1. Introduction
Pathogenic bacteria employ pore forming toxins (PFTs) to subvert, invade, or destroy host target cells. The factors defining the toxin-susceptibility of different cell types and the species variation for a given cell-type are only partially understood. This limits the development of anti-infective strategies targeting PFTs, which are increasingly in demand due to a dwindling pool of effective antibiotics. Generally, the susceptibility of host cells to a given toxin seems to depend on the presence of specific high-affinity receptors for binding of toxin monomers, subsequent oligomerisation, and pore formation [
1,
2]. Further, the efficiency of the host cell’s repair mechanisms co-determines the outcome of an attack [
3]. Yet another mechanism that may play a role for the impact of pore-forming toxins on target cells is the enhancement of damage by purinergic signaling [
4]. This mechanism is supported by several lines of evidence: in case of RTX-family-toxins (Repeats in ToXin-family), hemolysis was inhibited by inhibitors specific for ATP-gated purinergic receptors (P2XRs) or upon depletion of extracellular ATP [
5,
6]. Since P2XRs are activated by ATP, these observations point towards a role of these receptors for enhancing the effect of RTX-toxin-induced hemolysis. Increased levels of extracellular ATP were measured upon treatment of erythrocytes with the RTX-toxins HlyA (hemolysin A from
E. coli), and LTX (Leukotoxin from
Aggregatibacter Actinomycetemcomitans), and based on the absence of effect of various inhibitors, it was concluded that this increase could not be accounted for by egress through cellular membrane channels, but was likely due to leakage through toxin pores [
7]. Furthermore, leakage of ATP from liposomes loaded with ATP was observed upon addition of RTX toxins, apparently supporting the idea that ATP (mass 507.18 g/mol) can pass through the RTX toxin pores. Notably, under the same conditions, egress of calcein (mass 622.55 g/mol) was not detectable. The concept that P2XR-signaling enhances the hemolytic effect of pore formers was extended to other proteins, including Hla from
S. aureus [
8] and complement [
9]. The collective data were interpreted to indicate that P2XR-signalling is a general enhancer mechanism in hemolysis.
These reports led us to investigate whether P2XR-signaling is also relevant for Hla-dependent toxicity towards spontaneously transformed human adult skin keratinocytes (HaCaT). Our initial finding that pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) inhibited Hla-dependent cytotoxicity seemed to support this idea. However, subsequent binding assays indicated that PPADS already inhibited the interaction of Hla with cells. This raised the question of whether Hla-dependent action on erythrocytes, detected as hemolysis, might be affected by P2XR-inhibitors through similar non-canonical mechanisms. Accordingly, we decided to re-investigate the role of P2XR-signalling for Hla-dependent lysis of erythrocytes. We chose rabbit erythrocytes and Hla from
S. aureus as a model. Rabbit erythrocytes are the most sensitive erythrocytes with respect to Hla-induced hemolysis. Early experiments indicated that the amount of Hla molecules irreversibly bound to rabbit erythrocytes are about 10 monomers per cell at a level of 50% lysis (after 6 h), corresponding to 1–2 pores [
10]. It seems plausible that in order to obtain hemolysis by such a small number of pores, cellular mechanisms enhancing the permeability of the plasma-membrane could be involved. Just as in the studies mentioned above, we investigated the extent of hemolysis in the absence or presence of inhibitors and activators of P2XRs. Furthermore, in order to exclude unspecific interactions between the P2XR-inhibitors, lipid-membranes and Hla, we also studied calcein efflux from liposomes in the presence of these substances. In addition, oligomerisation of Hla in the presence of inhibitors was investigated by gel-electrophoresis, using liposomes or erythrocyte membranes, supplemented by a calorimetric study of the PPADS/Hla binding in absence of liposomes or cells. The results of this study indicate that P2XR-antagonists interfere with binding and/or oligomerisation of Hla to target membranes, raising doubts that P2XRs play a general role in pore-forming toxin-dependent hemolysis.
3. Discussion
Previous observations that P2XR-antagonists inhibit hemolysis caused by several pore forming proteins, including Hla [
5,
6,
8,
9,
13,
14], led to the now widely accepted idea that P2XRs are critical for pore-forming toxin-dependent hemolysis [
4]. This role has been discussed as one of the main functions of these receptors in erythrocytes [
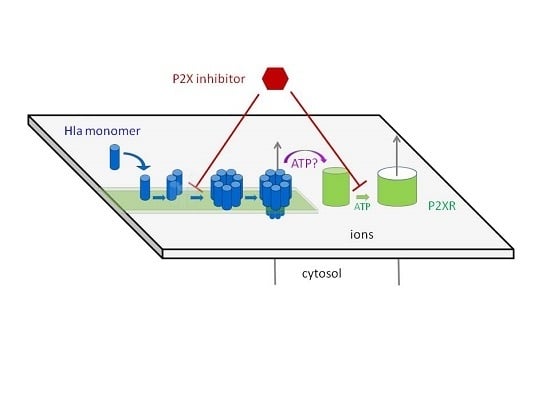
15]. In this context, it is upheld that a few small toxin pores are insufficient to directly cause hemolysis, whereas the release of ATP, and the subsequent activation of P2XRs would trigger substantial ion fluxes, colloid-osmotic swelling of cells, and would ultimately lead to hemolysis.
