Tenuifolide B from Cinnamomum tenuifolium Stem Selectively Inhibits Proliferation of Oral Cancer Cells via Apoptosis, ROS Generation, Mitochondrial Depolarization, and DNA Damage
Abstract
:1. Introduction
2. Results
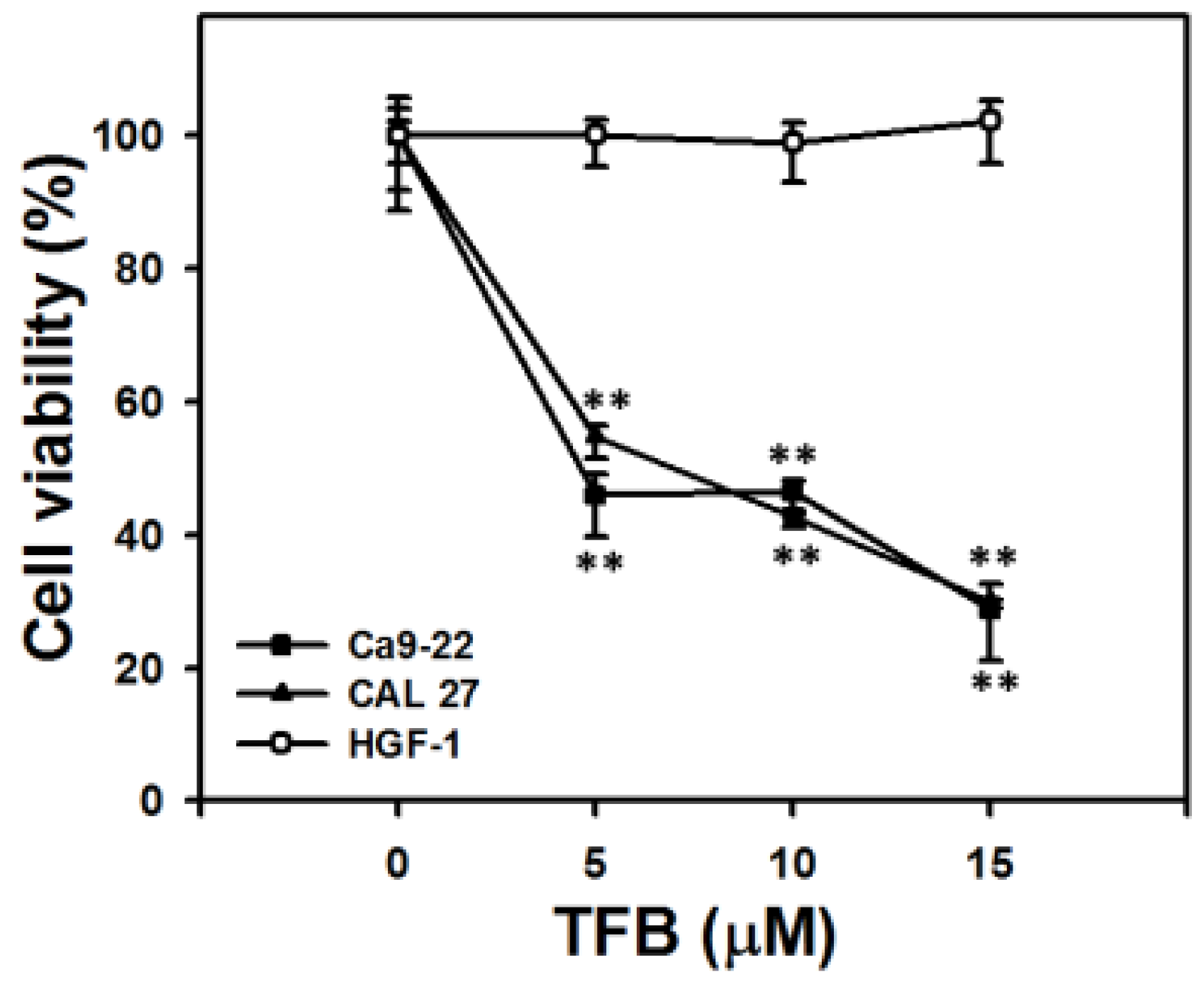
2.1. Cell Viability and ATP Cellular Content
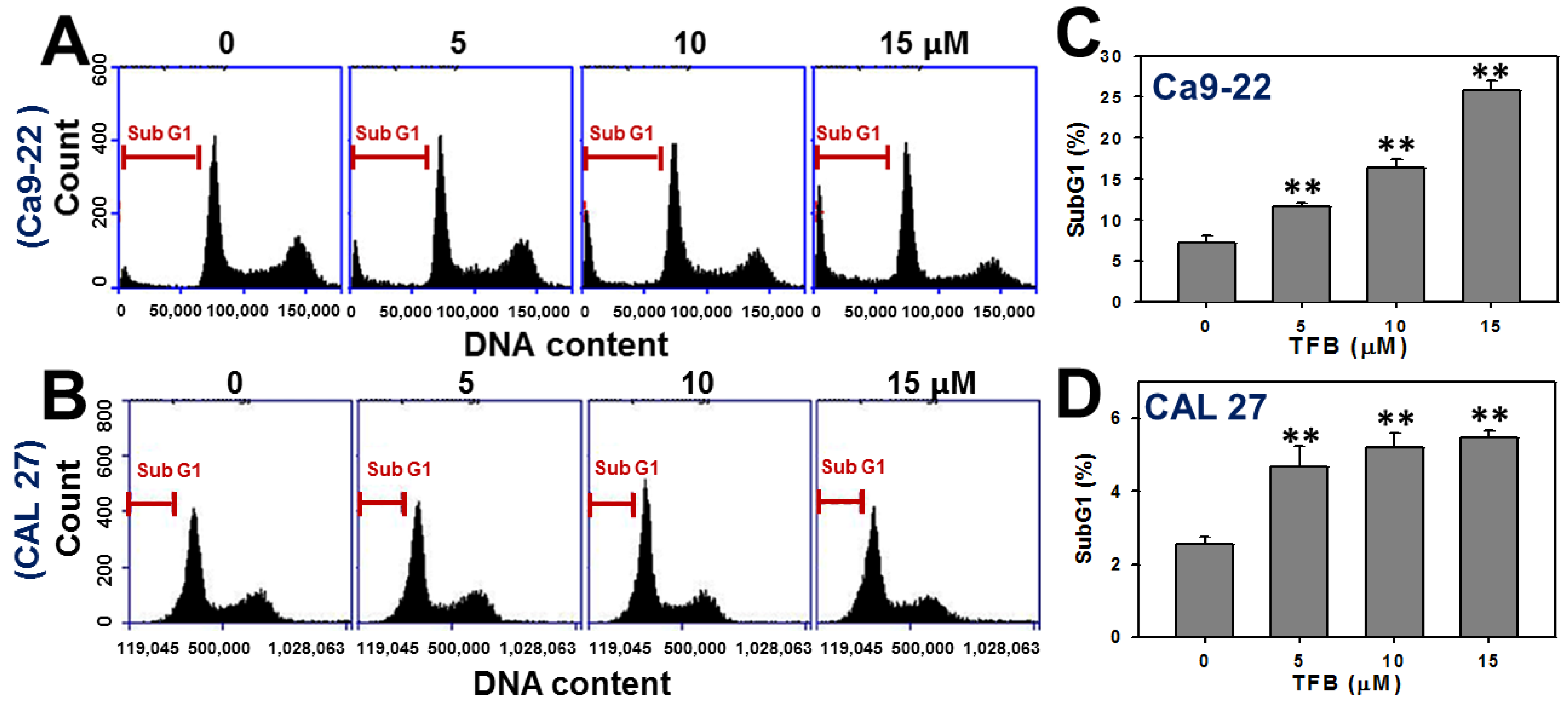
