Handheld Inkjet Printing Paper Chip Based Smart Tetracycline Detector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Milk Samples
2.3. Inkjet Printing Paper Chip Preparation
2.4. Paper Chip based ELISA Testing
2.5. Tetracycline Detection using Commercial ELISA Kit
3. Results
3.1. Smartphone Based Paper Chip Reader and Application
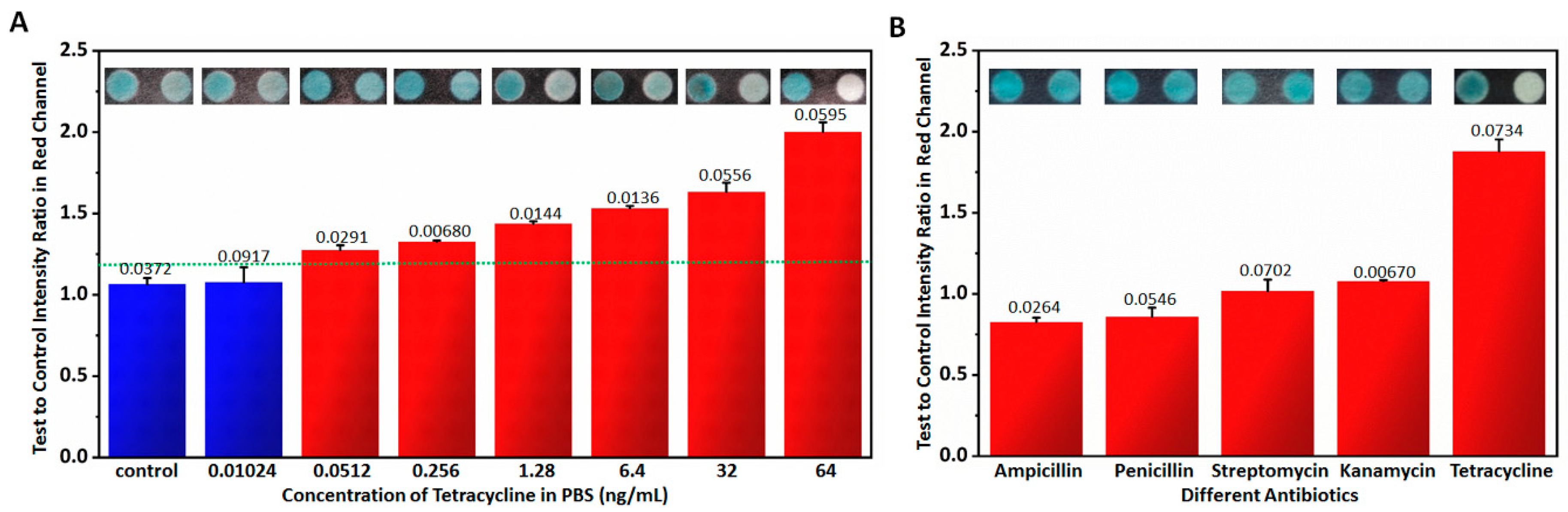
3.2. Sensitivity, Specificity and Stability Testing
3.3. Practical Applications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. 2016, 88, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammie, S.L.; Hughes, J.M. Antimicrobial Resistance, Food Safety, and One Health: The Need for Convergence. Annu. Rev. Food Sci. Technol. 2016, 7, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Ortelli, D.; Sporri, A.S.; Edder, P. Veterinary Drug Residue in Food of Animal Origin in Switzerland: A Health Concern? Chimia 2018, 72, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, S.; Yu, W.; Liu, Z.; Lei, L.; Li, N.; Zhang, H.; Yu, Y. Ionic liquid-anionic surfactant based aqueous two-phase extraction for determination of antibiotics in honey by high-performance liquid chromatography. Talanta 2014, 124, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Wang, Y.; Nie, C.; Ran, D.; Jia, X. Preparation of magnetic mixed-templates molecularly imprinted polymer for the separation of tetracycline antibiotics from egg and honey samples. Anal. Methods 2012, 4, 1005–1011. [Google Scholar] [CrossRef]
- Lv, Y.-K.; Zhang, J.-Q.; He, Y.-D.; Zhang, J.; Sun, H.-W. Adsorption-controlled preparation of molecularly imprinted hybrid composites for selective extraction of tetracycline residues from honey and milk. New J. Chem. 2014, 38, 802–808. [Google Scholar] [CrossRef]
- Vidal, J.L.; Aguilera-Luiz Mdel, M.; Romero-Gonzalez, R.; Frenich, A.G. Multiclass analysis of antibiotic residues in honey by ultraperformance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2009, 57, 1760–1767. [Google Scholar] [CrossRef]
- Aga, D.S.; O’Connor, S.; Ensley, S.; Payero, J.O.; Snow, D.; Tarkalson, D. Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2005, 53, 7165–7171. [Google Scholar] [CrossRef]
- Tarapoulouzi, M.; Papachrysostomou, C.; Constantinou, S.; Kanari, P.; Hadjigeorgiou, M. Determinative and confirmatory method for residues of tetracyclines in honey by LC-MS/MS. Food Addit. Contam. 2013, 30, 1728–1732. [Google Scholar] [CrossRef]
- De Wasch, K.; Okerman, L.; Croubels, S.; De Brabander, H.; Van Hoof, J.; De Backer, P. Detection of residues of tetracycline antibiotics in pork and chicken meat: Correlation between results of screening and confirmatory tests. Analyst 1998, 123, 2737–2741. [Google Scholar] [CrossRef]
