Efficacy and Safety of Intravesical OnabotulinumtoxinA Injection in Patients with Detrusor Hyperactivity and Impaired Contractility
Abstract
:1. Introduction
2. Results
2.1. Therapeutic Effectiveness
2.2. Adverse Events
3. Discussion
4. Conclusions
5. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| OAB | overactive bladder |
| UAB | underactive bladder |
| PVR | post-void residual urine |
| DHIC | detrusor hyperactivity and impaired contractility |
| VE | voiding efficiency |
| AE | adverse events |
| Qmax | maximal flow rate |
| Vol | voided volume |
| BOO | bladder outlet obstruction |
| DM | diabetes mellitus |
| DU | detrusor underactivity |
References
- Milsom, I.; Kaplan, S.A.; Coyne, K.S.; Sexton, C.C.; Kopp, Z.S. Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: Results from EpiLUTS. Urology 2012, 80, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.I.; Chapple, C.R.; Abrams, P.; Dmochowski, R.; Haab, F.; Nitti, V.; Koelbl, H.; van Kerrebroeck, P.; Wein, A.J. Detrusor underactivity and the underactive bladder: A new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur. Urol. 2014, 65, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.W.; Cannon, A.; Bartlett, E.; Ellis-Jones, J.; Abrams, P. The natural history of lower urinary tract dysfunction in men: Minimum 10-year urodynamic follow-up of untreated detrusor underactivity. BJU Int. 2005, 96, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, M.B. The overactive bladder progression to underactive bladder hypothesis. Int. Urol. Nephrol. 2014, 46 (Suppl. 1), S23–S27. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, T.; Miyazato, M.; Saito, S. Relationship between connexin43-derived gap junction proteins in the bladder and age-related detrusor underactivity in rats. Life Sci. 2014, 116, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Daneshgari, F. Diabetic bladder dysfunction: Current translational knowledge. J. Urol. 2009, 182, S18–S26. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Kuo, H.C. Botulinum toxin injection for lower urinary tract dysfunction. Int. J. Urol. 2013, 20, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Kuo, H.C. Increased risk of large post-void residual urine and decreased long-term success rate after intravesical onabotulinumtoxinA injection for refractory idiopathic detrusor overactivity. J. Urol. 2013, 189, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Urodynamic evidence of effectiveness of botulinum A toxin injection in treatment of detrusor overactivity refractory to anticholinergic agents. Urology 2004, 63, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Yoshimura, N.; Chancellor, M.B. The other bladder syndrome: Underactive bladder. Rev. Urol. 2013, 15, 11–20. [Google Scholar] [PubMed]
