The Scorpion Toxin Analogue BmKTX-D33H as a Potential Kv1.3 Channel-Selective Immunomodulator for Autoimmune Diseases
Abstract
:1. Introduction
2. Results
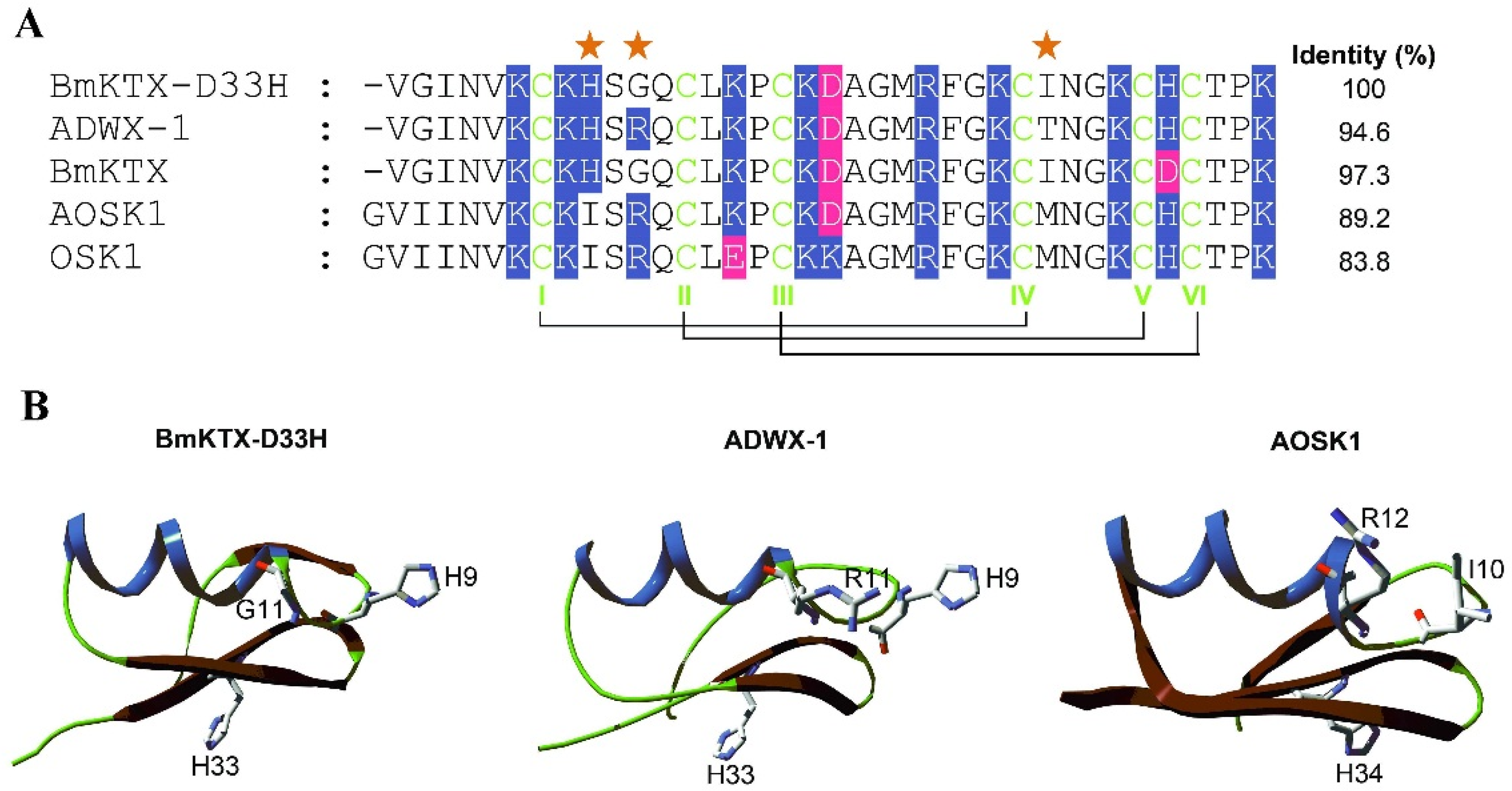
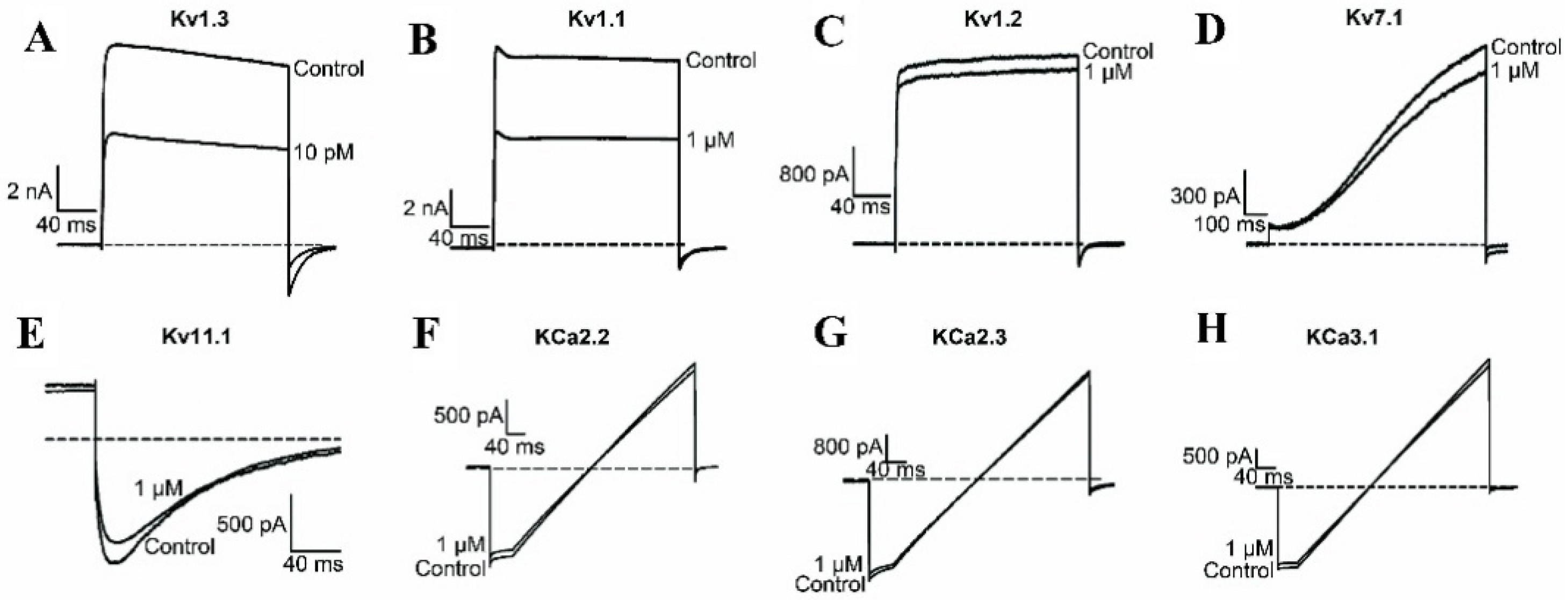
2.1. BmKTX-D33H is a Highly Selective Blocker of the Kv1.3 Channel
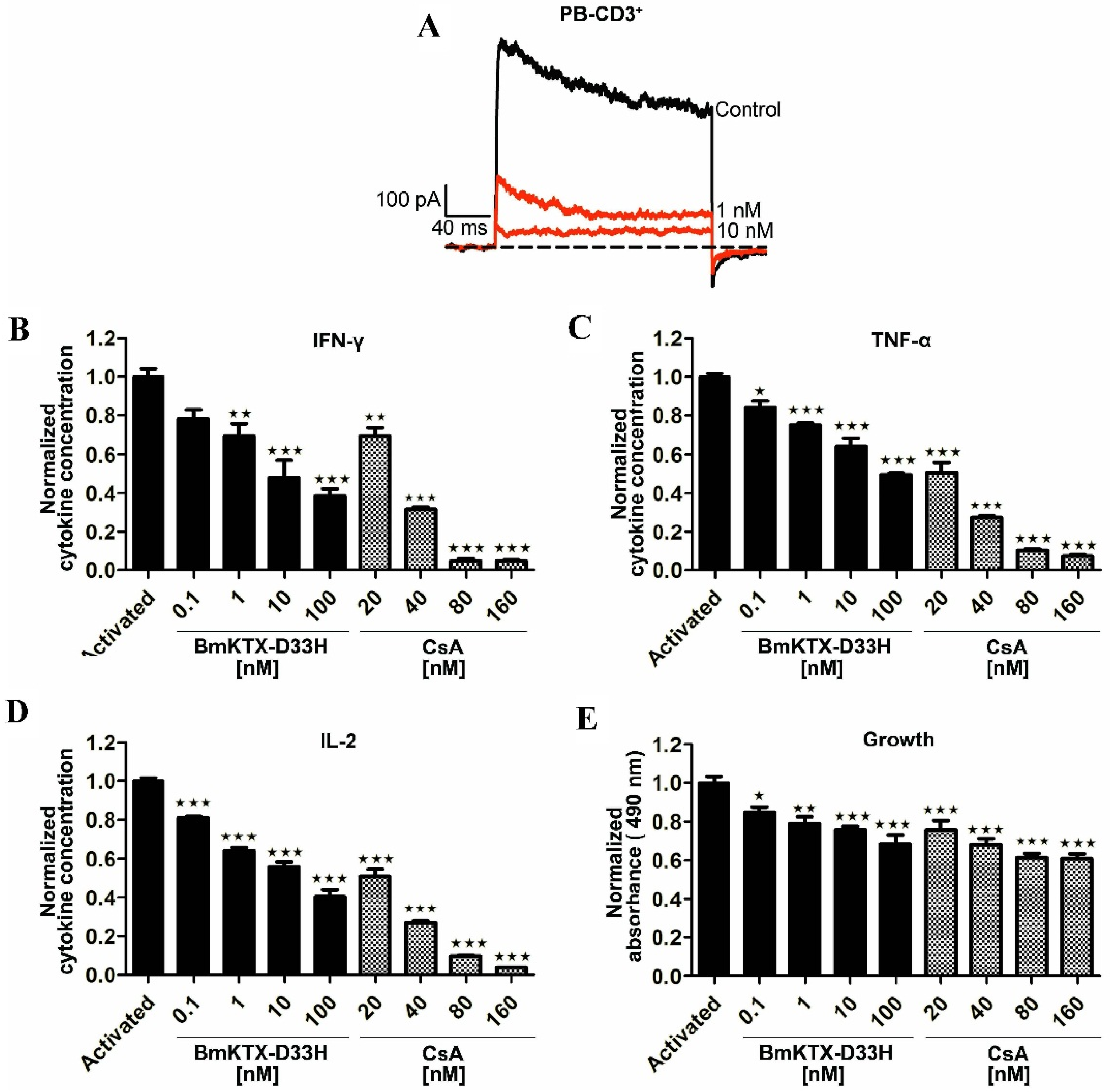
2.2. Immunosuppressive Effect of BmKTX-D33H in Jurkat Cells
2.3. BmKTX-D33H Efficiently Inhibits Kv1.3 Channel Currents, Cytokine Secretion and Proliferation of Human T Cells
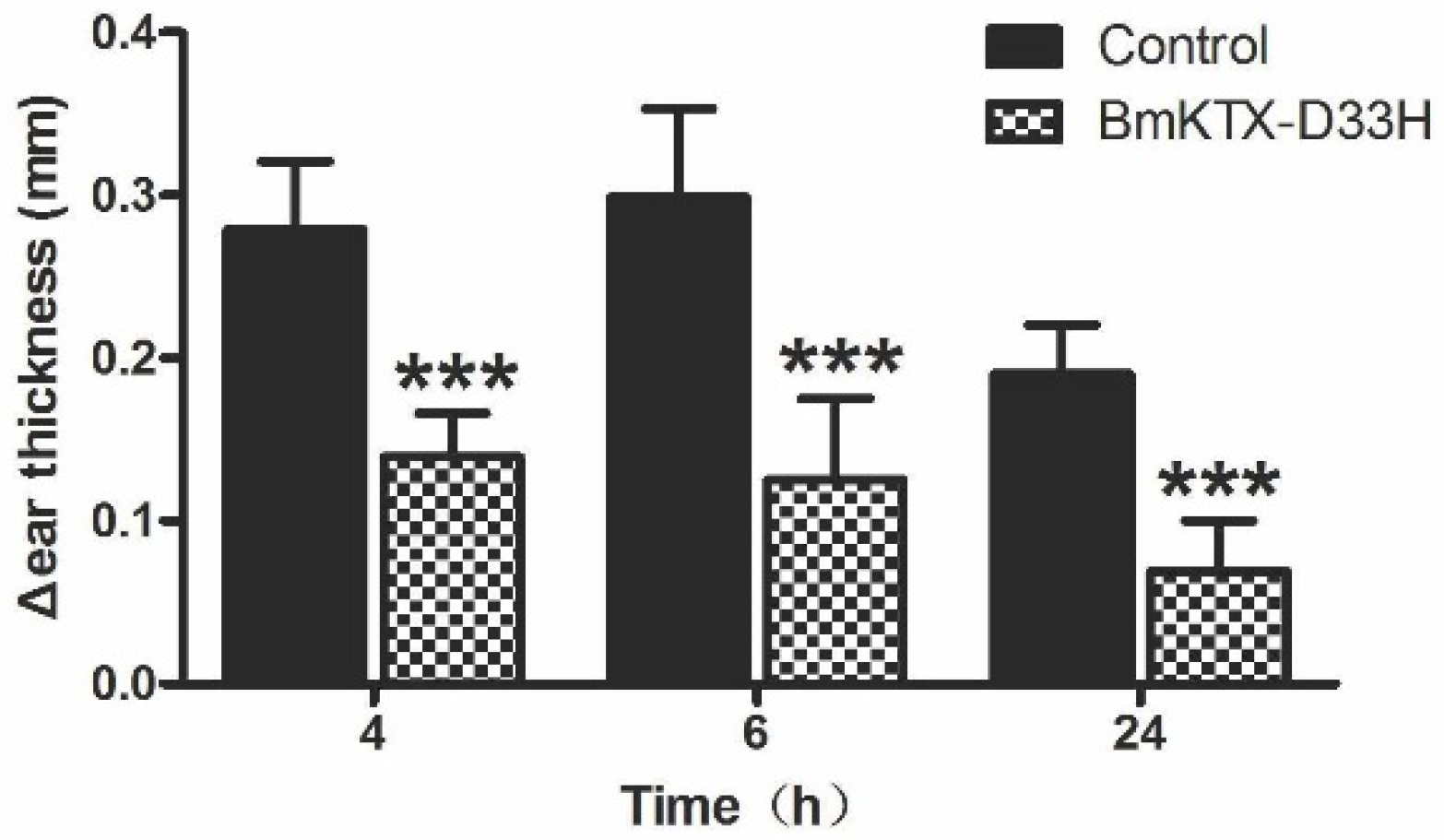
2.4. BmKTX-D33H Inhibits DTH Reactions in Vivo
3. Discussion
4. Experimental Section
4.1. Peptide Expression and Purification
4.2. Electrophysiological Studies
4.3. Cell Preparation and Culture Condition
4.4. Measurement of Cytokine Production and Cell Proliferation
4.5. Delayed-Type Hypersensitivity in Rats
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DTH | Delayed type hypersensitivity |
| eGFP | Enhanced green fluorescent protein |
| h | Human |
| IPTG | Isopropyl thio-b-d-galactoside |
| ION | Ionmycin |
| MS | Multiple sclerosis |
| OSK1 | Orthochirus scrobiculosus toxin 1 |
| PB | Peripheral blood |
| PBS | Phosphate-buffered saline |
| BmKTX | Potassium channel blocking toxin from scorpion Buthus martensii |
| Asp33 to His, BmKTX-D33H | Potassium channel blocking toxin from scorpion Buthus martensii with a mutated position 33 |
| PMA | Propylene glycol monomethyl ether acetate |
| ShK | Stichodactyla helianthus toxin |
| T1DM | Type-1 diabetes mellitus |
| CsA | Cyclosporin A |
References