2.2. Cell Cycle Progression
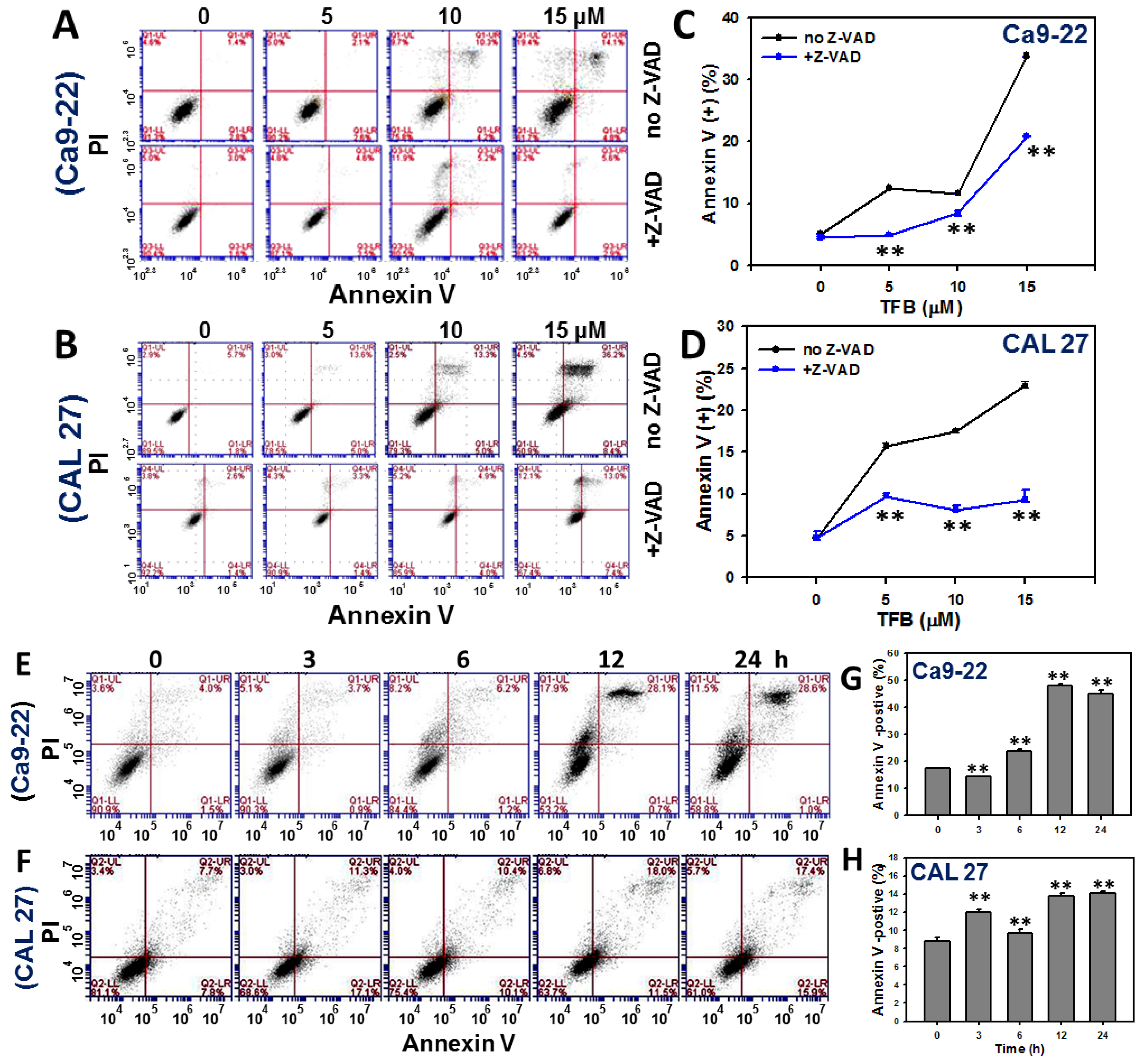
2.3. Annexin V-Based Apoptosis