- Yildirim, A.; Onol, F.F.; Haklar, G.; Tarcan, T. The role of free radicals and nitric oxide in the ischemia-reperfusion injury mediated by acute bladder outlet obstruction. Int. Urol. Nephrol. 2008, 40, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chancellor, M.B.; Lin, J.M.; Hsieh, J.H.; Yu, H.J. Type 2 diabetes but not metabolic syndrome is associated with an increased risk of lower urinary tract symptoms and erectile dysfunction in men aged <45 years. BJU Int. 2010, 105, 1136–1140. [Google Scholar] [PubMed]
- Yu, H.J.; Lee, W.C.; Liu, S.P.; Tai, T.Y.; Wu, H.P.; Chen, J. Unrecognized voiding difficulty in female type 2 diabetic patients in the diabetes clinic: A prospective case-control study. Diabetes Care 2004, 27, 988–989. [Google Scholar] [CrossRef] [PubMed]
- Gormley, E.A.; Lightner, D.J.; Burgio, K.L.; Chai, T.C.; Clemens, J.Q.; Culkin, D.J.; Das, A.K.; Foster, H.E., Jr.; Scarpero, H.M.; Tessier, C.D.; et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J. Urol. 2012, 188 (Suppl. 6), 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chan, L.; Tse, V. Clinical outcome in male patients with detrusor overactivity with impaired contractility. Int. Neurourol. J. 2014, 18, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Baukloh, H.; Weiss, C.; Stolze, T.; Herholz, J.; Stürzebecher, B.; Miller, K.; Knispel, H.H. Botulinum-A toxin detrusor and sphincter injection in treatment of overactive bladder syndrome: Objective outcome and patient satisfaction. Eur. Urol. 2005, 48, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.M.; Lin, H.H.; Kuo, H.C. Factors associated with a better therapeutic effect of solifenacin in patients with overactive bladder syndrome. Neurourol. Urodyn. 2014, 33, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Hanna-Mitchell, A.T.; Ruiz, G.W.; Daneshgari, F.; Liu, G.; Apodaca, G.; Birder, L.A. Impact of diabetes mellitus on bladder uroepithelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R84–R93. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A.; Standardisation Sub-committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Yang, S.S.; Chen, Y.T.; Hsieh, J.H. Videourodynamics identifies the causes of young men with lower urinary tract symptoms and low uroflow. Eur. Urol. 2003, 4, 386–390. [Google Scholar] [CrossRef]