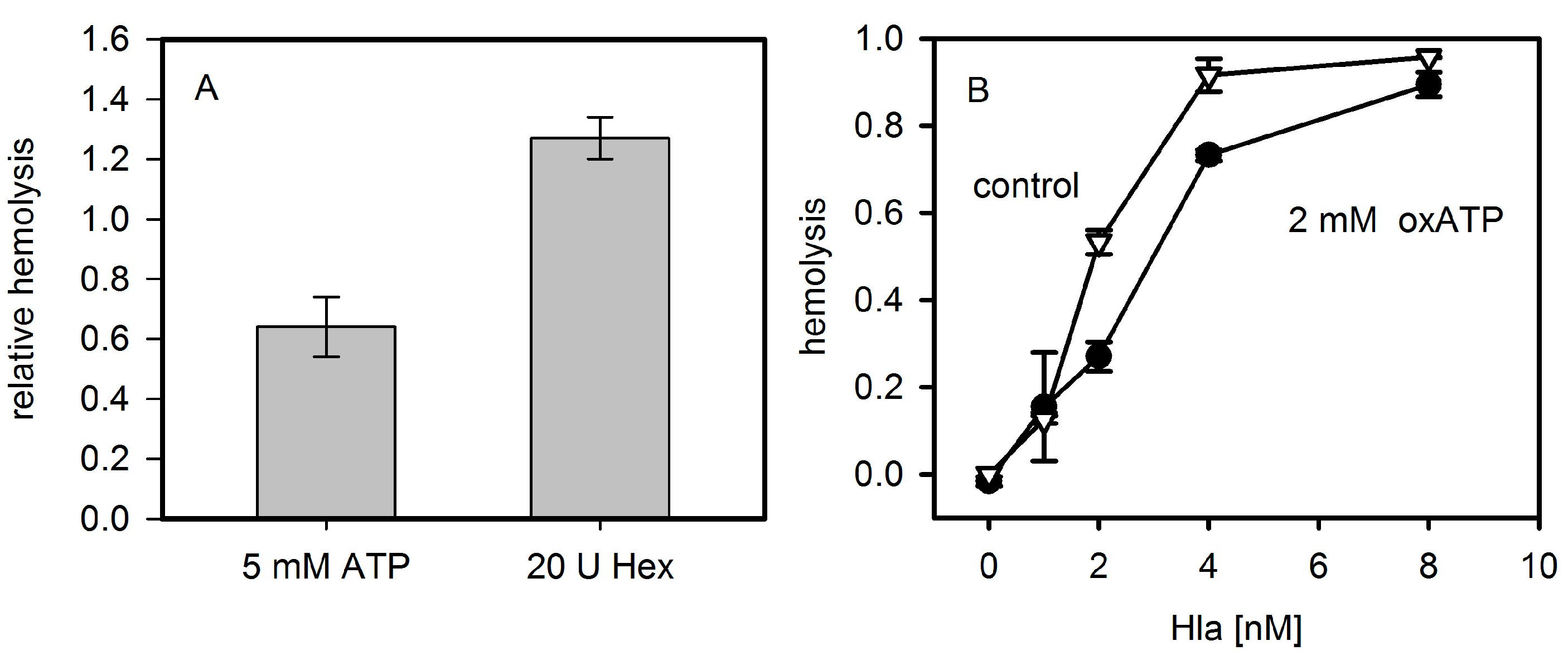
This hypothesis is based on the reduction of hemolysis in the presence of ATP-converting enzymes or P2XR-antagonists, and knock-down experiments [
5,
6,
8,
9]. Interestingly, data reporting an increase of toxin-induced hemolysis due to presence of ATP can only be found for Hla and LTX [
6,
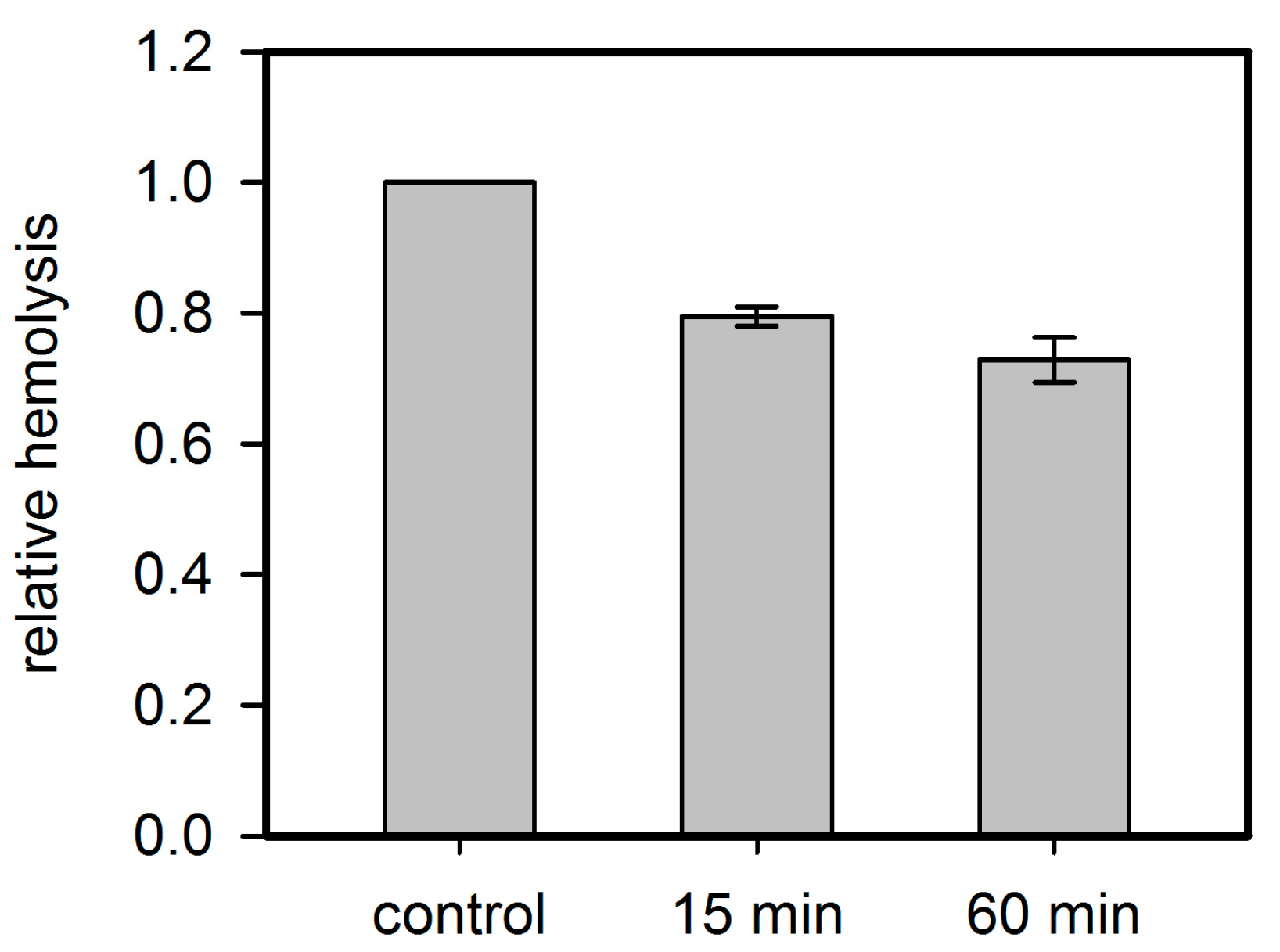
8] and not for other agonists of P2XR. In absence of toxins activation of P2XR-receptors by the addition of ATP to the extracellular medium leads to flux of monovalent ions and to PS-exposure, but not to significant levels of hemolysis at the time scale of typical hemolysis experiments, namely up to 60 min [
16]. Thus, P2XR-receptor activation alone does not lead to hemolysis, indicating that the toxin pores themselves must fulfill a significant part in destabilizing membrane stability and ion homeostasis. In case of Hla, the increase in hemolysis due to presence of 3 mM ATP occurred exclusively at very early points in time (2.5 min): here, the hemolysis level increased from 4% to 6%. Later on, a decrease in hemolysis was observed [
8]. In contrast, an about 3-fold increase of hemolysis at 1 mM ATP after 60 min incubation was reported for human erythrocytes exposed to LTX. To sum up, a general effect of extracellular ATP on PFT-dependent hemolysis has not been unequivocally documented.
