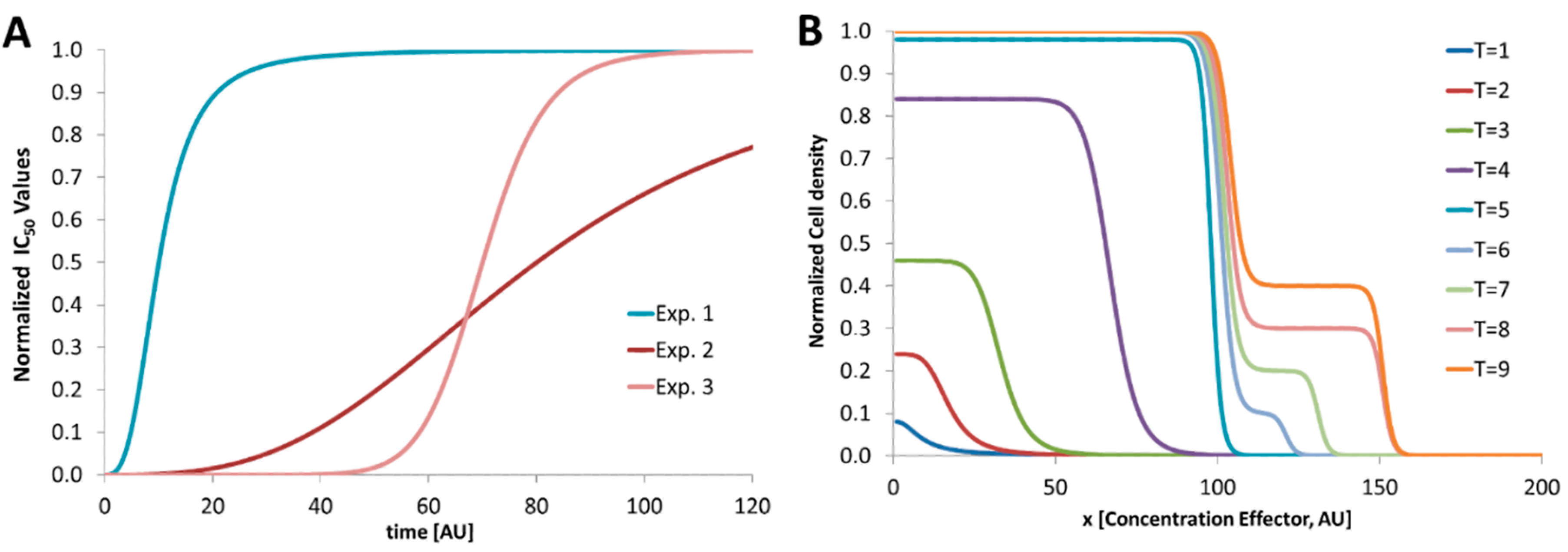
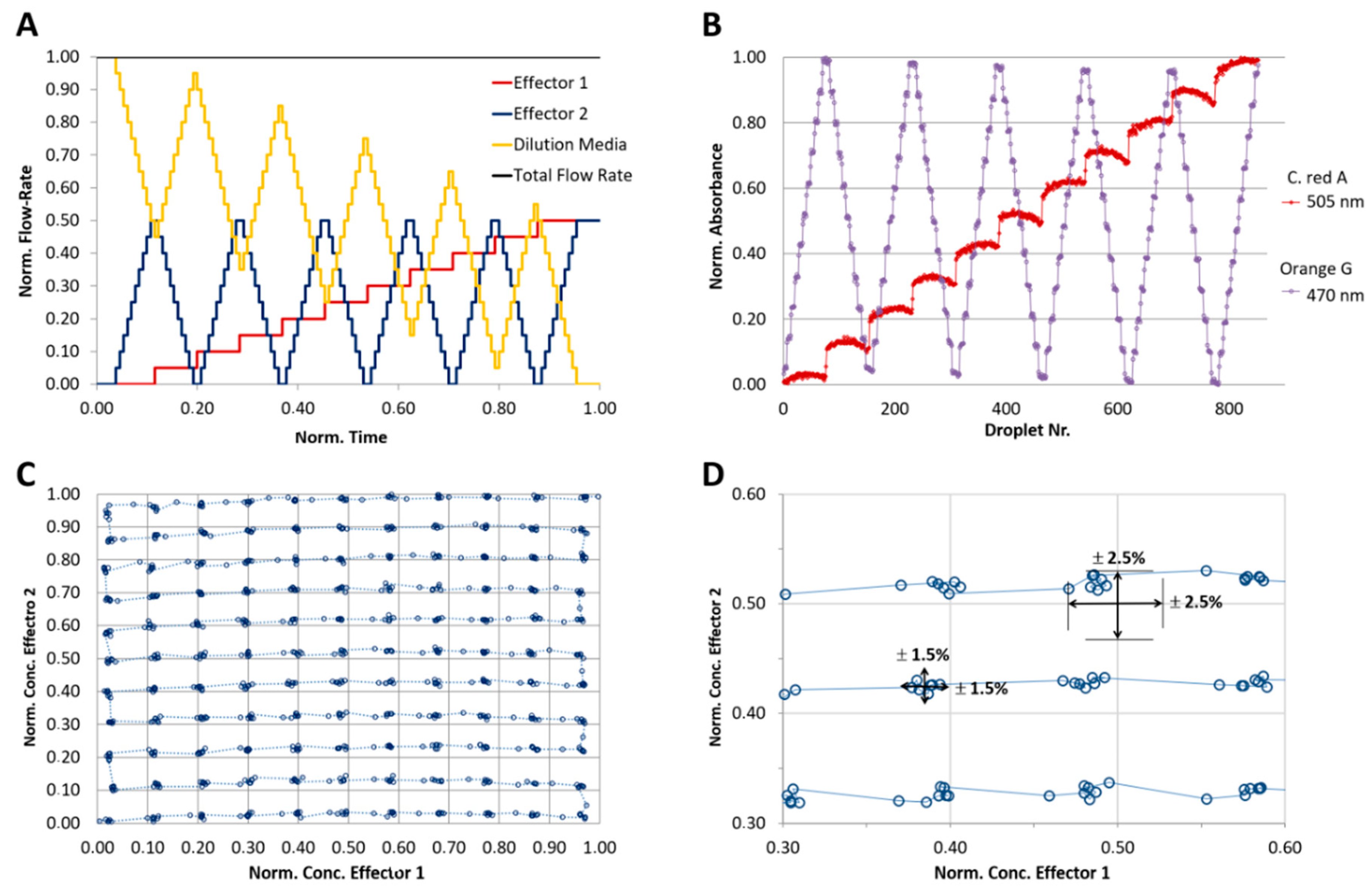
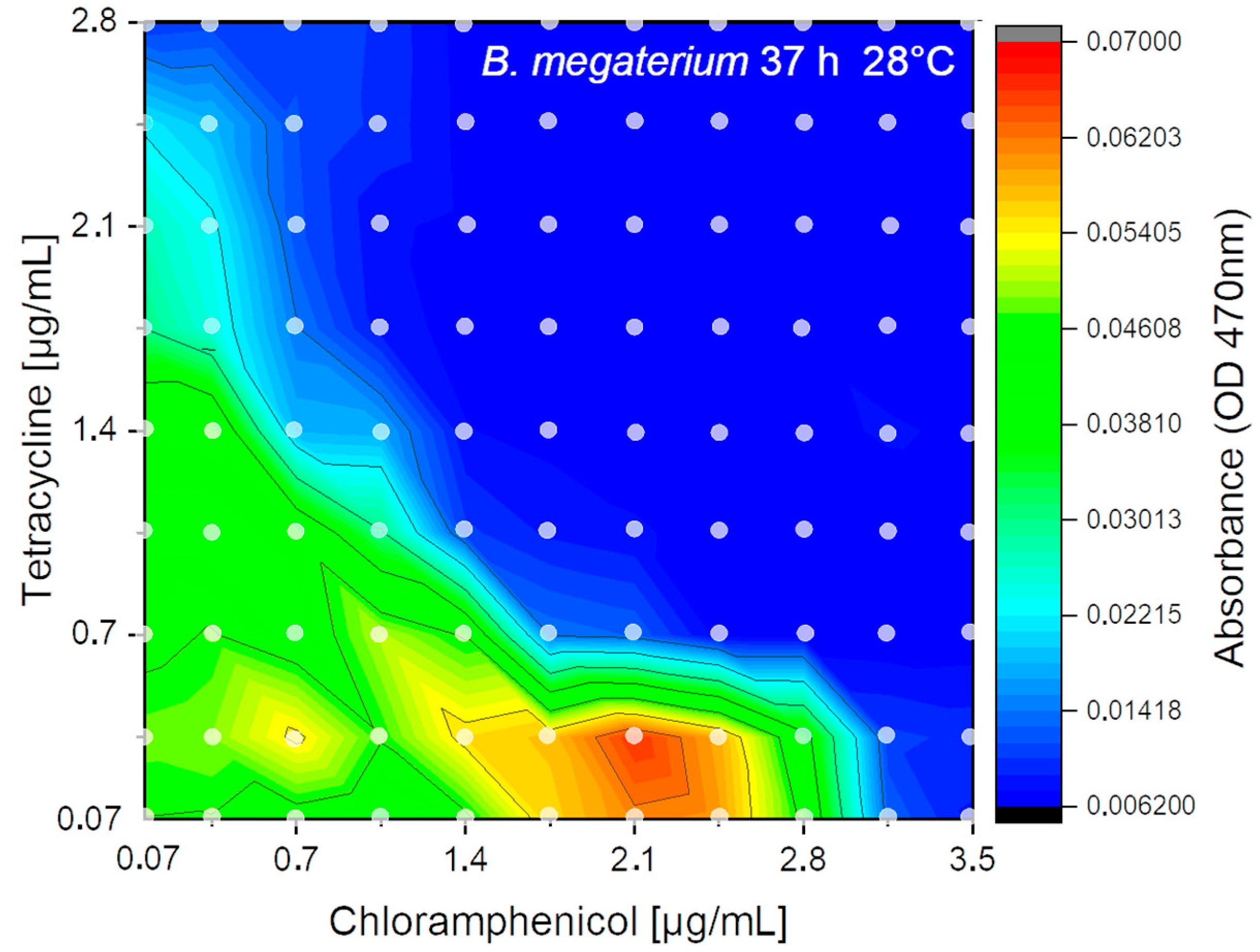
4.4. 2D-Dose Response Screening
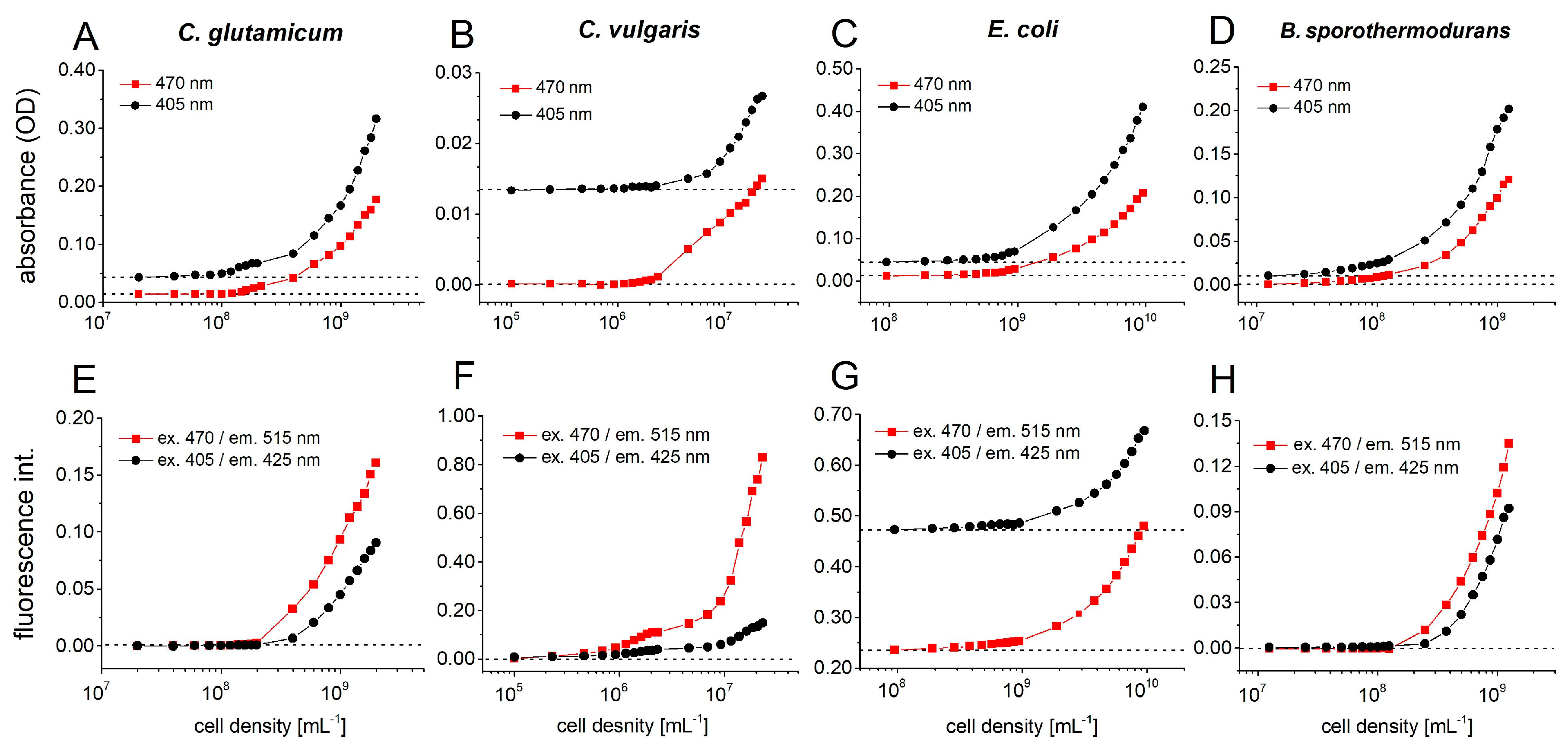
4.5. Photo-/Fluorimetric Sensor Calibration for Different Microbial Cultures
4.6. Dose–Response Screening of B. megaterium Growth Against Antibiotics
4.7. 2D-Combination Effects of Two Antibiotics on the Growth of B. megaterium
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Foudeh, A.M.; Didar, T.F.; Veres, T.; Tabrizian, M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip 2012, 12, 3249–3266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lin, J.-M. Microfluidic Platforms for Microbial; Springer: New York, NY, USA, 2018. [Google Scholar]
- Wetzel, K.; Cao, J.; Kothe, E.; Köhler, J.M. Changing growth behavior of heavy-metal tolerant bacteria: Media optimization using droplet-based microfluidics. Eng. Life Sci. 2015, 15, 327–332. [Google Scholar] [CrossRef]
- Gokulakrishnan, P.; Vergis, J. Molecular methods for microbiological quality control of meat and meat products: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Surrette, C.; Scherer, B.; Corwin, A.; Grossmann, G.; Kaushik, A.M.; Hsieh, K.; Zhang, P.; Liao, J.C.; Wong, P.K.; Wang, T.-H.; et al. Rapid microbiology screening in pharmaceutical workflows. SLAS Technol. Transl. Life Sci. Innov. 2018, 23, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Boedicker, J.Q.; Li, L.; Kline, T.R.; Ismagilov, R.F. Detecting bacteria and determining their susceptibility to antibiotics by stochastic confinement in nanoliter droplets using plug-based microfluidics. Lab Chip 2008, 8, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Churski, K.; Kaminski, T.S.; Jakiela, S.; Kamysz, W.; Baranska-Rybak, W.; Weibel, D.B.; Garstecki, P. Rapid screening of antibiotic toxicity in an automated microdroplet system. Lab Chip 2012, 12, 1629–1637. [Google Scholar] [CrossRef]
