Investigation of Non-Covalent Interactions of Aflatoxins (B1, B2, G1, G2, and M1) with Serum Albumin
Abstract
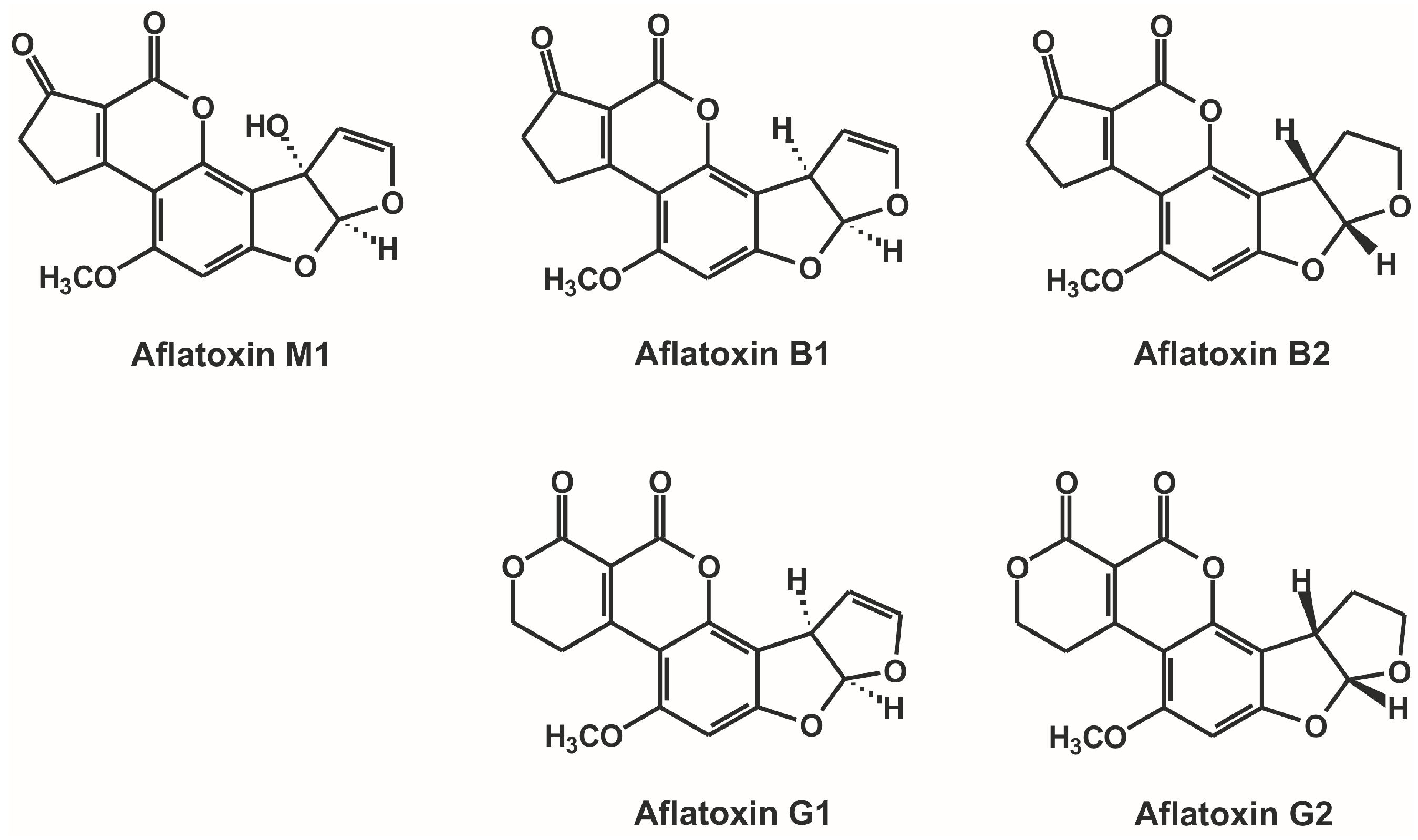
:1. Introduction
2. Results and Discussion
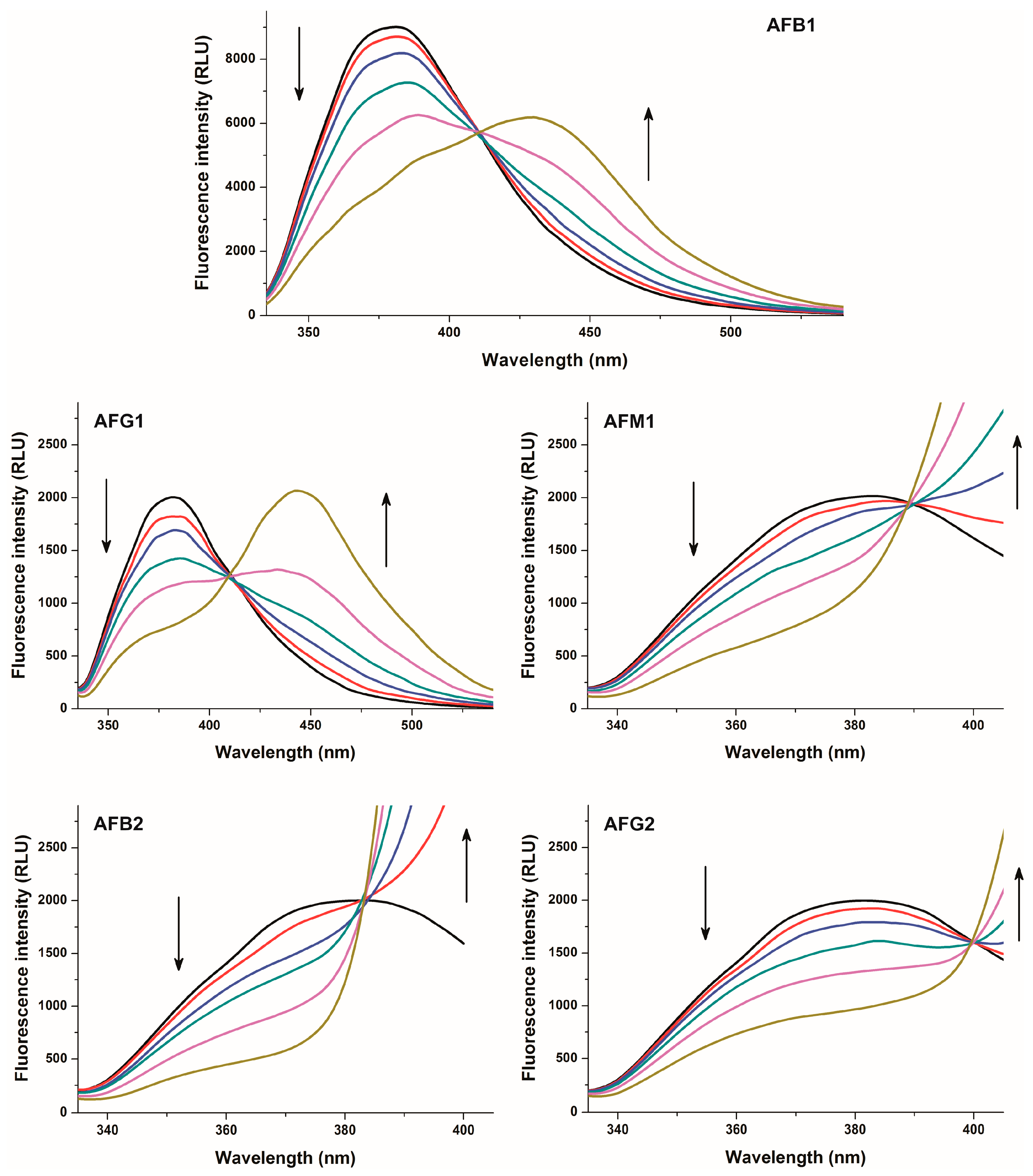
2.1. Fluorescence Properties of AFB1 in the Absence and Presence of HSA
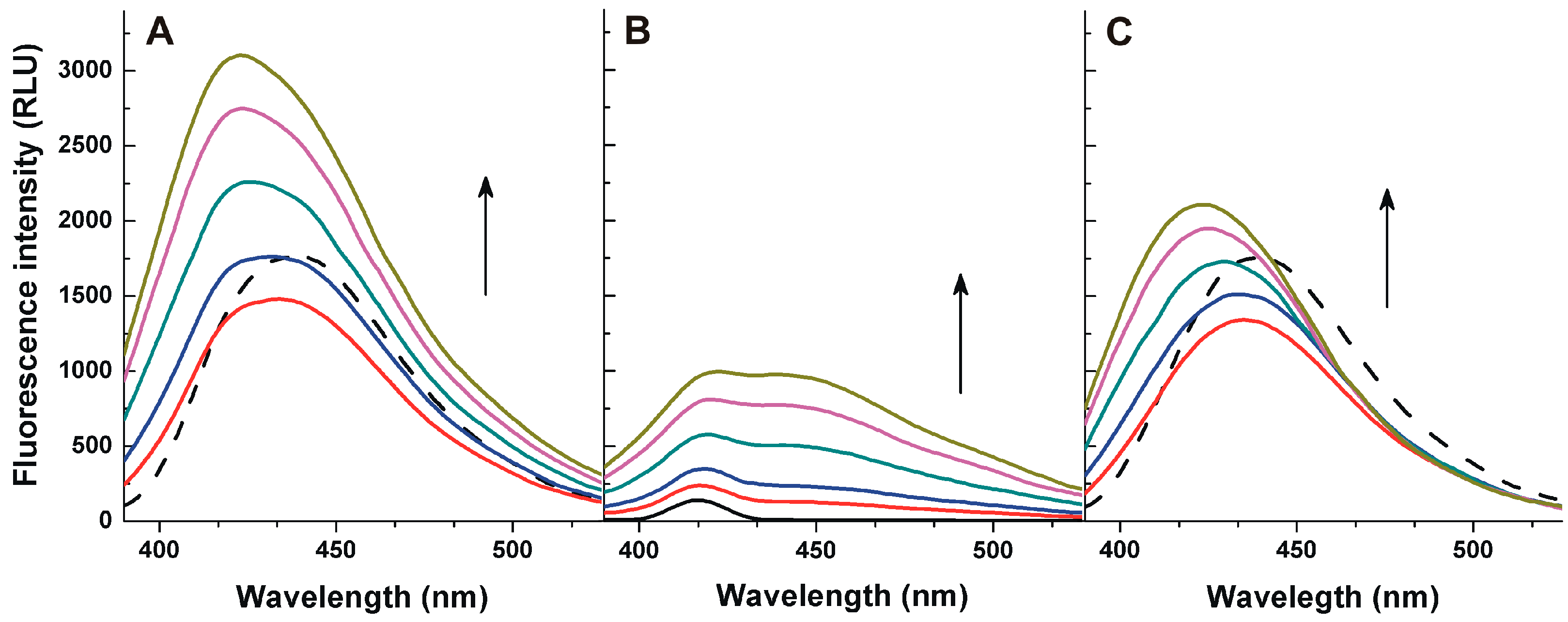
2.2. Investigation of Aflatoxin-HSA Interactions by Fluorescence Quenching Method
2.3. Thermodynamic Studies
2.4. Molecular Modeling of the Binding of Aflatoxins to HSA
2.5. Investigation of the Binding Site of Aflatoxins on HSA Using Site Markers
2.6. Interaction of AFB1 with Bovine, Porcine, and Rat Serum Albumins
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Spectroscopic Measurements
4.3. Thermodynamic Studies
4.4. Structural Calculations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Da Rocha, M.E.B.; da Chagas Oliveira Freire, F.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120, S28–S48. [Google Scholar] [CrossRef] [PubMed]
- Mishra, H.N.; Das, C. A review on biological control and metabolism of aflatoxin. Crit. Rev. Food Sci. Nutr. 2003, 43, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Ok, H.E.; Kim, H.J.; Shim, W.B.; Lee, H.; Bae, D.H.; Chung, D.H.; Chun, H.S. Natural occurrence of aflatoxin B1 in marketed foods and risk estimates of dietary exposure in Koreans. J. Food Prot. 2007, 70, 2824–2828. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Toscano, P.; van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Naturally Occurring Substances, Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; International Agency for Research on Cancer (IARC): Lyon, France, 1993; Volume 56, p. 362. [Google Scholar]