- Wulff, H.; Calabresi, P.A.; Allie, R.; Yun, S.; Pennington, M.; Beeton, C.; Chandy, K.G. The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J. Clin. Invest. 2003, 111, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Beeton, C.; Chandy, K.G. Potassium channels as therapeutic targets for autoimmune disorders. Curr. Opin. Drug Discov. Devel. 2003, 6, 640–647. [Google Scholar] [PubMed]
- Chandy, K.G.; Wulff, H.; Beeton, C.; Pennington, M.; Gutman, G.A.; Cahalan, M.D. K+ channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 2004, 25, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Beeton, C.; Pennington, M.W.; Wulff, H.; Singh, S.; Nugent, D.; Crossley, G.; Khaytin, I.; Calabresi, P.A.; Chen, C.Y.; Gutman, G.A.; et al. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol. Pharmacol. 2005, 67, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R.; et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 17414–17419. [Google Scholar] [CrossRef] [PubMed]
- Tudor, J.E.; Pallaghy, P.K.; Pennington, M.W.; Norton, R.S. Solution structure of ShK toxin, a novel potassium channel inhibitor from a sea anemone. Nat. Struct. Biol. 1996, 3, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Kalman, K.; Pennington, M.W.; Lanigan, M.D.; Nguyen, A.; Rauer, H.; Mahnir, V.; Paschetto, K.; Kem, W.R.; Grissmer, S.; Gutman, G.A.; et al. ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide. J. Biol. Chem. 1998, 273, 32697–32707. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Sankaranarayanan, A.; Azam, P.; Schmidt-Lassen, K.; Homerick, D.; Hansel, W.; Wulff, H. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol. Pharmacol. 2005, 68, 1254–1270. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, W.H.; Han, S.; Peng, B.W.; Yin, J.; Wu, Y.L.; He, X.H.; Li, W.X. Selective inhibition of CCR7− effector memory T cell activation by a novel peptide targeting Kv1.3 channel in a rat experimental autoimmune encephalomyelitis model. J. Biol. Chem. 2012, 287, 29479–29494. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Chang, S.C.; Chauhan, S.; Huq, R.; Tajhya, R.B.; Chhabra, S.; Norton, R.S.; Beeton, C. Development of highly selective Kv1.3-blocking peptides based on the sea anemone peptide ShK. Mar. Drugs 2015, 13, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, J.A.; Kamenz, C.; Scholtz, G. Reinterpreting the morphology of the Jurassic scorpion Liassoscorpionides. Arthropod Struct. Dev. 2007, 36, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genomics Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Mouhat, S.; Jouirou, B.; Mosbah, A.; De Waard, M.; Sabatier, J.M. Diversity of folds in animal toxins acting on ion channels. Biochem. J. 2004, 378, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Mouhat, S.; Andreotti, N.; Jouirou, B.; Sabatier, J.M. Animal toxins acting on voltage-gated potassium channels. Curr. Pharm. Des. 2008, 14, 2503–2518. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, Y.; Zhao, R.; Zhang, W.; He, Y.; Wu, Y.; Cao, Z.; Guo, L.; Li, W. Molecular diversity of toxic components from the scorpion Heterometrus petersii venom revealed by proteomic and transcriptome analysis. Proteomics 2010, 10, 2471–2485. [Google Scholar] [CrossRef] [PubMed]