- Eun, Y.J.; Utada, A.S.; Copeland, M.F.; Takeuchi, S.; Weibel, D.B. Encapsulating bacteria in agarose microparticles using microfluidics for high-throughput cell analysis and isolation. ACS Chem. Biol. 2010, 6, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Wheat, P.F. History and development of antimicrobial susceptibility testing methodology. J. Antimicrob. Chemother. 2001, 48, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Mahler, L.; Wink, K.; Beulig, R.J.; Scherlach, K.; Tovar, M.; Zang, E.; Martin, K.; Hertweck, C.; Belder, D.; Roth, M. Detection of antibiotics synthetized in microfluidic picolitre-droplets by various actinobacteria. Sci. Rep. 2018, 8, 13087. [Google Scholar] [CrossRef]
- Roth, M.; Martin, K.; Zang, E.; Nett, M.; Henkel, T. A microfluidics-based approach to drug discovery. Biomed. Eng. 2012, 57, 270. [Google Scholar] [CrossRef]
- Scanlon, T.C.; Dostal, S.M.; Griswold, K. A High-throughput screen for antibiotic drug discovery. Biotechnol. Bioeng. 2014, 111, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baquero, F.; Martínez, J.L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- E Falagas, M.; A Bliziotis, I. Pandrug-resistant Gram-negative bacteria: The dawn of the post-antibiotic era? Int. J. Antimicrob. Agents 2007, 29, 630–636. [Google Scholar] [CrossRef]
- Jones, G.; Patel, N.; Levy, M.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.; Carmeli, Y.; Falagas, M.E.