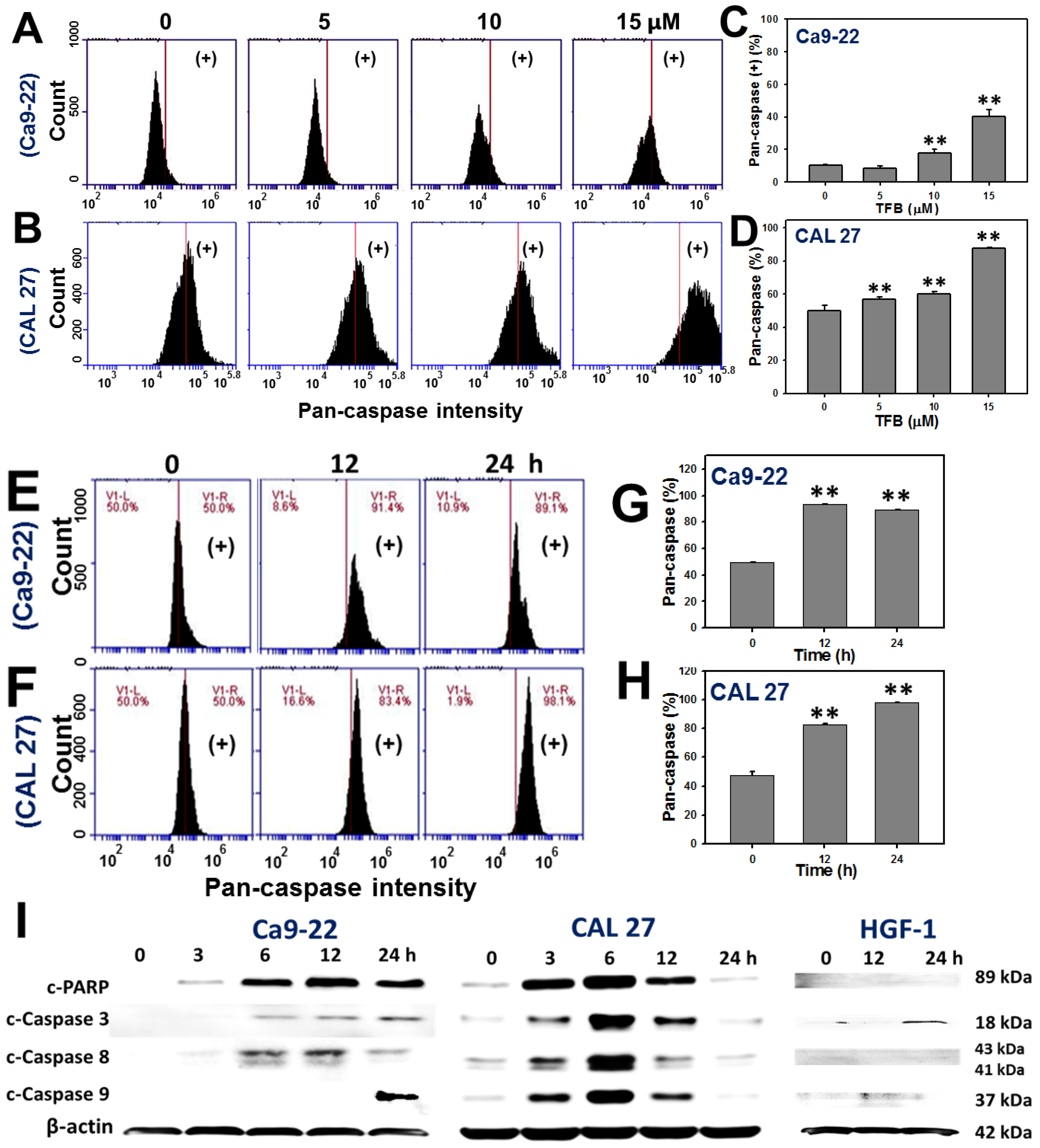
2.4. Caspases-Based Apoptosis
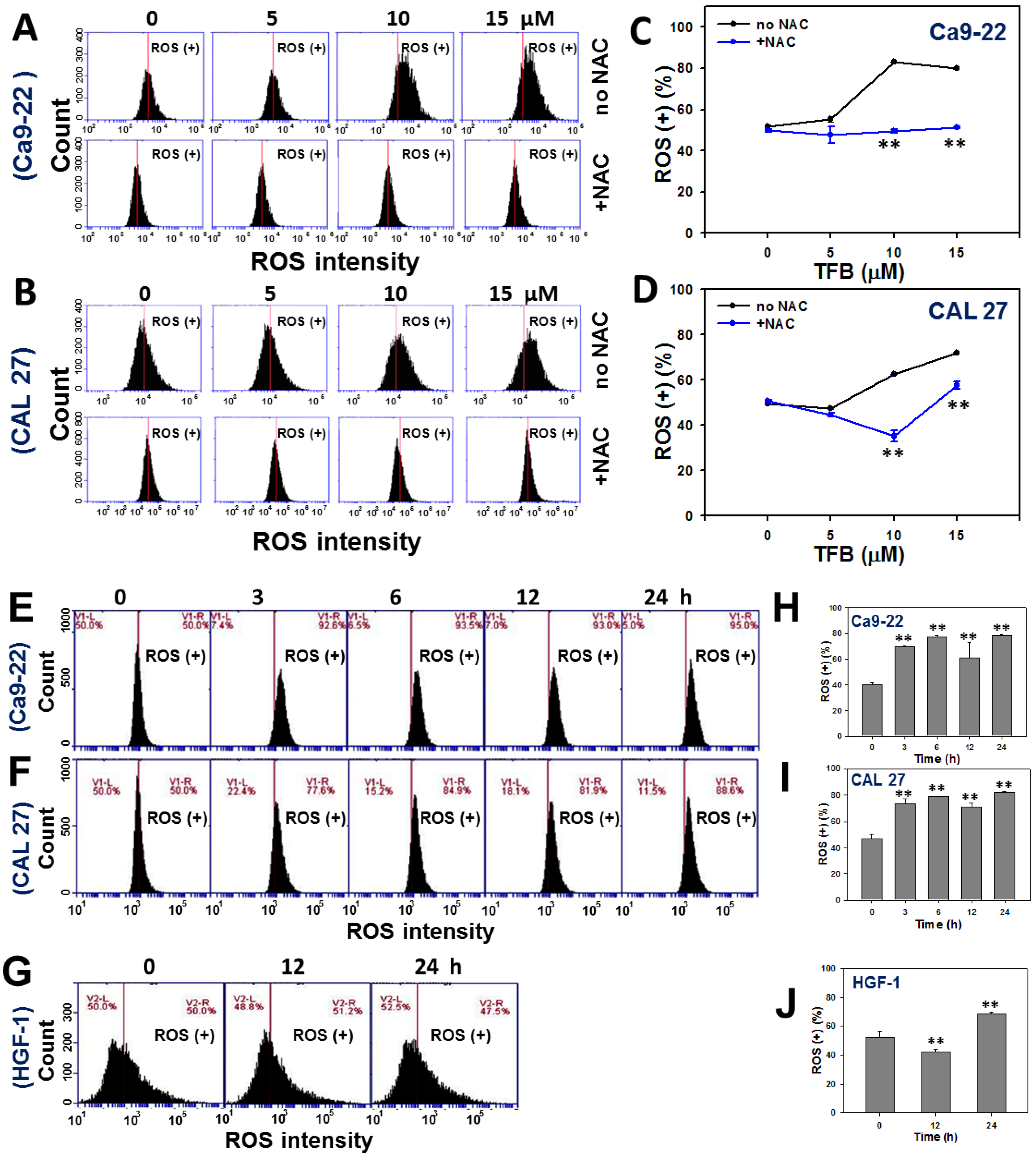
