Microneedle Patterning of 3D Nonplanar Surfaces on Implantable Medical Devices Using Soft Lithography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soft Lithographic Microfabrication of a Thin Flexible Microneedle Array
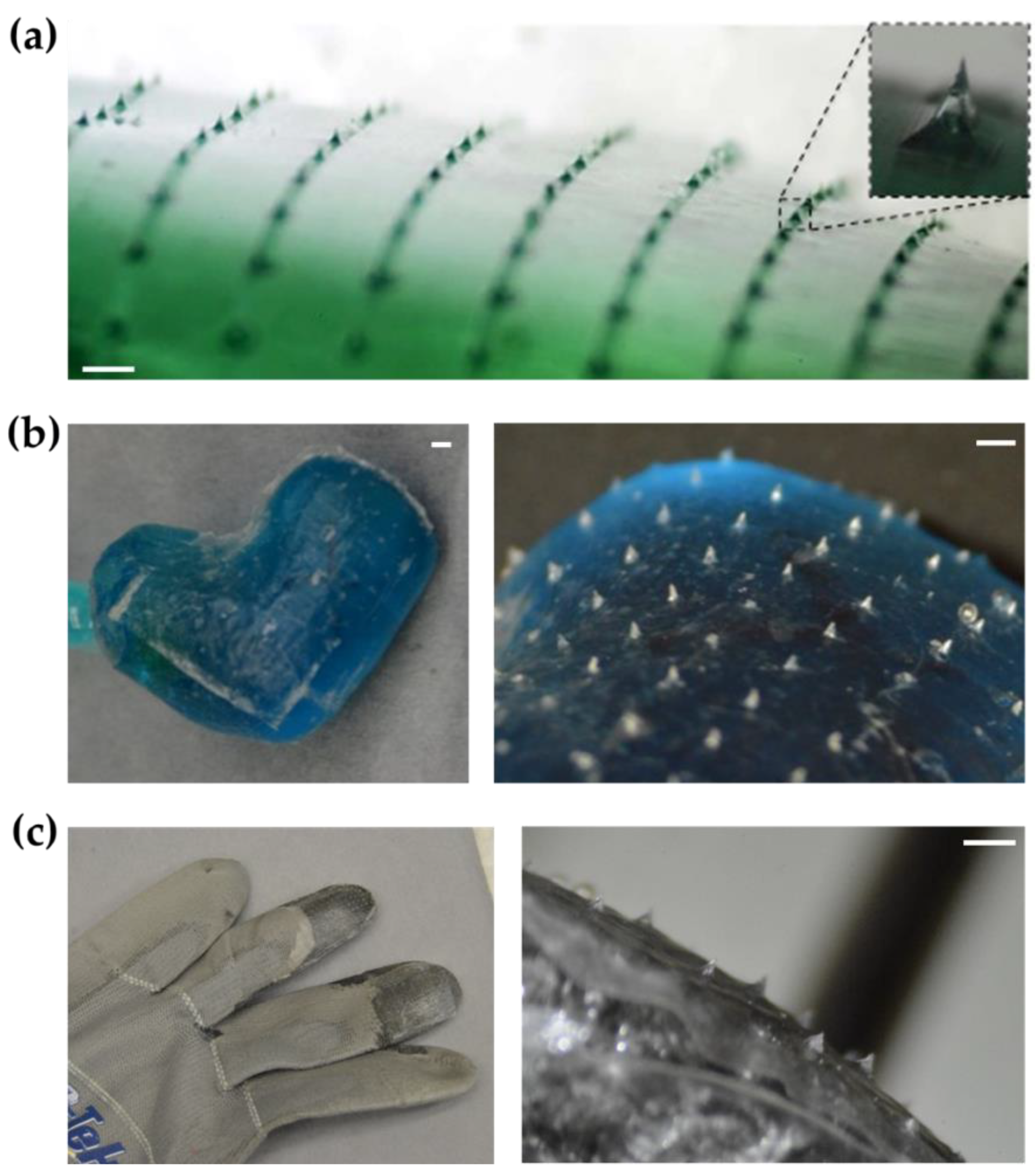
2.2. Transfer of Microneedle Arrays to Other Objects
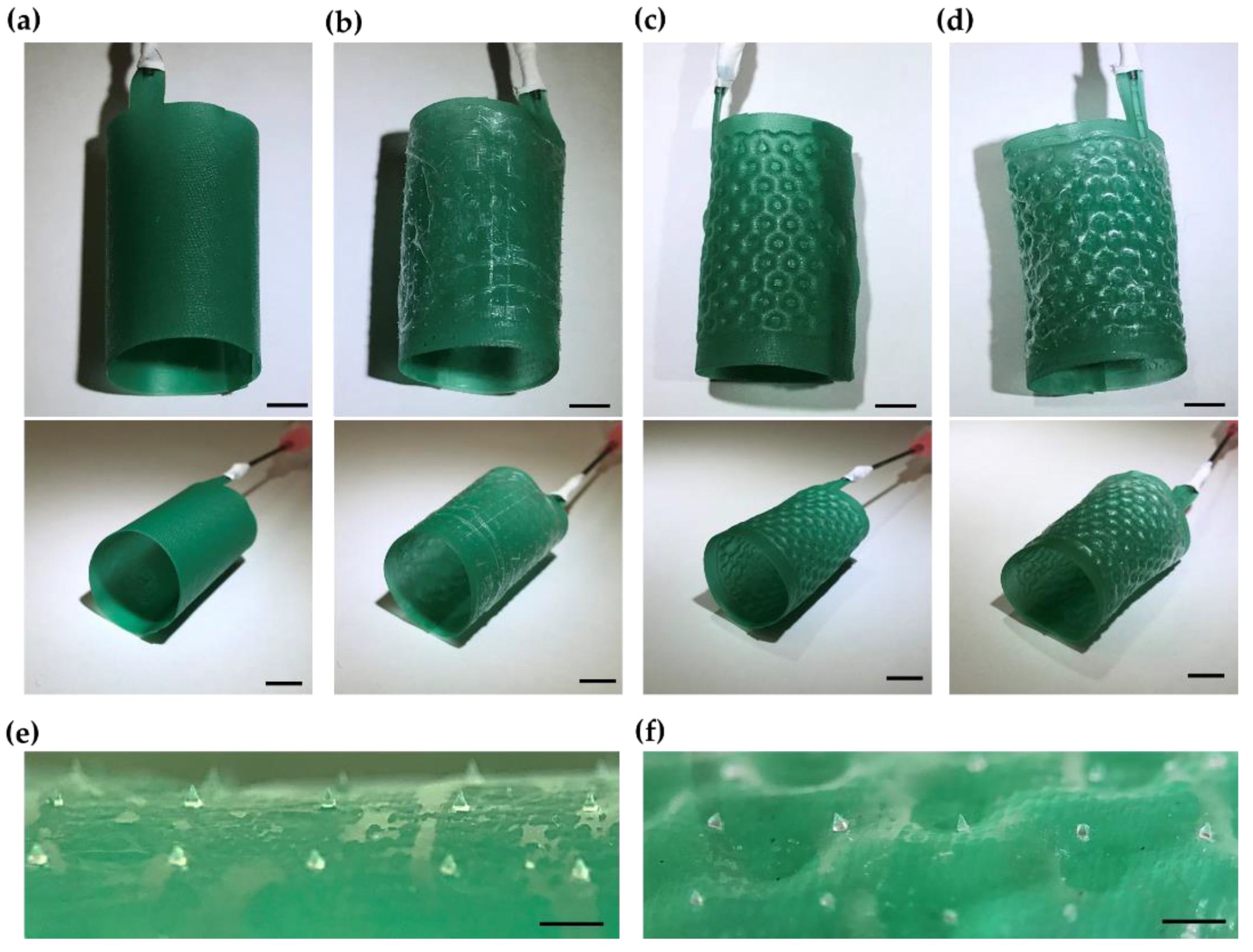
2.3. Rapid Prototyping of Microneedle-Patterned Soft Robotic Medical Device
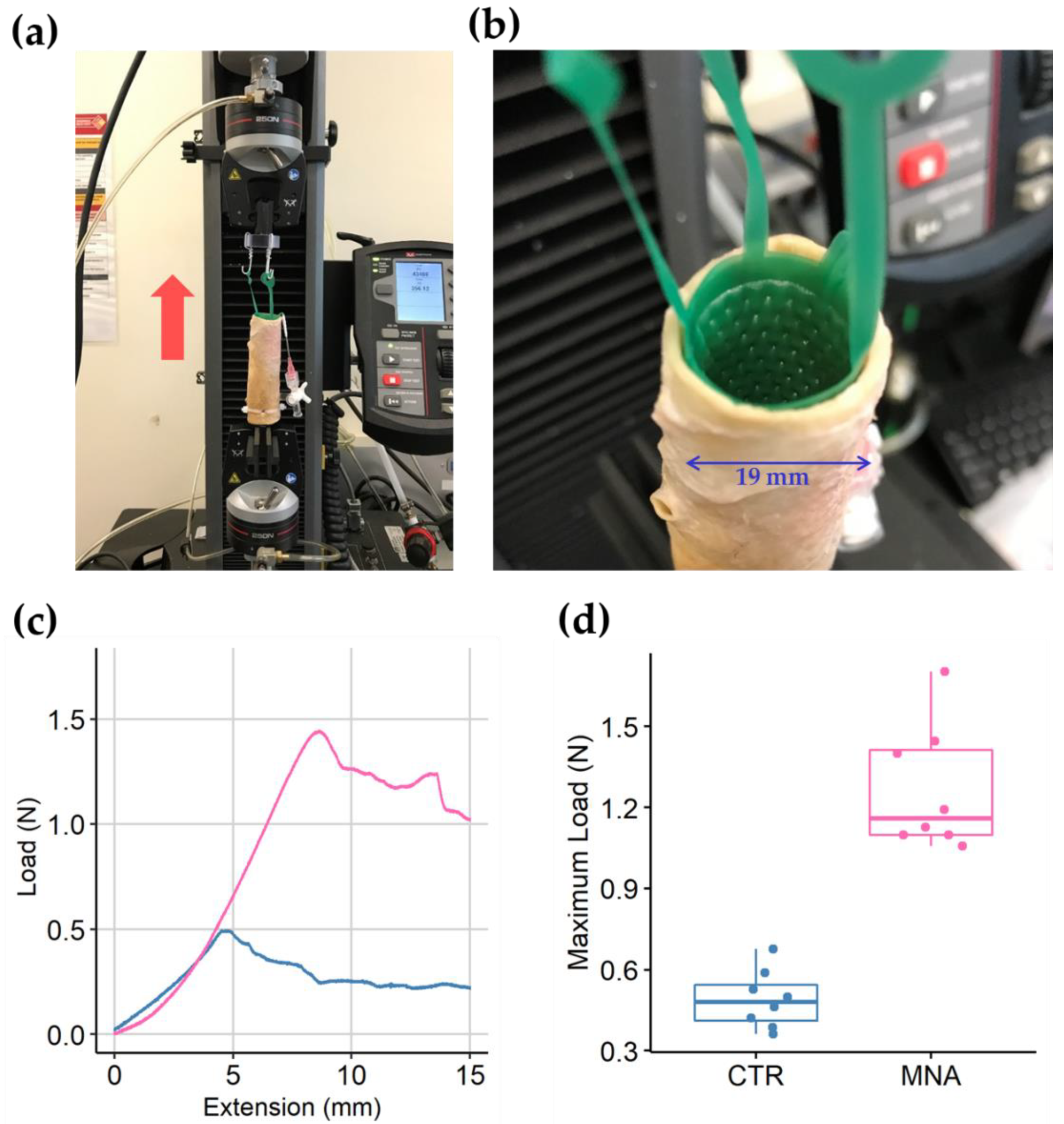
2.4. Anchorage Test of the Microneedle-Patterned Polyurethane Stent
2.5. Mechanical Integrity Test of the Microneedle Film
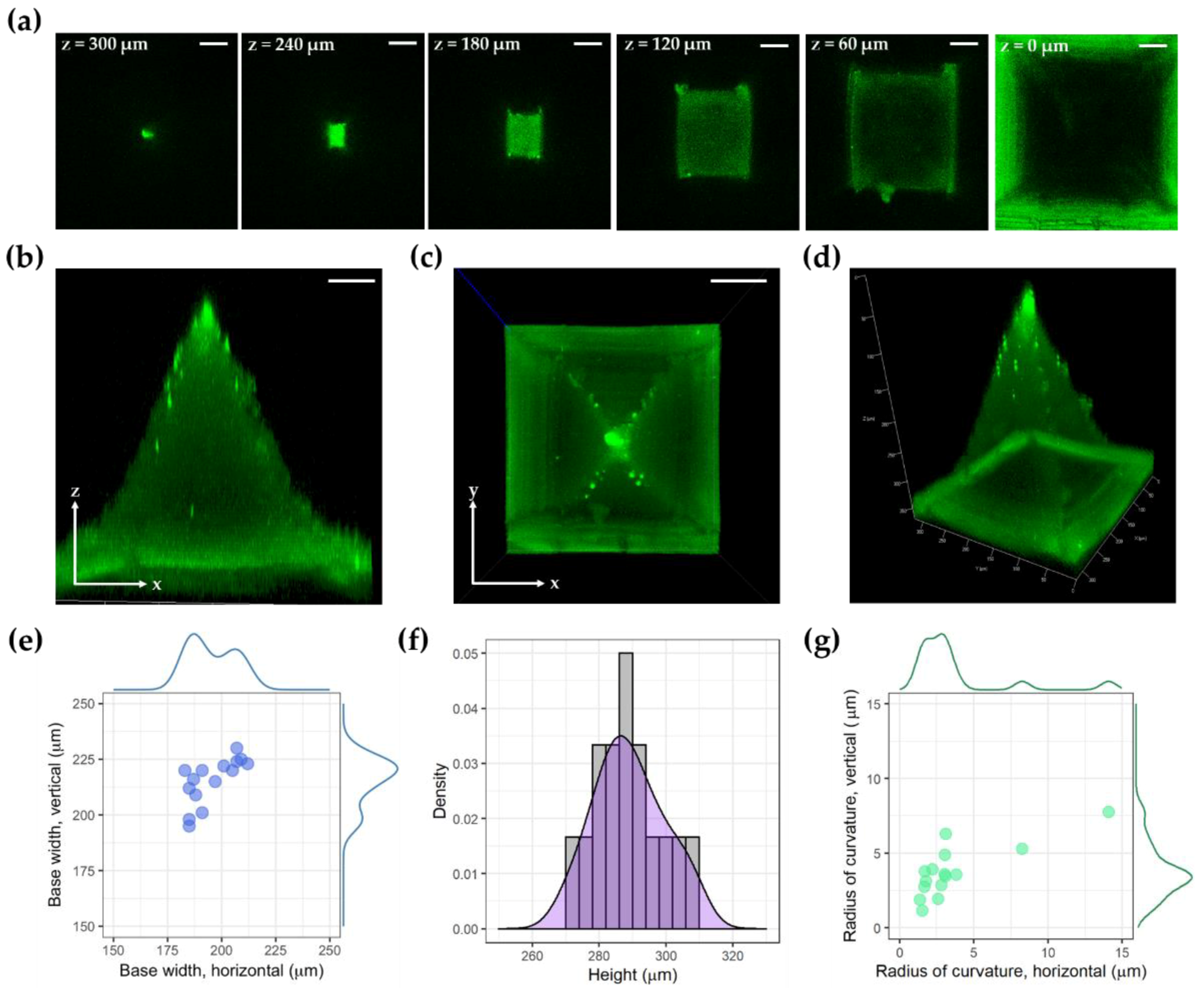
2.6. Imaging
2.7. Statistics
3. Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef]
- Tan, J.L.; Liu, W.; Nelson, C.M.; Raghavan, S.; Chen, C.S. Simple approach to micropattern cells on common culture substrates by tuning substrate wettability. Tissue Eng. 2004, 10, 865–872. [Google Scholar] [CrossRef]