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Hafermann, L.; Köhler, J.M. Stochastically reduced communities-Microfluidic compartments as model and investigation tool for soil microorganism growth in structured spaces. Eng. Life Sci. 2017, 17, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Kehe, J.; Kulesa, A.; Ortiz, A.; Ackerman, C.M.; Thakku, S.G.; Sellers, D.; Kuehn, S.; Gore, J.; Friedman, J.; Blainey, P.C. Massively parallel screening of synthetic microbial communities. Proc. Natl. Acad. Sci. USA 2019, 116, 12804–12809. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kerner, A.; Burns, M.A.; Lin, X.N. Microdroplet-enabled highly parallel co-cultivation of microbial communities. PLoS ONE 2011, 6, e17019. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Jiang, L.; Li, B.; Dong, Y.; He, Y.; Qiu, Y. Screening and evaluation of heavy metals facilitating antibiotic resistance gene transfer in a sludge bacterial community. Sci. Total Environ. 2019, 695, 133862. [Google Scholar] [CrossRef]
- Arias-Andres, M.; Klümper, U.; Rojas-Jimenez, K.; Grossart, H.-P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Genet. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Manaia, C.M.; Rocha, J.; Scaccia, N.; Marano, R.; Radu, E.; Biancullo, F.; Cerqueira, F.; Fortunato, G.; Iakovides, I.C.; Zammit, I.; et al. Antibiotic resistance in wastewater treatment plants: Tackling the black box. Environ. Int. 2018, 115, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.J.; Bailey, M.; Hansen, L.H.; Kroer, N.; Wuertz, S. Studying plasmid horizontal transfer in situ: A critical review. Nat. Rev. Microbiol. 2005, 3, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, A.Z.; Cen, T.; Li, X.; He, M.; Li, D.; Chen, J. Sub-inhibitory concentrations of heavy metals facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes in water environment. Environ. Pollut. 2018, 237, 74–82. [Google Scholar] [CrossRef]