- Nitti, V.W.; Lefkowitz, G.; Ficazzola, M.; Dixon, C.M. Lower urinary tract symptoms in young men: Video urodynamic findings and correlation with noninvasive measures. J. Urol. 2002, 168, 135–138. [Google Scholar] [CrossRef]
- Jeong, S.J.; Kim, H.J.; Lee, Y.J.; Lee, B.K.; Choo, Y.M.; Oh, J.J.; Lee, S.C.; Jeong, C.W.; Yoon, C.Y.; Hong, S.K.; et al. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: A comparison between men and women. Korean J. Urol. 2012, 53, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Resnick, N.M.; Yalla, S.V.; Laurino, E. The pathophysiology of urinary incontinence among institutionalized elderly persons. N. Engl. J. Med. 1989, 320, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Liao, C.H.; Chung, S.D. Adverse events of intravesical botulinum toxin A injections for idiopathic detrusor overactivity: Risk factors and influence on treatment outcome. Eur. Urol. 2010, 58, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Liao, C.H.; Kuo, H.C. Diabetes mellitus does not affect the efficacy and safety of intravesical onabotulinumtoxinA injection in patients with refractory detrusor overactivity. Neurourol. Urodyn. 2014, 33, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Baseline | 2 Weeks | 4 Weeks | 3 Months | 6 Months | |
|---|---|---|---|---|---|---|
| OABSS | DHIC | 12.3 ± 2.0 | 11.1 ± 2.56 * | 10.1 ± 2.77 * | 10.1 ± 3.80 * | 9.61 ± 3.24 * |
| OAB | 11.2 ± 2.9 | 9.48 ± 3.2 * | 9.52 ± 2.96 * | 9.19 ± 2.87 * | 8.06 ± 3.3 * | |
| USS | DHIC | 4.0 ± 0 | 3.48 ± 0.87 * | 3.40 ± 0.88 * | 3.7 ± 0.92 | 3.28 ± 0.96 * |
| OAB | 3.62 ± 0.74 | 3.14 ± 1.1 * | 3.29 ± 1.01 * | 3.10 ± 1.0 * | 3.06 ± 1.06 * | |
| GRA | DHIC | 0 | 1.19 ± 1.33 * | 1.30 ± 1.38 * | 1.10 ± 1.74 * | 1.50 ± 1.47 * |
| OAB | 0 | 0.95 ± 1.28 * | 1.43 ± 1.12 * | 1.52 ± 0.81 * | 1.72 ± 0.96 * | |
| PPBC | DHIC | 4.67 ± 1.77 | 3.90 ± 1.70 | 3.25 ± 1.65 * | 3.15 ± 1.76 * | 2.89 ± 1.75 * |
| OAB | 4.52 ± 1.66 | 3.10 ± 1.61 * | 2.48 ± 1.47 * | 2.81 ± 1.63 * | 2.56 ± 1.29 * | |
| UUI/3 days | DHIC | 7.44 ± 9.51 | 6.67 ± 11.1 | 5.84 ± 9.22 | 12.3 ± 20.9 | 9.53 ± 19.6 |
| OAB | 6.0 ± 13.6 | 3.65 ± 7.39 | 4.70 ± 9.09 | 3.62 ± 8.13 * | 2.88 ± 2.03 * | |
| Urgency/3 days | DHIC | 27.7 ± 17.7 | 27.9 ± 23.4 | 29.8 ± 30.2 | 27.5 ± 28.2 | 29.1 ± 33.5 |
| OAB | 27.3 ± 15.0 | 22.4 ± 14.5 * | 19.9 ± 15.7 * | 26.9 ± 19.8 | 15.1 ± 11.9 * | |