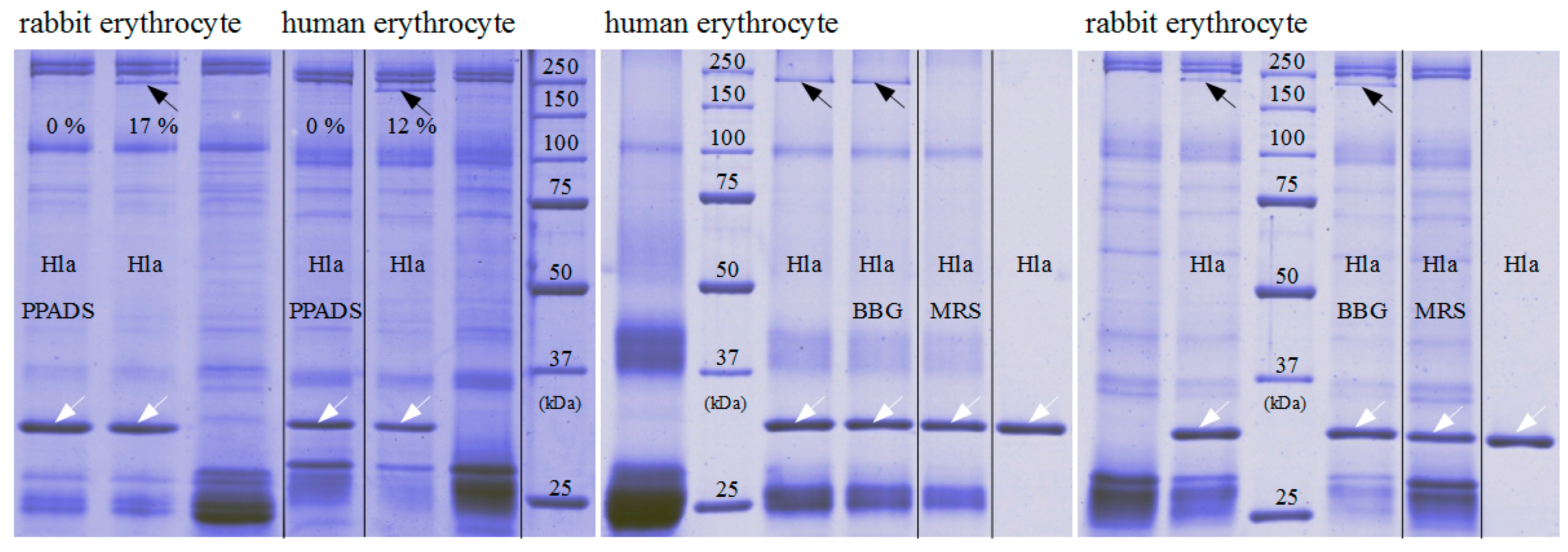
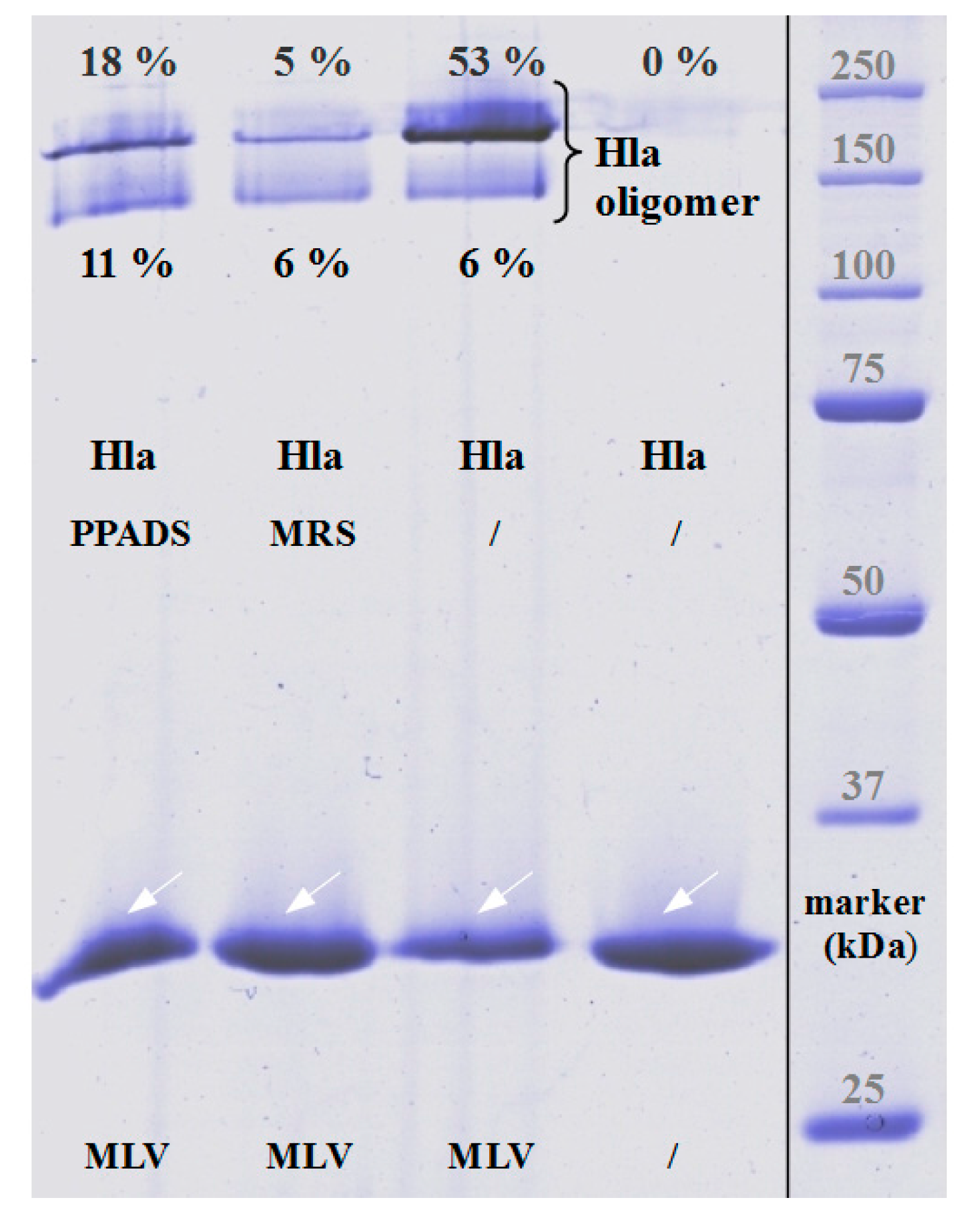
The fact that our data with rabbit erythrocytes deviate in some aspects from an earlier study might in part be related to the different purity levels of the Hla-preparations used: whereas, our preparations from cultures of
S. aureus contain very low amounts of contaminants (see
Figure 5, last lane shows Hla without erythrocytes), Hla from a commercial source consists of merely ~60% protein (according to the manufacturer). Moreover, only part of said protein content appears to be intact Hla [
17]; in the same study, it was shown that even heat-inactivated
S. aureus preparations—thus, being devoid of functional Hla-amplify the response to Hla-treatment in monocytes. Some variation in modulation of hemolytic activity has also been observed for HlyA, when crude and pure preparations are compared [
5].