- Tumini, M.; Nagel, O.G.; Althaus, R.L. Microbiological bioassay using Bacillus pumilus to detect tetracycline in milk. J. Dairy Res. 2015, 82, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Liu, H.; Xu, L.; Tian, L.; Luan, F. Matrix Solid-Phase Dispersion Extraction and Capillary Electrophoresis Determination of Tetracycline Residues in Milk. Food Anal. Methods 2012, 5, 148–153. [Google Scholar] [CrossRef]
- Tong, J.; Rao, Q.; Zhu, K.; Jiang, Z.; Ding, S. Simultaneous determination of five tetracycline and macrolide antibiotics in feeds using HPCE. J. Sep. Sci. 2009, 32, 4254–4260. [Google Scholar] [CrossRef] [Green Version]
- Reybroeck, W.; Ooghe, S.; Brabander, H.D.; Daeseleire, E. Validation of the Tetrasensor Honey Test Kit for the Screening of Tetracyclines in Honey. J. Agric. Food Chem. 2007, 55, 8359–8366. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, V.; Germagnoli, L.; Banfi, G. Point-of-care testing: Where is the evidence? A systematic survey. Clin. Chem. Lab. Med. 2014, 52, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Li, Y.; Xu, X.; Yuan, M.; Wang, S. Enzyme-linked immunosorbent assay and colloidal gold-based immunochromatographic assay for several (fluoro)quinolones in milk. Microchim. Acta 2011, 173, 307–316. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, J.; Paeng, K.-J.; Park, S.-W.; Paeng, I.R. Biotin–avidin mediated competitive enzyme-linked immunosorbent assay to detect residues of tetracyclines in milk. Microchem. J. 2008, 88, 26–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Liu, W.; Zhao, C.; Xi, R. Preparation of Anti-Tetracycline Antibodies and Development of an Indirect Heterologous Competitive Enzyme-Linked Immunosorbent Assay to Detect Residues of Tetracycline in Milk. J. Agric. Food Chem. 2007, 55, 211–218. [Google Scholar] [CrossRef]
- Chatterjee, K.; Kuo, C.W.; Chen, A.; Chen, P. Detection of residual rifampicin in urine via fluorescence quenching of gold nanoclusters on paper. J. Nanobiotechnol. 2015, 13, 46. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Gao, Z.; Luo, Y.; Liu, X.; Zhao, W.; Lin, B. Manual-slide-engaged paper chip for parallel SERS-immunoassay measurement of clenbuterol from swine hair. Electrophoresis 2016, 37, 418–424. [Google Scholar] [CrossRef]
- Cheng, C.-M.; Martinez, A.W.; Gong, J.; Mace, C.R.; Phillips, S.T.; Carrilho, E.; Mirica, K.A.; Whitesides, G.M. Paper-Based ELISA. Angew. Chem. 2010, 122, 4881–4884. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Luo, Y.; Shi, B.; Liu, X.; Gao, Z.; Du, Y.; Zhao, W.; Lin, B. Chemiluminescence diminishment on a paper-based analytical device: High throughput determination of β-agonists in swine hair. Anal. Methods 2014, 6, 9684–9690. [Google Scholar] [CrossRef]