- Ruiming, Z.; Yibao, M.; Yawen, H.; Zhiyong, D.; Yingliang, W.; Zhijian, C.; Wenxin, L. Comparative venom gland transcriptome analysis of the scorpion Lychas mucronatus reveals intraspecific toxic gene diversity and new venomous components. BMC Genom. 2010, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, Y.; Zhao, R.; Wu, Y.; Li, W.; Cao, Z. Extreme diversity of scorpion venom peptides and proteins revealed by transcriptomic analysis: Implication for proteome evolution of scorpion venom arsenal. J. Proteom. 2012, 75, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, R.; Di, Z.; Li, Z.; Xu, X.; Hong, W.; Wu, Y.; Zhao, H.; Li, W.; Cao, Z. Molecular diversity of Chaerilidae venom peptides reveals the dynamic evolution of scorpion venom components from Buthidae to non-Buthidae. J. Proteom. 2013, 89, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yu, Y.; Wu, Y.; Hao, P.; Di, Z.; He, Y.; Chen, Z.; Yang, W.; Shen, Z.; He, X.; et al. The genome of Mesobuthus martensii reveals a unique adaptation model of arthropods. Nat. Commun. 2013, 4, 2602. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Di, Z.; Wu, Y.; Li, W. Overview of scorpion species from China and their toxins. Toxins (Basel) 2014, 6, 796–815. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Duan, Z.; Di, Z.; He, Y.; Li, J.; Li, Z.; Xie, C.; Zeng, X.; Cao, Z.; Wu, Y.; et al. Proteomic analysis of the venom from the scorpion Mesobuthus martensii. J. Proteom. 2014, 106, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Robinson, A.; Gordon, D.; Chung, S.H. Modeling the binding of three toxins to the voltage-gated potassium channel (Kv1.3). Biophys. J. 2011, 101, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cao, Z.; Yi, H.; Jiang, D.; Mao, X.; Liu, H.; Li, W. Simulation of the interaction between ScyTx and small conductance