- Lok, C. Mining the microbial dark matter. Nature 2015, 522, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Rutledge, P.J.; Challis, G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Zengler, K.; Toledo, G.; Rappé, M.; Elkins, J.G.; Mathur, E.J.; Short, J.M.; Keller, M. Nonlinear partial differential equations and applications: Cultivating the uncultured. Proc. Natl. Acad. Sci. USA 2002, 99, 15681–15686. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, T.S.; Scheler, O.; Garstecki, P. Droplet microfluidics for microbiology: Techniques, applications and challenges. Lab Chip 2016, 16, 2168–2187. [Google Scholar] [CrossRef] [Green Version]
- Boitard, L.; Cottinet, D.; Bremond, N.; Baudry, J.; Bibette, J. Growing microbes in millifluidic droplets. Eng. Life Sci. 2015, 15, 318–326. [Google Scholar] [CrossRef]
- Jiang, L.; Boitard, L.; Broyer, P.; Chareire, A.-C.; Bourne-Branchu, P.; Mahe, P.; Tournoud, M.; Franceschi, C.; Zambardi, G.; Baudry, J.; et al. Digital antimicrobial susceptibility testing using the MilliDrop technology. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 415–422. [Google Scholar] [CrossRef]
- Kou, S.; Cheng, D.; Sun, F.; Hsing, I.-M. Microfluidics and microbial engineering. Lab Chip 2016, 16, 432–446. [Google Scholar] [CrossRef]
- Martin, K.; Henkel, T.; Baier, V.; Grodrian, A.; Schön, T.; Roth, M.; Köhler, J.M.; Metze, J. Generation of larger numbers of separated microbial populations by cultivation in segmented-flow microdevices. Lab Chip 2003, 3, 202–207. [Google Scholar] [CrossRef]
- Schaerli, Y.; Hollfelder, F. The potential of microfluidic water-in-oil droplets in experimental biology. Mol. BioSyst. 2009, 5, 1392. [Google Scholar] [CrossRef]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiology: An Introduction, 13th ed.; Pearson: Boston, MA, USA, 2019. [Google Scholar]