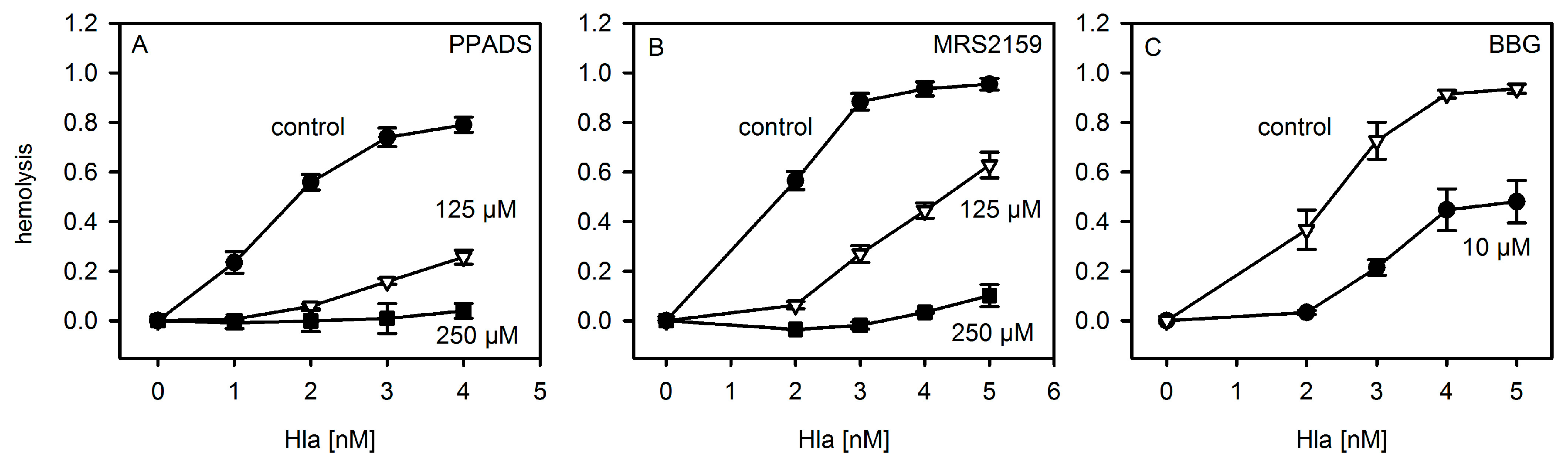
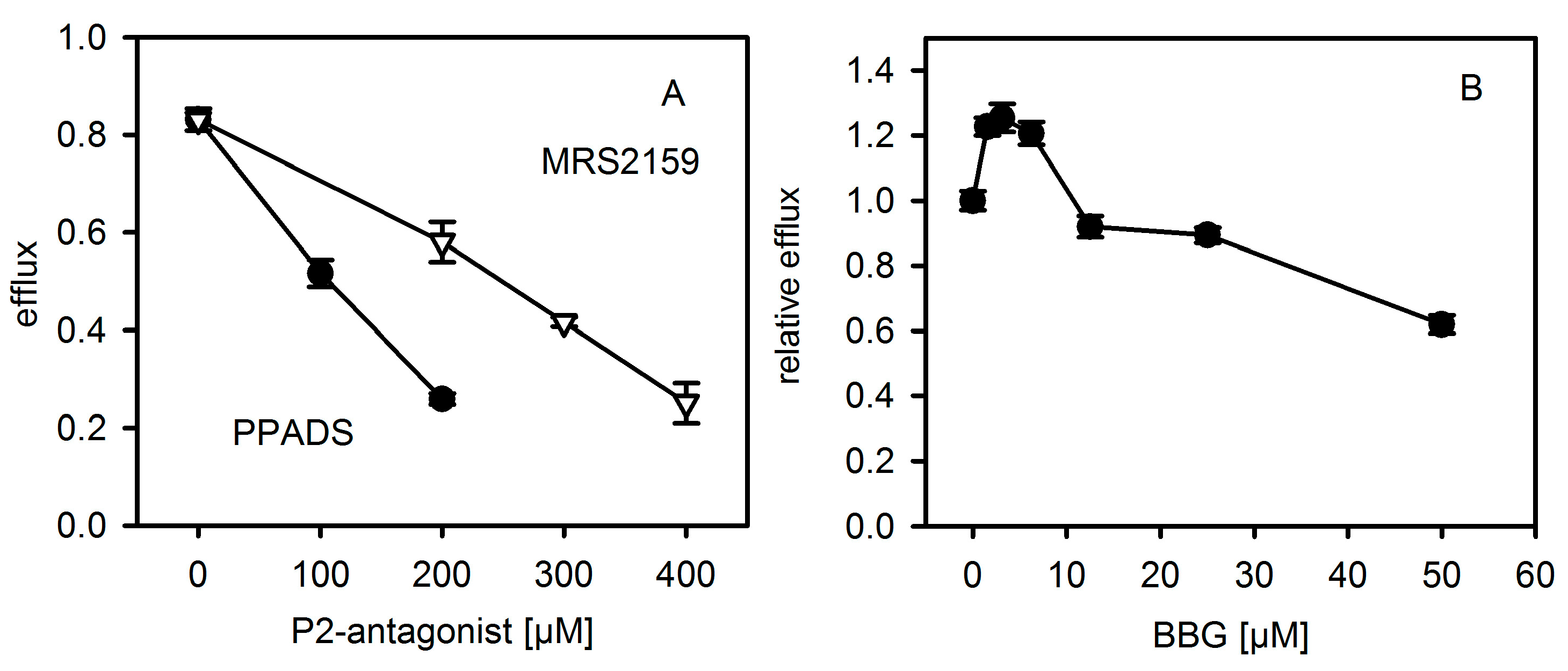
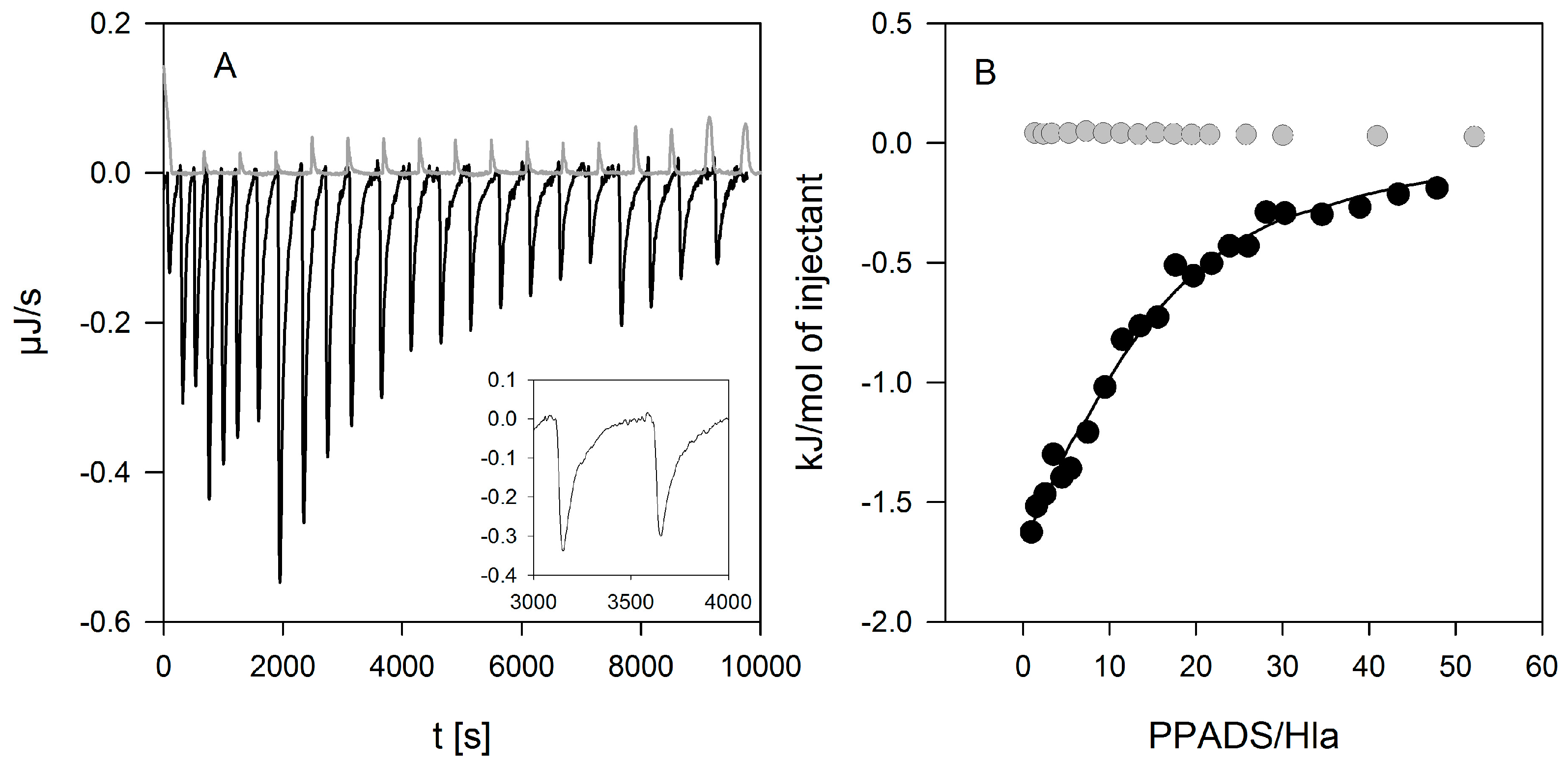
The results presented here cast doubt on the proposed role of P2XR-activation in enhancing hemolysis of erythrocytes by Hla. Previous results thought to support this concept could actually be explained by non-canonical effects of inhibitors. First, PPADS and MRS2159 do not only block Hla-dependent hemolysis, but markedly reduce calcein release from liposomes. Second, the oligomerisation of Hla is drastically reduced by PPADS, as shown on nucleated cells, erythrocytes, and liposomes. Finally, a direct interaction between Hla and PPADS could be revealed by ITC. Notably, reduction of the inhibitory action on Hla persisted after dilution of PPADS following treatment.
Our collective results suggest an interference of PPADS and MRS2159 with Hla binding and oligomer formation, apparently due to the competition between PPADS and membrane constituents for Hla. This nicely fits to the observation that binding of Hla to human erythrocytes—which have an overall lower affinity for Hla—is much more affected by PPADS than binding to rabbit erythrocytes, which carry binding sites of higher affinity [
10]. Inhibition by BBG seems to operate by a different mechanism. The minor effect observed due to the presence of oxATP is not necessarily related to P2XR-inactivation; as it has been shown to be not a specific inhibitor of P2XRs [
18,
19] and the effects might be due to the inhibition of other targets.