calcium-activated potassium channel by docking and MM-PBSA. Biophys. J. 2004, 87, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Cao, Z.; Yin, S.; Dai, C.; Wu, Y.; Li, W. Interaction simulation of hERG K+ channel with its specific BeKm-1 peptide: Insights into the selectivity of molecular recognition. J. Proteome Res. 2007, 6, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Qiu, S.; Cao, Z.; Wu, Y.; Li, W. Molecular basis of inhibitory peptide maurotoxin recognizing Kv1.2 channel explored by ZDOCK and molecular dynamic simulations. Proteins 2008, 70, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yi, H.; Yin, S.J.; Chen, Z.Y.; Liu, H.; Cao, Z.J.; Wu, Y.L.; Li, W.X. Structural basis of a potent peptide inhibitor designed for Kv1.3 channel, a therapeutic target of autoimmune disease. J. Biol. Chem. 2008, 283, 19058–19065. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Yi, H.; Liu, H.; Cao, Z.; Wu, Y.; Li, W. Molecular Information of charybdotoxin blockade in the large conductance calcium-activated potassium channel. J. Chem. Inf. Model 2009, 49, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Hu, Y.T.; Yang, W.S.; He, Y.W.; Feng, J.; Wang, B.; Zhao, R.M.; Ding, J.P.; Cao, Z.J.; Li, W.X.; et al. Hg1, novel peptide inhibitor specific for Kv1.3 channels from first scorpion Kunitz-type potassium channel toxin family. J. Biol. Chem. 2012, 287, 13813–13821. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Y.; Hu, J.; Yang, W.; Sabatier, J.M.; De Waard, M.; Cao, Z.; Li, W.; Han, S.; Wu, Y. Unusual binding mode of scorpion toxin BmKTX onto potassium channels relies on its distribution of acidic residues. Biochem. Biophys. Res. Commun. 