- Xu, B.; Wei, Q.; Mettetal, M.R.; Han, J.; Rau, L.; Tie, J.; May, R.M.; Pathe, E.T.; Reddy, S.T.; Sullivan, L.; et al. Surface micropattern reduces colonization and medical device-associated infections. J. Med. Microbiol. 2017, 66, 1692–1698. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Rivas, A.; González-Quijano, G.K.; Proa-Coronado, S.; Séverac, C.; Dague, E. Methods of Micropatterning and Manipulation of Cells for Biomedical Applications. Micromachines 2017, 8, 347. [Google Scholar] [CrossRef]
- Thery, M. Micropatterning as a tool to decipher cell morphogenesis and functions. J. Cell Sci. 2010, 123, 4201–4213. [Google Scholar] [CrossRef] [Green Version]
- Shirure, V.S.; George, S.C. Design considerations to minimize the impact of drug absorption in polymer-based organ-on-a-chip platforms. Lab Chip 2017, 17, 681–690. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H.J.; Son, K.J.; Koh, W.G. Fabrication of hydrogel-micropatterned nanofibers for highly sensitive microarray-based immunosensors having additional enzyme-based sensing capability. J. Mater. Chem. 2011, 21, 4476–4483. [Google Scholar] [CrossRef]
- Chatzimichail, S.; Supramaniam, P.; Ces, O.; Salehi-Reyhani, A. Micropatterning of planar metal electrodes by vacuum filling microfluidic channel geometries. Sci. Rep. 2018, 8, 14380. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Phan, D.T.; Bender, R.H.F.; Andrejecsk, J.W.; Sobrino, A.; Hachey, S.J.; George, S.C.; Hughes, C.C. Blood-brain barrier-on-a-chip: Microphysiological systems that capture the complexity of the blood-central nervous system interface. Exp. Biol. Med. 2017, 242, 1669–1678. [Google Scholar] [CrossRef]
- Alaie, S.; Robinson, S.S.; Amiri Moghadam, A.A.; Auge, J.; Datye, A.; Sidoti, H.; Doshi, T.; Gharaie, S.H.; Min, J.K.; Mosadegh, B.; et al. Micropatterning of Nonplanar Surfaces on 3D Devices Using Conformal Template Vacuum Bagging. Adv. Mater. Technol. 2018, 3, 1700353. [Google Scholar] [CrossRef]
- Resnik, D.; Možek, M.; Pečar, B.; Janež, A.; Urbančič, V.; Iliescu, C.; Vrtačnik, D. In Vivo Experimental Study of Noninvasive Insulin Microinjection through Hollow Si