To our knowledge, the impact of P2-receptor antagonists on pore formation on pure lipid membranes has only been tested thus far on black lipid membranes; and merely RTX-toxins were studied [
13]. In that work, membrane perforation appeared to not be altered. Yet, the applied concentrations of PPADS might have been simply too low, since under these conditions hemolysis was inhibited by merely 50%. Also, the mode of pore formation by RTX-toxins is likely to be quite different from pore formation by Hla. It is well possible that antagonists do not interact with RTX, but with structurally unrelated Hla. A number of small molecular weight compounds have been identified that seem to interfere with the oligomerisation of Hla [
20,
21,
22], but we are not aware of compounds interfering with RTX-toxin activity.
The present work sheds light on how P2XR-antagonists interfere with the function of a membrane pore-forming toxin through non-canonical mechanisms. The data warrant a critical reassessment of the widely accepted idea that P2XRs are general enhancers of membrane pore formation, and thus they call for further studies in this field.
4. Materials and Methods
4.1. Chemicals
Egg yolk 1,2-diacyl-sn-glycero-3-phosphoethanolamine (ePE), 1,2 -diacyl-sn-glycero-3-phospho-L-serine from bovine brain (bPS), egg yolk N-acyl-d-sphingosine-1-phosphocholine (eSM), and cholesterol (Chol) were from Sigma Aldrich (Deisenhofen, Germany). N-(9Z-octadecenoyl)-sphing-4-enine-1-phosphocholine (OSM) was purchased from Avanti Polar Lipids (Alabaster, AL, USA). All of the lipids were already dissolved in chloroform except for cholesterol, which was obtained as powder and dissolved in chloroform for use. All of the chemicals for gel-electrophoresis and buffer solutions were purchased from Roth GmbH (Karlsruhe, Germany). Hexokinase, PPADS (pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid, tetrasodium salt hydrate), calcein, oxATP, BBG (Brilliant Blue G), and MRS2159 (a derivative of PPADS) were obtained from Sigma Aldrich (Deisenhofen, Germany). ATP Bioluminescence Assay Kit CLSII was purchased from ROCHE (Mannheim, Germany).
4.2. Toxin (Hla)
Except for fluorescence microscopy all of the experiments were carried out with wild type Hla, which was purified from supernatant of
S. aureus cultures (Hla-deficient strain DU1090 transformed with Hla-encoding plasmid pAC), as previously described [
23]. For fluorescence microscopy, the mutant S3C was labeled with 5-iodoacetamidofluorescein (5-IAF, Molecular Probes, Eugene, OR, USA) according to the protocol described earlier [
23]. Internally labeled toxin (
35S-Hla) was prepared as described before [
24].
4.3. ATP-Assay
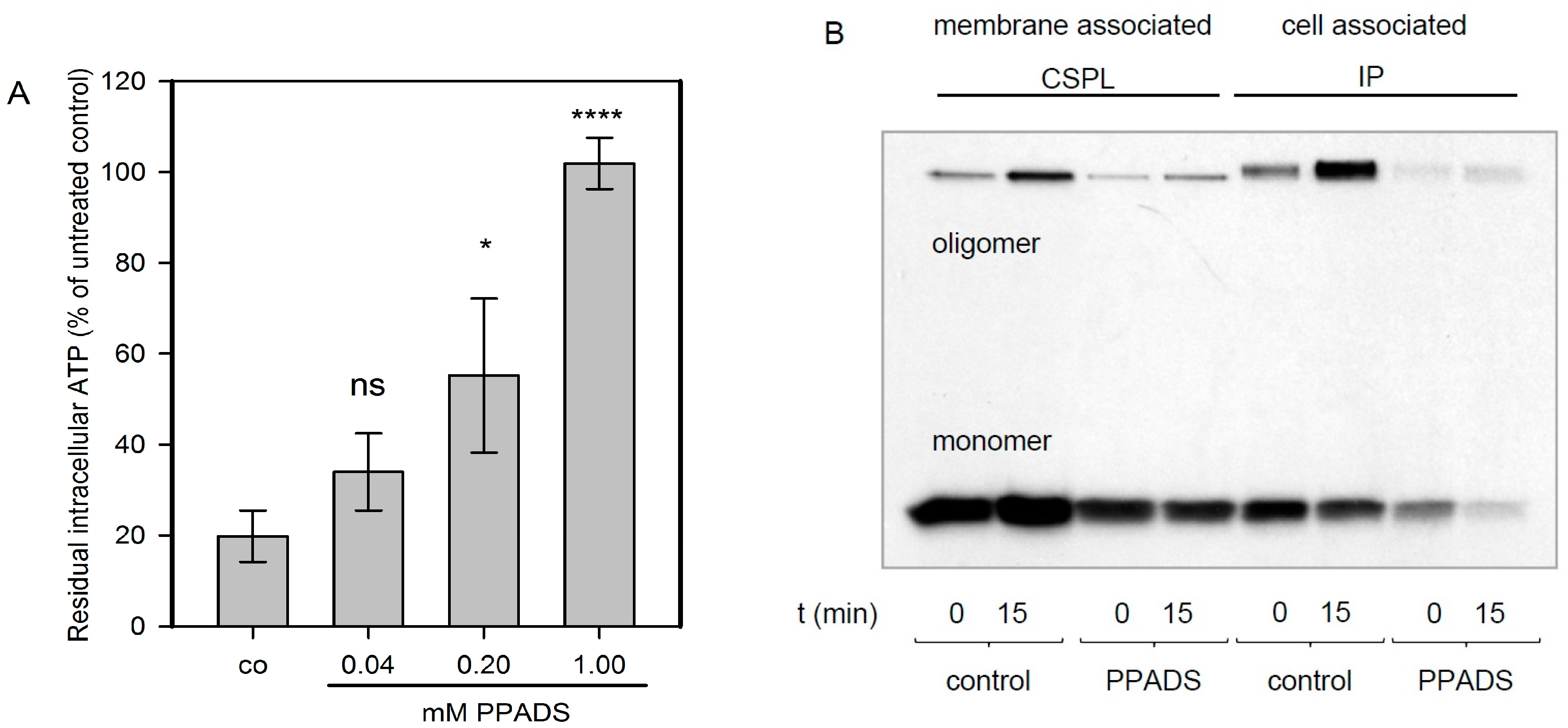
Intracellular ATP levels in cells were measured as described elsewhere [
11]. In brief, HaCaT-cells were seeded at a density of 10
4 cells per well in 96-well tissue culture plates. The next day, treatments were performed and ATP was measured using the ATP Bioluminescence Assay Kit CLSII from ROCHE. The samples were analyzed with a Lumat 9705 instrument from Berthold (Bad Wildbad, Germany).