2014, 447, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Y.; Hong, J.; Hu, J.; Yang, W.; Xiang, F.; Yang, F.; Xie, Z.; Cao, Z.; Li, W.; et al. Toxin acidic residue evolutionary function-guided design of de novo peptide drugs for the immunotherapeutic target, the Kv1.3 channel. Sci. Rep. 2015, 5, 9881. [Google Scholar] [CrossRef] [PubMed]
- Mouhat, S.; Teodorescu, G.; Homerick, D.; Visan, V.; Wulff, H.; Wu, Y.; Grissmer, S.; Darbon, H.; de Waard, M.; Sabatier, J.M. Pharmacological profiling of Orthochirus scrobiculosus toxin 1 analogs with a trimmed N-terminal domain. Mol. Pharmacol. 2006, 69, 354–362. [Google Scholar] [PubMed]
- Rashid, M.H.; Huq, R.; Tanner, M.R.; Chhabra, S.; Khoo, K.K.; Estrada, R.; Dhawan, V.; Chauhan, S.; Pennington, M.W.; Beeton, C.; et al. A potent and Kv1.3-selective analogue of the scorpion toxin HsTX1 as a potential therapeutic for autoimmune diseases. Sci. Rep. 2014, 4, 4509. [Google Scholar] [PubMed]
- Mouhat, S.; Visan, V.; Ananthakrishnan, S.; Wulff, H.; Andreotti, N.; Grissmer, S.; Darbon, H.; de Waard, M.; Sabatier, J.M. K+ channel types targeted by synthetic OSK1, a toxin from Orthochirus scrobiculosus scorpion venom. Biochem. J. 2005, 385, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.A.; Roux, B. Modeling the structure of agitoxin in complex with the Shaker K+ channel: A computational approach based on experimental distance restraints extracted from thermodynamic mutant cycles. Biophys. J. 2002, 83, 2595–2609. [Google Scholar] [CrossRef]