- Gallagher, E.P.; Wienkers, L.C.; Stapleton, P.L.; Kunze, K.L.; Eaton, D.L. Role of human microsomal and human complementary DNA-expressed cytochromes P4501A2 and P4503A4 in the bioactivation of aflatoxin B1. Cancer Res. 1994, 54, 101–108. [Google Scholar] [PubMed]
- Ueng, Y.F.; Shimada, T.; Yamazaki, H.; Guengerich, F.P. Oxidation of aflatoxin B1 by bacterial recombinant human cytochrome P450 enzymes. Chem. Res. Toxicol. 1995, 8, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.A.; Essigmann, J.M.; Wogan, G.N. Excretion of an aflatoxin-guanine adduct in the urine of aflatoxin B1-treated rats. Cancer Res. 1981, 41, 650–654. [Google Scholar] [PubMed]
- Sabbioni, G.; Skipper, P.L.; Buchi, G.; Tannenbaum, S.R. Isolation and characterization of the major serum albumin adduct formed by aflatoxin B1 in vivo in rats. Carcinogenesis 1987, 8, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Becker-Algeri, T.A.; Castagnaro, D.; de Bortoli, K.; de Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81, R544–R552. [Google Scholar] [CrossRef] [PubMed]
- Lamplugh, S.M.; Hendrickse, R.G.; Apeagyei, F.; Mwanmut, D.D. Aflatoxins in breast milk, neonatal cord blood, and serum of pregnant women. Br. Med. J. 1988, 296, 968. [Google Scholar] [CrossRef]
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Albumin-drug interaction and its clinical implication. Biochim. Biophys. Acta 2013, 1830, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F. Subdomain IB is the third major drug binding region of human serum albumin: Toward the three-sites model. Mol. Pharm. 2013, 10, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Poór, M.; Lemli, B.; Bálint, M.; Hetényi, C.; Sali, N.; Kőszegi, T.; Kunsági-Máté, S. Interaction of citrinin with human serum albumin. Toxins 2015, 7, 5155–5166. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Jia, B.; Liu, C.; Liu, K.; Qi, Y.; Hu, Z. Study of the interaction of deoxynivalenol with human serum albumin by spectroscopic technique and molecular modelling. Food Addit. Contam. Part A 2013, 30, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Yuqin, L.; Guirong, Y.; Zhen, Y.; Caihong, L.; Baoxiu, J.; Jiao, C.; Yurong, G. Investigation of the interaction between patulin and human serum albumin by a spectroscopic method, atomic force microscopy, and molecular modeling. Biomed. Res. Int. 2014, 2014, 734850. [Google Scholar] [CrossRef] [PubMed]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111. [Google Scholar]
- Poór, M.; Kunsági-Máté, S.; Bálint, M.; Hetényi, C.; Gerner, Z.; Lemli, B. Interaction of mycotoxin zearalenone with human serum albumin. J. Photochem. Photobiol. B 2017, 170, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.S.; Skipper, P.L.; Peng, X.; Groopman, J.D.; Chen, J.S.; Wogan, G.N.; Tannenbaum, S.R. Serum albumin adducts in the molecular epidemiology of aflatoxin carcinogenesis: Correlation with aflatoxin B1 intake and urinary excretion of aflatoxin M1. Carcinogenesis 1988, 9, 1323–1325. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Hudson, G.J.; Sabbioni, G.; Chapot, B.; Hall, A.J.; Wogan, G.N.; Whittle, H.; Montesano, R.; Groopman, J.D. Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiol. Biomark. Prev. 1992, 1, 229–234. [Google Scholar]
- Dirr, H.W.; Schabort, J.C. Aflatoxin B1 transport in rat blood plasma. Binding to albumin in vivo and in vitro and spectrofluorimetric studies into the nature of the interaction. Biochim. Biophys. Acta 1986, 881, 383–390. [Google Scholar] [CrossRef]