4.4. Oligomerisation of Hla on HaCaT-Cells
The basic procedure was as described elsewhere [
3]. To assess the impact of PPADS on binding and oligomerisation of Hla on nucleated cells, we used HaCaT-cells, human non-virally transformed keratinocytes [
25]. The cell line, obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany), was free of mycoplasma. Cells were plated on tissue culture dishes 24 h before start of the experiment. After the addition of 1 mM PPADS, plates were incubated at 37 °C for 30 min. Then, the plates were placed on ice and
35S-Hla (~30 nM) was added. After 40 min incubation on ice, cells were briefly washed. Directly after washing (0 min) or after 15 min of incubation at 37 °C, bound toxin was determined. Cell surface protein labeling (CSPL) with biotin and subsequent precipitation via Neutravidin-pull-down was employed to detect Hla on the cell surface, while immunoprecipitation (IP) with Hla-antibody was used to detect total Hla, i.e., toxin associated with both cell surface and cytosol. Neutravidin-precipitates were separated by SDS-PAGE and
35S-Hla-containing bands, representing monomers and SDS-stable oligomers, were visualized by fluorography. Samples for SDS-PAGE were heated to 60 °C for 10 min prior to loading.
4.5. Erythrocytes and Hemolysis Assay
Rabbit erythrocytes were purchased from Fiebig Nährstofftechnik (Idstein, Germany). Human erythrocytes were collected from leucocyte-enriched buffy coats of healthy donors (Transfusion Center Mainz, Germany) and were kindly provided by Dr. Iris Bellinghausen (Department of Dermatology, University Medical Center Mainz, Germany). Informed consent was obtained from all of the donors. All of the experiments with erythrocytes were performed in a buffer containing 14 mM Hepes, 0.8 mM MgSO2, 1.8 mM CaCl2, 5.3 mM KCl, 124 mM NaCl, 5.6 mM glucose, titrated with NaOH to obtain a pH of 7.2 at 37 °C. Erythrocytes were washed by centrifugation (4 °C, 500 g) in this buffer until the supernatant was clear. For the hemolysis assay, the erythrocytes were diluted to about 2.5% yielding an OD of ~0.8 at 415 nm and 1 mm path-length for fully lysed samples. The components of the assay were mixed directly before starting the experiment. The erythrocytes were added at the end at a sufficiently large volume to avoid local concentration effects. Samples were shaken at 200 rpm for 60 min at 37 °C, transferred to ice, centrifuged (4 °C, 500 g) and extracellular hemoglobin in the supernatant was quantified by absorption spectroscopy (NanoDrop ND-1000 Spectrophotometer, peqLab, VWR International, LLC). Hemolysis was determined based on the concentration of hemoglobin measured at 415 nm. At this wavelength, absorption of BBG at the concentrations used is negligible. In the case of PPADS and MRS2159 there is a measurable contribution at this wavelength, but in the concentrations used the contribution it is not very high and could be subtracted by preparing appropriate controls. Additionally, the results were checked by comparison with determination of hemoglobin concentration at 576 nm. Erythrocytes incubated in absence of Hla served as negative control (0% hemolysis), while erythrocytes osmotically lysed by dilution with water served as positive control (100% hemolysis) at the corresponding wavelength. Due to the lack of effect upon the addition of hexokinase, the activity of hexokinase was checked and found to be about 60% of the level reported by the supplier. The buffer for the experiments in the presence of hexokinase contained increased levels of glucose (10 mM) and MgSO4 (2.5 mM). In order to maintain osmolarity, the concentration of NaCl was adjusted accordingly. For the experiments addressing the residual activity of PPADS after dilution to non-active concentrations, PPADS (1 mM) and Hla (2.5 µM) were incubated on ice for the time given. Then, the solution was diluted quickly 1000-fold in two steps, and the diluted solution was incubated with erythrocytes and was prepared for determination of free hemoglobin, as described above. Hla (2.5 µM) incubated in buffer and treated exactly the same way as the PPADS-containing sample served as reference.
4.6. Hla-Oligomerisation on Pure Lipid Membranes (MLVs) and Erythrocytes
MLV preparation was adapted from the thin-film method [
26]. A “pseudo ternary” lipid mixture [
27] made of 40% cholesterol, 20% OSM, 20% ePE, and 20% bPS with a total lipid amount of 1mg was prepared in chloroform. After chloroform evaporation under nitrogen, the lipid film was dried for 3 h under vacuum. The lipid film was then rehydrated by addition of 1 mL 70 mM sodium-phosphate-buffer (pH 7.2). 20 µL of this MLV-solution were mixed with 20 µL solution containing 2.5 µM Hla in absence or presence of 500 µM MRS or PPADS, respectively. After 30 min of incubation at room temperature, the samples were subjected to SDS-gel-electrophoresis [
28] with some modification: the samples were not heated prior to loading, the loading buffer does not contain SDS, and the running buffer contained only 0.1% SDS. Under these conditions, monomeric Hla runs true in the gel, but Hla-heptamers (pores and presumably also some types of pre-pores) retain their oligomeric structure and lead to characteristic high molecular bands. Finally, the gels were stained according to [
29].
Oligomer formation on erythrocytes was determined similarly, employing 0.5% human or rabbit erythrocytes in phosphate buffered saline, which were incubated with 2.5 µM Hla in a final volume of 40 µL for 30 min at room temperature in the presence or absence of P2XR-inhibitors, as indicated. Erythrocytes were then concentrated by centrifugation with 1000 g for 15 min at 4 °C. The supernatant was discarded and the erythrocyte-pellet was supplemented with SDS loading puffer containing 2% SDS in final. The samples were loaded on 10% SDS-gels for electrophoresis, without prior heat-denaturation.
4.7. Hla-Induced Calcein Efflux
Liposomes (LUVs, 100 nm diameter) were prepared, as described before [
27], using a solution of 50 mM calcein and 70 mM phosphate-buffer, pH 7.2 (final) as hydration buffer. Free calcein was removed with a desalting column (PD10, Amersham Biosciences Europe GmbH, Freiburg, Germany). Efflux experiments were performed with a lipid concentration of about 50 µM (20 mol % eSM, 40 mol % cholesterol, 20 mol % ePE, 20 mol % bPS) and 200–300 nM Hla, with P2XR-inhibitor concentrations, as indicated. Liposomes were added at the end into the assay mixture. Fluorescence change due to the dequenching of calcein was followed at 515 nm (excitation 495 nm) with a Hitachi F4500 (Binninger Analytic, Schwaebisch Gmuend, Germany) at 37 °C for 30 min. The level of fluorescence at full leakage was determined by the addition of Triton-X at a final concentration of 2%. Dilution and the inner-filter effect due to the absorbance of P2XR-antagonists was taken into account.