- Prado, I.C.; Souza, A.L.A.; Provance, D.W., Jr.; Cassella, R.J.; De-Simone, S.G. Ultrasensitive and rapid immuno-detection of human IgE anti-therapeutic horse sera using an electrochemical immunosensor. Anal. Biochem. 2017, 538, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D. Rapid Detection of Enterobacter Sakazakii in milk Powder using amino modified chitosan immunomagnetic beads. Int. J. Biol. Macromol. 2016, 93, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Suzuki, K.; Citterio, D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal. Biochem. 2008, 80, 6928–6934. [Google Scholar] [CrossRef] [PubMed]
- Wickstrom, H.; Hilgert, E.; Nyman, J.O.; Desai, D.; Sen Karaman, D.; de Beer, T.; Sandler, N.; Rosenholm, J.M. Inkjet Printing of Drug-Loaded Mesoporous Silica Nanoparticles-A Platform for Drug Development. Molecules 2017, 22, 2020. [Google Scholar] [CrossRef] [PubMed]
- Apilux, A.; Ukita, Y.; Chikae, M.; Chailapakul, O.; Takamura, Y. Development of automated paper-based devices for sequential multistep sandwich enzyme-linked immunosorbent assays using inkjet printing. Lab Chip 2013, 13, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wei, Q. Plasmonic molecular assays: Recent advances and applications for mobile health. Nano Res. 2018, 11, 5439–5473. [Google Scholar] [CrossRef]
- Wei, Q.; Luo, W.; Chiang, S.; Kappel, T.; Mejia, C.; Tseng, D.; Chan, R.Y.; Yan, E.; Qi, H.; Shabbir, F.; et al. Imaging and sizing of single DNA molecules on a mobile phone. ACS Nano 2014, 8, 12725–12733. [Google Scholar] [CrossRef]
- Wei, Q.; Nagi, R.; Sadeghi, K.; Feng, S.; Yan, E.; Ki, S.J.; Caire, R.; Tseng, D.; Ozcan, A. Detection and spatial mapping of mercury contamination in water samples using a smart-phone. ACS Nano 2014, 8, 1121–1129. [Google Scholar] [CrossRef]
- Meng, X.; Huachuan, H.; Yan, K.; Tian, X.; Yu, W.; Cui, H.; Kong, Y.; Xue, L.; Liu, C.; Wang, S. Smartphone based hand-held quantitative phase microscope using transport of intensity equation method. Lab Chip 2016, 17, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Huang, C.H.; Lee, K.; Yoo, Y.E.; Castro, C.M.; Weissleder, R.; Lee, H. Rapid identification of health care-associated infections with an integrated fluorescence anisotropy system. Sci. Adv. 2016, 2, e1600300. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; O’Dell, D.; Jiang, L.; Oncescu, V.; Gumus, A.; Lee, S.; Mancuso, M.; Mehta, S. Smartphone technology can be transformative to the deployment of lab-on-chip diagnostics. Lab Chip 2014, 14, 3159–3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Ge, L.; Song, X.; Yu, J.; Ge, S.; Huang, J.; Zeng, F. Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens. Bioelectron. 2012, 31, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Long, G.L.; Winefordner, J.D. Limit of Detection a Closer Look at the IUPAC Definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar] [CrossRef]