- Vuignier, K.; Schappler, J.; Veuthey, J.L.; Carrupt, P.A.; Martel, S. Drug–protein binding: A critical review of analytical tools. Anal. Bioanal. Chem. 2010, 398, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, B.; Spiteller, M. Raman Spectroscopic and Mass Spectrometric Determination of Aflatoxins. Food Anal. Meth. 2014, 7, 242–256. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Z.; Tan, X.; Xiao, X.; Wang, P.; Wu, L.; Shao, K.; Yin, W.; Han, H. Ultrasensitive detection of aflatoxin B1 by SERS aptasensor based on exonuclease-assisted recycling amplification. Biosens. Bioelectron. 2017, 97, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, M.; Yang, X.; Li, H.; Guo, Z.; Rahma, M.H. A large Raman scattering cross-section molecular embedded SERS aptasensor for ultrasensitive Aflatoxin B1 detection using CS-Fe3O4 for signal enrichment. Spectrochim. Acta A 2018, 189, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Poór, M.; Kunsági-Máté, S.; Bencsik, T.; Petrik, J.; Vladimir-Knežević, S.; Kőszegi, T. Flavonoid aglycones can compete with Ochratoxin A for human serum albumin: A new possible mode of action. Int. J. Biol. Macromol. 2012, 51, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Poór, M.; Li, Y.; Kunsági-Máté, S.; Varga, Z.; Hunyadi, A.; Dankó, B.; Chang, F.R.; Wu, Y.C.; Kőszegi, T. Protoapigenone derivatives: Albumin binding properties and effects on HepG2 cells. J. Photochem. Photobiol. B 2013, 124, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Czibulya, Z.; Poór, M.; Lecomte, S.; Kiss, L.; Harte, E.; Kőszegi, T.; Kunsági-Máté, S. Thermodynamic study of the effects of ethanol on the interaction of ochratoxin A with human serum albumin. J. Lumin. 2014, 148, 18–25. [Google Scholar] [CrossRef]