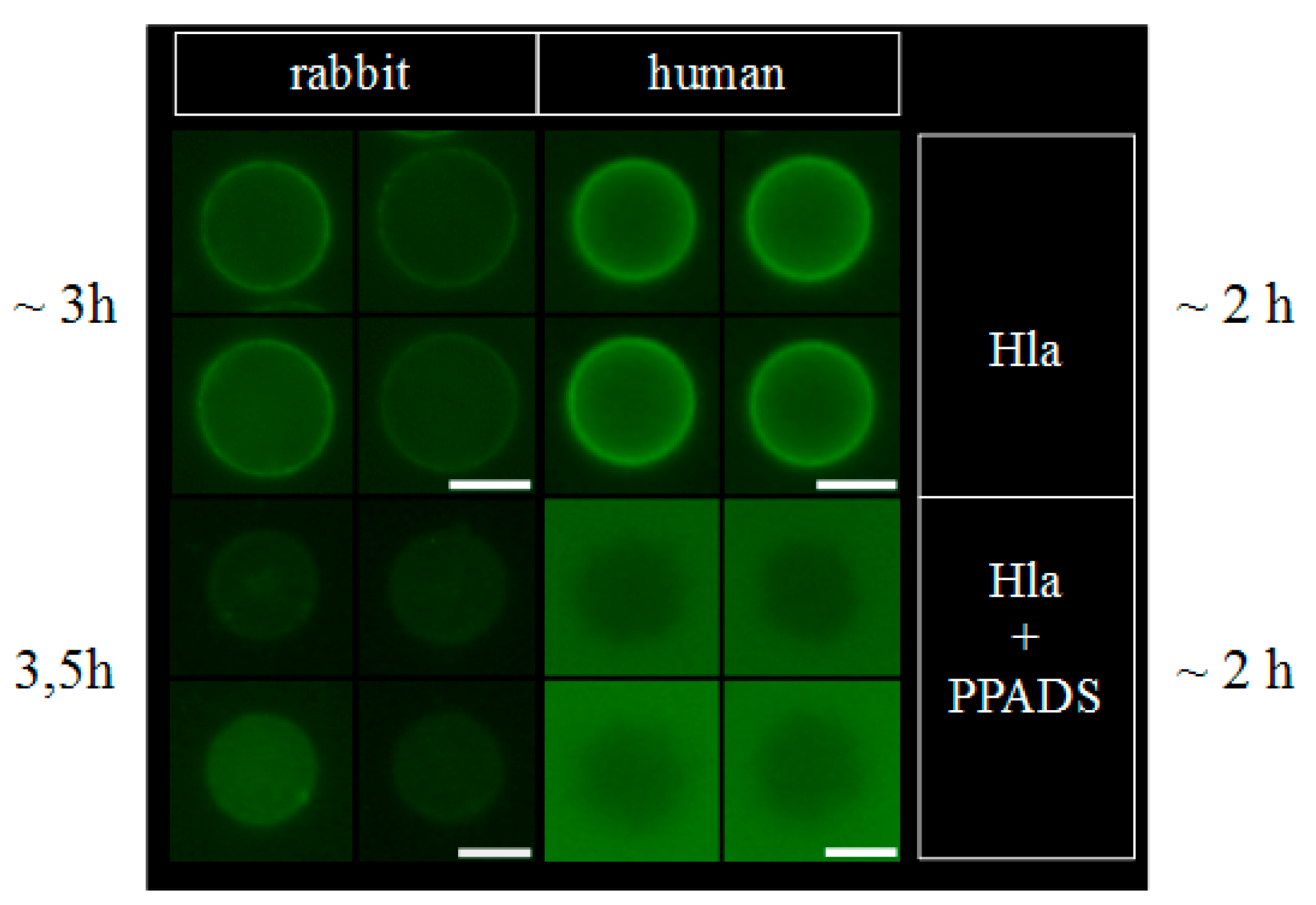
4.8. Fluorescence Microscopy
Fluorescence micrographs were taken with a Keyence BZ 8000K (Keyence Corporation, Osaka, Japan). Samples were incubated in reaction tubes at room temperature before observation. The high amount of Hla (2.5 µM) was necessary to yield a detectable fluorescence signal. Under these conditions, both types of erythrocytes were already lysed when investigated. After the indicated times, an aliquot of the sample was transferred to the slide, covered and observed after the erythrocytes had settled, which takes about 5 min. From the micrographs, which contained many erythrocytes, four individual, representative cells were selected for presentation. In case of rabbit erythrocytes, the population was rather heterogeneous, thus cells with different levels of fluorescence were included.
4.9. Isothermal Titration Calorimetry
Hla at a concentration between 10 and 30 µM in the cell was titrated with PPADS (2.5–5 mM) in a 250 µL syringe, both dissolved in 70 mM sodium-phosphate buffer (VP-ITC, Malvern, Worcestershire, UK) at 20 and 37 °C. PPADS was injected in 3–10 μL-steps, at a stirring speed of 200 rpm, with a reference power of 60 μJ/s, and a filter period of 2 s. Baselines were set automatically by the software, and re-adjusted manually. Peak integration and calculation of [PPADS]/[Hla] was performed automatically by the software. Corresponding baselines (titration of PPADS in buffer) were subtracted; titration of buffer in protein did not yield measureable heats.
Single experiments were analyzed based on a model with one binding site for PPADS on a Hla monomer. The error for the parameters of a single experiment is given as evaluated by the fitting routine included in the instrument (OriginLab). Finally, the results of three experiments were averaged and the SEM is given.
4.10. Statistics
For the hemolysis experiments, between two and five curves were averaged. The error bars correspond to the standard error of the mean; in the case of two replicates it is the deviation of the values from the mean. For each individual curve, the extracellular hemoglobin concentration was measured twice. Control experiments (absence of inhibitors) were measured the same day, mostly directly in parallel with the inhibited sample. The effect of the three inhibitors was consistently observed in experiments employing other erythrocytes charges. Liposomal efflux experiments are the average of three experiments with the same liposome preparation.