Microneedle Array. Micromachines 2018, 9, 40. [Google Scholar] [CrossRef]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [Green Version]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef]
- Luo, Z.; Sun, W.; Fang, J.; Lee, K.; Li, S.; Gu, Z.; Dokmeci, M.R.; Khademhosseini, A. Biodegradable Gelatin Methacryloyl Microneedles for Transdermal Drug Delivery. Adv. Healthc. Mater. 2019, 8, e1801054. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 177–200. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Modi, G.; Pillay, V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release 2014, 185, 130–138. [Google Scholar] [CrossRef]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [Green Version]
- Sadeqi, A.; Nejad, H.R.; Kiaee, G.; Sonkusale, S. Cost-effective Fabrication of Chitosan Microneedles for Transdermal Drug Delivery. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 5737–5740. [Google Scholar]
- Lee, J.W.; Han, M.R.; Park, J.H. Polymer microneedles for transdermal drug delivery. J. Drug Target. 2013, 21, 211–223. [Google Scholar] [CrossRef]
- Kochhar, J.S.; Goh, W.J.; Chan, S.Y.; Kang, L. A simple method of microneedle array fabrication for transdermal drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 299–309. [Google Scholar] [CrossRef]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Norman, J.J.; Choi, S.O.; Tong, N.T.; Aiyar, A.R.; Patel, S.R.; Prausnitz, M.R.; Allen, M.G. Hollow microneedles for intradermal injection fabricated by sacrificial micromolding and selective electrodeposition. Biomed. Microdevices 2013, 15, 203–210. [Google Scholar] [CrossRef]
- Choi, S.O.; Kim, Y.C.; Park, J.H.; Hutcheson, J.; Gill, H.S.; Yoon, Y.K.; Prausnitz, M.R.; Allen, M.G. An electrically active microneedle array for electroporation. Biomed. Microdevices 2010, 12, 263–273. [Google Scholar] [CrossRef]
- Lim, C.Y. Metallic Microneedles. U.S. Patent No. US20150335871A1, 26 November 2015. Available online: https://patents.google.com/patent/US20150335871A1/en?oq=US20150335871A1 (accessed on 15 October 2019).