- Poór, M.; Li, Y.; Kunsági-Máté, S.; Petrik, J.; Vladimir-Knežević, S.; Kőszegi, T. Molecular displacement of warfarin from human serum albumin by flavonoid aglycones. J. Lumin. 2013, 142, 122–127. [Google Scholar] [CrossRef]
- Ahlfors, C.E. Competitive interaction of biliverdin and bilirubin only at the primary bilirubin binding site on human albumin. Anal. Biochem. 1981, 110, 295–307. [Google Scholar] [CrossRef]
- Hu, T.; Liu, Y. Probing the interaction of cefodizime with human serum albumin using multi-spectroscopic and molecular docking techniques. J. Pharm. Biomed. Anal. 2015, 107, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Hetényi, C.; van der Spoel, D. Efficient docking of peptides to proteins without prior knowledge of the binding site. Protein Sci. 2002, 11, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Hetényi, C.; van der Spoel, D. Blind docking of drug-sized compounds to proteins with up to a thousand residues. FEBS Lett. 2006, 580, 1447–1450. [Google Scholar] [CrossRef] [PubMed]


| logKSV | logKa | n | logK | |
|---|---|---|---|---|
| AFB1–HSA | 4.44 ± 0.06 | 4.46 ± 0.08 | 1.005 ± 0.029 | 4.65 ± 0.01 |
| AFB2–HSA | 4.21 ± 0.02 | 4.40 ± 0.08 | 1.054 ± 0.000 | 4.55 ± 0.01 |
| AFG1–HSA | 4.33 ± 0.04 | 4.43 ± 0.03 | 1.020 ± 0.019 | 4.58 ± 0.01 |
| AFG2–HSA | 3.96 ± 0.04 | 4.04 ± 0.07 | 1.017 ± 0.025 | 4.34 ± 0.01 |
| AFM1–HSA | 4.28 ± 0.01 | 4.26 ± 0.00 | 1.004 ± 0.013 | 4.52 ± 0.01 |
| Complex | ΔH kJ mol−1 | ΔS JK−1 mol−1 | ΔG (298.16 K) kJ mol−1 |
|---|---|---|---|
| AFB1-HSA 1 | −10.48 | 55.09 | −26.90 |
| citrinin-HSA 2 | −24.15 | 20.90 | −29.96 |
| zearalenone-HSA 3 | −30.09 | −3.50 | −29.06 |