- Cao, J.; Schneider, S.; Schultheiß, R.; Schober, A.; Köhler, J.M.; Groß, G.A. “From microtiter plates to droplets” tools for micro-fluidic droplet processing. Microsyst. Technol. 2015, 21, 539–548. [Google Scholar] [CrossRef]
- Groß, G.A.; Hamann, C.; Günther, P.M.; Köhler, J.M. Formation of polymer and nanoparticle doped polymer Minirods by use of the Microsegmented flow principle. Chem. Eng. Technol. 2007, 30, 341–346. [Google Scholar] [CrossRef]
- Debski, P.R.; Sklodowska-Jaros, K.; Michalski, J.; Korczyk, P.M.; Dolata, M.; Jakiela, S. Continuous recirculation of microdroplets in a closed loop tailored for screening of bacteria cultures. Micromachines 2018, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Horka, M.; Sun, S.; Ruszczak, A.; Garstecki, P.; Mayr, T. Lifetime of phosphorescence from nanoparticles yields accurate measurement of concentration of oxygen in microdroplets, allowing one to monitor the metabolism of bacteria. Anal. Chem. 2016, 88, 12006–12012. [Google Scholar] [CrossRef]
- Abate, A.R.; Chen, C.-H.; Agresti, J.J.; Weitz, D.A. Beating poisson encapsulation statistics using close-packed ordering. Lab Chip 2009, 9, 2628–2631. [Google Scholar] [CrossRef]
- Collins, D.; Neild, A.; Demello, A.; Liu, A.-Q.; Ai, Y. The Poisson distribution and beyond: Methods for microfluidic droplet production and single cell encapsulation. Lab Chip 2015, 15, 3439–3459. [Google Scholar] [CrossRef]
- Sakakura, T.; Nishimura, K.; Suzuki, H.; Yomo, T. Statistical analysis of discrete encapsulation of nanomaterials in colloidal capsules. Anal. Methods 2012, 4, 1648–1655. [Google Scholar] [CrossRef]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biotechnol. 2005, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tugcu, G.; Ertürk, M.D.; Sacan, M. On the aquatic toxicity of substituted phenols to Chlorella vulgaris: QSTR with an extended novel data set and interspecies models. J. Hazard. Mater. 