| Frequency/3 days | DHIC | 26.6 ± 14.2 | 30.0 ± 14.4 | 31.1 ± 24.6 | 28.6 ± 22.4 | 29.7 ± 28.9 |
| OAB | 38.0 ± 12.9 | 37.6 ± 22.5 | 31.5 ± 9.27 * | 36.5 ± 19.8 | 31.4 ± 12.3 * | |
| Nocturia/3 days | DHIC | 10.5 ± 5.41 | 9.39 ± 3.29 | 8.16 ± 3.01 | 8.50 ± 2.09 | 7.59 ± 3.12 * |
| OAB | 11.4 ± 5.40 | 9.80 ± 4.57 | 8.55 ± 3.80 * | 10.5 ± 5.0 | 9.44 ± 3.01 | |
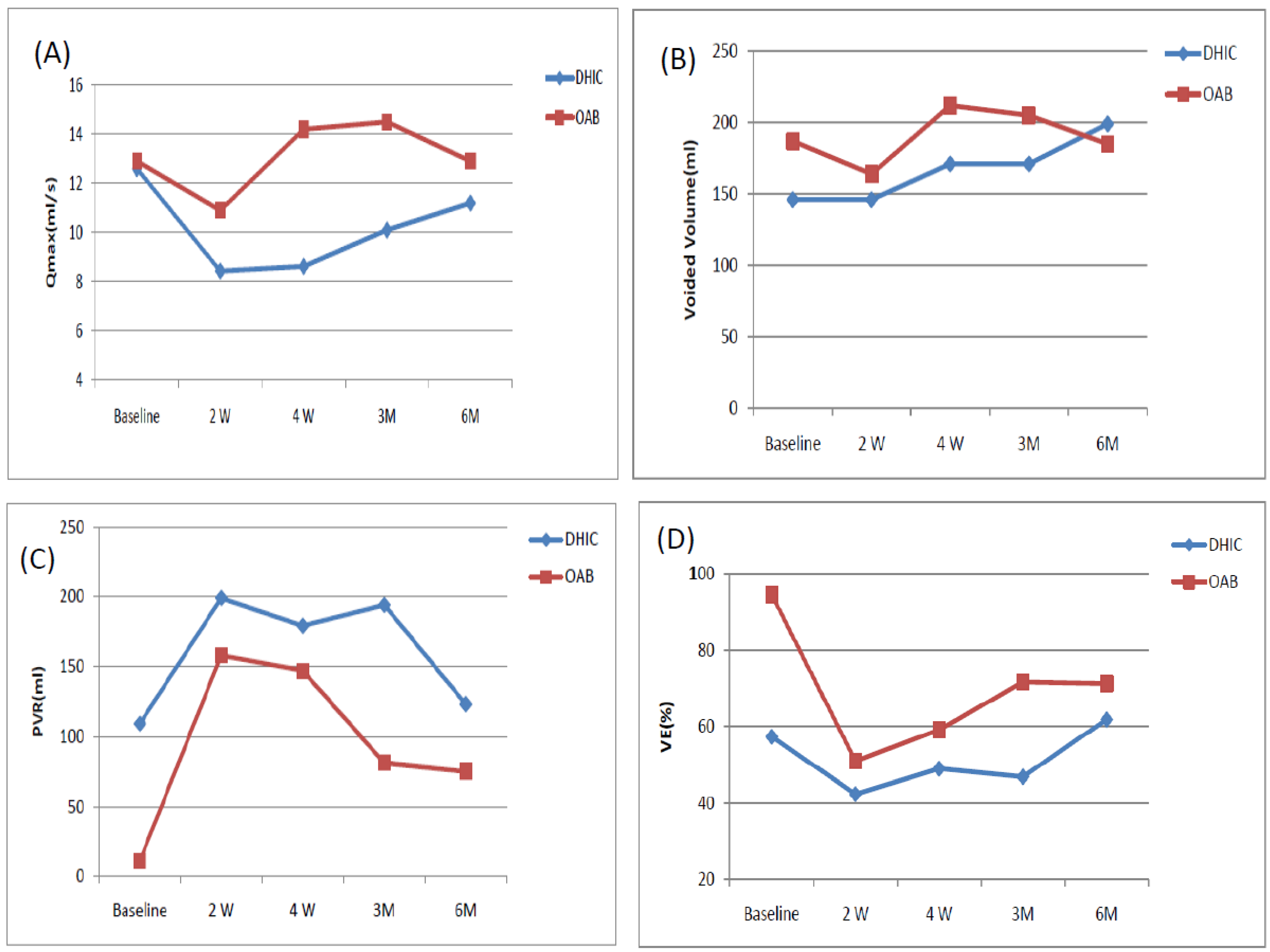
| Qmax (mL/s) | DHIC | 12.6 ± 10.7 | 8.43 ± 4.23 | 8.62 ± 3.34 | 10.1 ± 5.32 | 11.2 ± 6.33 |
| OAB | 12.9 ± 7.1 | 10.9 ± 7.9 | 14.2 ± 7.02 | 14.5 ± 8.54 | 12.9 ± 8.2 | |
| Voided volume (mL) | DHIC | 146 ± 69 | 146 ± 97 | 171 ± 99 | 171 ± 125 | 199 ± 126 |
| OAB | 187 ± 106 | 164 ± 136 | 212 ± 93 | 205 ± 96 | 185 ± 106 | |
| PVR volume (mL) | DHIC | 109 ± 149 | 199 ± 118 * | 179 ± 93 * | 194 ± 150 | 123 ± 79 |
| OAB | 11 ± 15 | 158 ± 184 * | 147 ± 123 * | 81 ± 75 * | 75 ± 72 * | |
| VE (%) | DHIC | 57.3 ± 24.8 | 42.3 ± 24.8 * | 48.9 ± 19.8 * | 46.8 ± 26.8 | 61.8 ± 20.1 |
| OAB | 94.6 ± 7.9 | 50.9 ± 29.4 * | 59.1 ± 21.8 * | 71.6 ± 20.1 * | 71.2 ± 22.4 * | |
| Adverse Events | DHIC (n = 21) | OAB (n = 21) | p Value |
|---|---|---|---|
| AUR | 7 (33.3%) | 3 (14.3%) | 0.277 |
| PVR volume > 200 mL | 12 (57.1%) | 7 (33.3%) | 0.215 |
| UTI | 8 (38.1%) | 4 (19.0%) | 0.306 |
| Gross hematuria | 0 (0%) | 1 (4.8%) | 1.000 |
| General weakness | 1 (4.8%) | 0 (0%) | 1.000 |
| Therapeutic duration (months) | 4.9 ± 4.8 | 7.2 ± 3.3 | 0.03 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Lee, C.-L.; Kuo, H.-C. Efficacy and Safety of Intravesical OnabotulinumtoxinA Injection in Patients with Detrusor Hyperactivity and Impaired Contractility. Toxins 2016, 8, 82. https://doi.org/10.3390/toxins8030082
Wang C-C, Lee C-L, Kuo H-C. Efficacy and Safety of Intravesical OnabotulinumtoxinA Injection in Patients with Detrusor Hyperactivity and Impaired Contractility. Toxins. 2016; 8(3):82. https://doi.org/10.3390/toxins8030082
Chicago/Turabian StyleWang, Chung-Cheng, Cheng-Ling Lee, and Hann-Chorng Kuo. 2016. "Efficacy and Safety of Intravesical OnabotulinumtoxinA Injection in Patients with Detrusor Hyperactivity and Impaired Contractility" Toxins 8, no. 3: 82. https://doi.org/10.3390/toxins8030082
APA StyleWang, C.-C., Lee, C.-L., & Kuo, H.-C. (2016). Efficacy and Safety of Intravesical OnabotulinumtoxinA Injection in Patients with Detrusor Hyperactivity and Impaired Contractility. Toxins, 8(3), 82. https://doi.org/10.3390/toxins8030082