| Ligand | Blind Docking | Blind Docking | Focused Docking | Focused Docking |
|---|---|---|---|---|
| RBE | #Rank | RBE | #Rank | |
| AFB1 | 1 | 2 | - | - |
| AFB2 | 0.98 | 2 | 0.99 | 1 |
| AFG1 | 0.95 | 2 | 0.95 | 1 |
| AFG2 | 0.98 | 4 | 0.99 | 1 |
| AFM1 | 0.98 | 2 | 0.97 | 1 |
| logKSV | logKa | n | logK | |
|---|---|---|---|---|
| AFB1–HSA | 4.44 ± 0.06 | 4.46 ± 0.08 | 1.005 ± 0.029 | 4.65 ± 0.01 |
| AFB1–BSA | 4.41 ± 0.06 | 4.44 ± 0.01 | 1.006 ± 0.015 | 4.63 ± 0.01 |
| AFB1–PSA | 4.45 ± 0.00 | 4.52 ± 0.10 | 1.023 ± 0.034 | 4.62 ± 0.01 |
| AFB1–RSA | 4.82 ± 0.01 | 4.90 ± 0.11 | 0.998 ± 0.051 | 4.91 ± 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poór, M.; Bálint, M.; Hetényi, C.; Gődér, B.; Kunsági-Máté, S.; Kőszegi, T.; Lemli, B. Investigation of Non-Covalent Interactions of Aflatoxins (B1, B2, G1, G2, and M1) with Serum Albumin. Toxins 2017, 9, 339. https://doi.org/10.3390/toxins9110339
Poór M, Bálint M, Hetényi C, Gődér B, Kunsági-Máté S, Kőszegi T, Lemli B. Investigation of Non-Covalent Interactions of Aflatoxins (B1, B2, G1, G2, and M1) with Serum Albumin. Toxins. 2017; 9(11):339. https://doi.org/10.3390/toxins9110339
Chicago/Turabian StylePoór, Miklós, Mónika Bálint, Csaba Hetényi, Beatrix Gődér, Sándor Kunsági-Máté, Tamás Kőszegi, and Beáta Lemli. 2017. "Investigation of Non-Covalent Interactions of Aflatoxins (B1, B2, G1, G2, and M1) with Serum Albumin" Toxins 9, no. 11: 339. https://doi.org/10.3390/toxins9110339
APA StylePoór, M., Bálint, M., Hetényi, C., Gődér, B., Kunsági-Máté, S., Kőszegi, T., & Lemli, B. (2017). Investigation of Non-Covalent Interactions of Aflatoxins (B1, B2, G1, G2, and M1) with Serum Albumin. Toxins, 9(11), 339. https://doi.org/10.3390/toxins9110339