2017, 339, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, L.; Tian, K.; Kumar, A.; Singh, S.; Prior, B.A.; Wang, Z. Metabolic engineering of Escherichia coli: A sustainable industrial platform for bio-based chemical production. Biotechnol. Adv. 2013, 31, 1200–1223. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Kürsten, D.; Schneider, S.; Köhler, J.M. Stimulation and inhibition of bacterial growth by caffeine dependent on chloramphenicol and a phenolic uncoupler—A ternary toxicity study using microfluid segment technique. J. Biomed. Nanotechnol. 2012, 8, 770–778. [Google Scholar] [CrossRef]
- Cao, J.; Köhler, J.M. Droplet-based microfluidics for microtoxicological studies. Eng. Life Sci. 2015, 15, 306–317. [Google Scholar] [CrossRef]
- Owusu-Darko, R.; Allam, M.; De Oliveira, S.D.; Ferreira, C.A.S.; Grover, S.; Mtshali, S.; Ismail, A.; Mallappa, R.H.; Tabit, F.T.; Buys, E.M. Genome sequences of Bacillus sporothermodurans strains isolated from ultra-high-temperature milk. Microbiol. Resour. Announc. 2019, 8, e00145-19. [Google Scholar] [CrossRef] [Green Version]
- Vary, P.S.; Biedendieck, R.; Fuerch, T.; Meinhardt, F.; Rohde, M.; Deckwer, W.-D.; Jahn, D. Bacillus megaterium—From simple soil bacterium to industrial protein production host. Appl. Microbiol. Biotechnol. 2007, 76, 957–967. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M.C. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [Green Version]
- Meissner, H.C.; Smith, A.L. The current status of chloramphenicol. Pediatrics 1979, 64, 348–356. [Google Scholar]
- Ford, S.R.; Switzer, R.L. Stimulation of derepressed enzyme synthesis in bacteria by growth on sublethal concentrations of chloramphenicol. Antimicrob. Agents Chemother. 1975, 7, 555–563. [Google Scholar] [CrossRef] [Green Version]