- Ren, M.; Xu, H.; Huang, X.; Kuang, M.; Xiong, Y.; Xu, H.; Xu, Y.; Chen, H.; Wang, A. Immunochromatographic assay for ultrasensitive detection of aflatoxin B(1) in maize by highly luminescent quantum dot beads. ACS Appl. Mater. Interfaces 2014, 6, 14215–14222. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Gao, Y.; Li, Y.; Liu, S.; Su, X. A novel sensing strategy for the detection of Staphylococcus aureus DNA by using a graphene oxide-based fluorescent probe. Analyst 2013, 138, 2749–2754. [Google Scholar] [CrossRef]
- Hu, J.; Wen, C.Y.; Zhang, Z.L.; Xie, M.; Hu, J.; Wu, M.; Pang, D.W. Optically encoded multifunctional nanospheres for one-pot separation and detection of multiplex DNA sequences. Anal. Chem. 2013, 85, 11929–11935. [Google Scholar] [CrossRef]





| Sample | Concentration (ng/mL) | Proposed Method (n = 5) | ELISA (n = 5) |
|---|---|---|---|
| PBS | 0 | − − − − − | − − − − − |
| 0.01024 | − − − − − | − − − − − | |
| 0.0512 | + + + + + | + + + + + | |
| 0.256 | + + + + + | + + + + + | |
| 1.28 | + + + + + | + + + + + | |
| 6.4 | + + + + + | + + + + + | |
| 32 | + + + + + | + + + + + | |
| 64 | + + + + + | + + + + + |
| Tetracycline (ng/mL) | Paper Chips Fabricated in 5 Batches | Paper Chips Conserved with Different Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Batch 1 | Batch 2 | Batch 3 | Batch 4 | Batch 5 | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | |
| 0 | − | − | − | − | − | − | − | − | − | − |
| 0.0512 | + | + | + | + | + | + | + | + | + | + |
| 0.256 | + | + | + | + | + | + | + | + | + | + |
| 1.28 | + | + | + | + | + | + | + | + | + | + |
| Sample | Concentration (ng/mL) | Proposed Method (n = 5) | ELISA (n = 5) |
|---|---|---|---|
| Milk | 0 | − − − − − | − − − − − |
| 0 | − − − − − | − − − − − | |
| 0 | − − − − − | − − − − − | |
| 0.01024 | − − − − − | − − − − − | |
| 0.0512 | + + + + + | + + + + + | |
| 6.4 | + + + + + | + + + + + | |
| 160 | + + + + + | + + + + + | |
| 800 | + + + + + | + + + + + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, X.; Shan, Y.; Huang, H.; Jian, D.; Xue, L.; Wang, S.; Liu, F. Handheld Inkjet Printing Paper Chip Based Smart Tetracycline Detector. Micromachines 2019, 10, 27. https://doi.org/10.3390/mi10010027
Li J, Wang X, Shan Y, Huang H, Jian D, Xue L, Wang S, Liu F. Handheld Inkjet Printing Paper Chip Based Smart Tetracycline Detector. Micromachines. 2019; 10(1):27. https://doi.org/10.3390/mi10010027
Chicago/Turabian StyleLi, Jiahao, Xin Wang, Yanke Shan, Huachuan Huang, Dan Jian, Liang Xue, Shouyu Wang, and Fei Liu. 2019. "Handheld Inkjet Printing Paper Chip Based Smart Tetracycline Detector" Micromachines 10, no. 1: 27. https://doi.org/10.3390/mi10010027