- Chen, R.; Chung, S.H. Engineering a potent and specific blocker of voltage-gated potassium channel Kv1.3, a target for autoimmune diseases. Biochemistry 2012, 51, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.J.; Jiang, L.; Yi, H.; Han, S.; Yang, D.W.; Liu, M.L.; Liu, H.; Cao, Z.J.; Wu, Y.L.; Li, W.X. Different residues in channel turret determining the selectivity of ADWX-1 inhibitor peptide between Kv1.1 and Kv1.3 channels. J. Proteome Res. 2008, 7, 4890–4897. [Google Scholar] [CrossRef] [PubMed]
- Beeton, C.; Chandy, K.G. Induction and monitoring of active delayed type hypersensitivity (DTH) in rats. J. Vis. Exp. 2007, e237. [Google Scholar] [CrossRef] [PubMed]


| Cell Types | Reagents (nM) | IC50 for Cytokines | ||
|---|---|---|---|---|
| IFN-γ | TNF-α | IL-2 | ||
| Jurkat | BmKTX-D33H | NA | NA | 23.9 ± 20.1 |
| CsA | NA | NA | 32.5 ± 0.4 | |
| CD3+ | BmKTX-D33H | 7.9 ± 7.8 | 58.5 ± 48.6 | 11.2 ± 12.7 |
| CsA | 28.3 ± 1.2 | 20.0 ± 1.1 | 20.4 ± 0.4 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, F.; Hu, Y.; Yu, W.; Xie, Z.; Hu, J.; Cao, Z.; Li, W.; Wu, Y. The Scorpion Toxin Analogue BmKTX-D33H as a Potential Kv1.3 Channel-Selective Immunomodulator for Autoimmune Diseases. Toxins 2016, 8, 115. https://doi.org/10.3390/toxins8040115
Ye F, Hu Y, Yu W, Xie Z, Hu J, Cao Z, Li W, Wu Y. The Scorpion Toxin Analogue BmKTX-D33H as a Potential Kv1.3 Channel-Selective Immunomodulator for Autoimmune Diseases. Toxins. 2016; 8(4):115. https://doi.org/10.3390/toxins8040115
Chicago/Turabian StyleYe, Fang, Youtian Hu, Weiwei Yu, Zili Xie, Jun Hu, Zhijian Cao, Wenxin Li, and Yingliang Wu. 2016. "The Scorpion Toxin Analogue BmKTX-D33H as a Potential Kv1.3 Channel-Selective Immunomodulator for Autoimmune Diseases" Toxins 8, no. 4: 115. https://doi.org/10.3390/toxins8040115