- Nejad, H.R.; Sadeqi, A.; Kiaee, G.; Sonkusale, S. Low-cost and cleanroom-free fabrication of microneedles. Microsyst. Nanoeng. 2018, 4, 17073. [Google Scholar] [CrossRef]
- Robinson, S.S.; Alaie, S.; Sidoti, H.; Auge, J.; Baskaran, L.; Avilés-Fernández, K.; Hollenberg, S.D.; Shepherd, R.F.; Min, J.K.; Dunham, S.N.; et al. Patient-specific design of a soft occluder for the left atrial appendage. Nat. Biomed. Eng. 2018, 2, 8–16. [Google Scholar] [CrossRef]
- Amiri Moghadam, A.A.; Jang, S.J.; Caprio, A.; Liu, J.; Singh, H.S.; Min, J.K.; Dunham, S.; Mosadegh, B. Using Soft Robotic Technology to Fabricate a Proof-of-Concept Transcatheter Tricuspid Valve Replacement (TTVR) Device. Adv. Mater. Technol. 2019, 4, 1800610. [Google Scholar] [CrossRef]
- Amiri Moghadam, A.A.; Alaie, S.; Gharaie, S.; Nath, S.D.; Vawda, S.; Al’Aref, S.J.; Romito, E.A.; Kolli, K.K.; Min, J.K.; Dunham, S.; et al. Toward Development of Inflatable Stents with Application in Endovascular Treatments. Adv. Funct. Mater. 2018, 28, 1804147. [Google Scholar] [CrossRef]
- Wiggins, M.J.; MacEwan, M.; Anderson, J.M.; Hiltner, A. Effect of soft-segment chemistry on polyurethane biostability during in vitro fatigue loading. J. Biomed. Mater. Res. A 2004, 68, 668–683. [Google Scholar] [CrossRef]
- Chen, Z.; Ward, R.; Tian, Y.; Malizia, F.; Gracias, D.H.; Shen, Y.R.; Somorjai, G.A. Interaction of fibrinogen with surfaces of end-group-modified polyurethanes: A surface-specific sum-frequency-generation vibrational spectroscopy study. J. Biomed. Mater. Res. 2002, 62, 254–264. [Google Scholar] [CrossRef]
- Norland Optical Adhesive 63. Available online: https://www.norlandprod.com/adhesives/NOA 63.html (accessed on 15 October 2019).
- Johnson, A.R.; Caudill, C.L.; Tumbleston, J.R.; Bloomquist, C.J.; Moga, K.A.; Ermoshkin, A.; Shirvanyants, D.; Mecham, S.J.; Luft, J.C.; DeSimone, J.M. Single-Step Fabrication of Computationally Designed Microneedles by Continuous Liquid Interface Production. PLoS ONE 2016, 11, e0162518. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.-J.; Doshi, T.; Nerayo, J.; Caprio, A.; Alaie, S.; Auge, J.; Min, J.K.; Mosadegh, B.; Dunham, S. Microneedle Patterning of 3D Nonplanar Surfaces on Implantable Medical Devices Using Soft Lithography. Micromachines 2019, 10, 705. https://doi.org/10.3390/mi10100705
Jang S-J, Doshi T, Nerayo J, Caprio A, Alaie S, Auge J, Min JK, Mosadegh B, Dunham S. Microneedle Patterning of 3D Nonplanar Surfaces on Implantable Medical Devices Using Soft Lithography. Micromachines. 2019; 10(10):705. https://doi.org/10.3390/mi10100705
Chicago/Turabian StyleJang, Sun-Joo, Tejas Doshi, Jerusalem Nerayo, Alexandre Caprio, Seyedhamidreza Alaie, Jordyn Auge, James K. Min, Bobak Mosadegh, and Simon Dunham. 2019. "Microneedle Patterning of 3D Nonplanar Surfaces on Implantable Medical Devices Using Soft Lithography" Micromachines 10, no. 10: 705. https://doi.org/10.3390/mi10100705