| Inoculum | Compartmentalized in Droplets of Volume: | ||||||
|---|---|---|---|---|---|---|---|
| Cell/mL | 1 nL | 10 nL | 100 nL | 500 nL | 1 µL | 10 µL | 100 µL |
| 1 | |||||||
| 10 | 1 | ||||||
| 100 | 1 | 10 | |||||
| 1000 | 1 | 1 | 10 | 100 | |||
| 10,000 | 1 | 5 | 10 | 100 | 1000 | ||
| 100,000 | 1 | 10 | 50 | 100 | 1000 | 10,000 | |
| 1.0 × 106 | 1 | 10 | 100 | 500 | 1000 | 10,000 | 100,000 |
| 1.0 × 107 | 10 | 100 | 1000 | 5000 | 10,000 | 100,000 | 1.0 × 106 |
| 5.0 × 107 | 50 | 500 | 5000 | 25,000 | 50,000 | 500,000 | 5.0 × 106 |
| 1.0 × 108 | 100 | 1000 | 10,000 | 50,000 | 100,000 | 1.0 × 106 | 1.0 × 107 |
| 1.0 × 109 | 1000 | 10,000 | 100,000 | 500,000 | 1.0 × 106 | 1.0 × 107 | 1.0 × 108 |
| 5.0 × 109 | 5000 | 50,000 | 500,000 | 2.5 × 106 | 5.0 × 106 | 5.0 × 107 | 5.0 × 108 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Richter, F.; Kastl, M.; Erdmann, J.; Burgold, C.; Dittrich, D.; Schneider, S.; Köhler, J.M.; Groß, G.A. Droplet-Based Screening for the Investigation of Microbial Nonlinear Dose–Response Characteristics System, Background and Examples. Micromachines 2020, 11, 577. https://doi.org/10.3390/mi11060577
Cao J, Richter F, Kastl M, Erdmann J, Burgold C, Dittrich D, Schneider S, Köhler JM, Groß GA. Droplet-Based Screening for the Investigation of Microbial Nonlinear Dose–Response Characteristics System, Background and Examples. Micromachines. 2020; 11(6):577. https://doi.org/10.3390/mi11060577
Chicago/Turabian StyleCao, Jialan, Felix Richter, Michael Kastl, Jonny Erdmann, Christian Burgold, David Dittrich, Steffen Schneider, J. Michael Köhler, and G. Alexander Groß. 2020. "Droplet-Based Screening for the Investigation of Microbial Nonlinear Dose–Response Characteristics System, Background and Examples" Micromachines 11, no. 6: 577. https://doi.org/10.3390/mi11060577