2.5. ROS
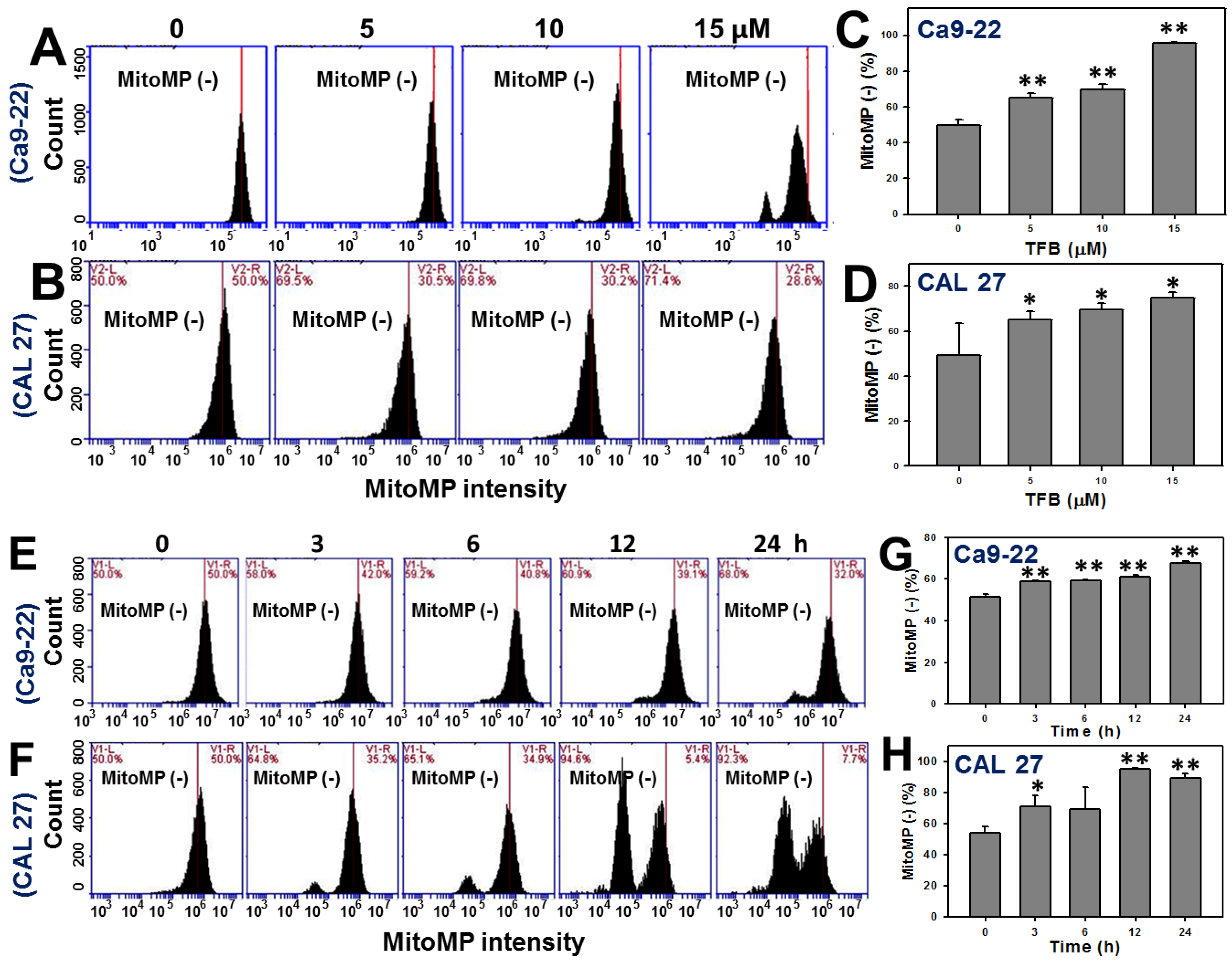
2.6. Mitochondrial Membrane Potentials (MitoMP)
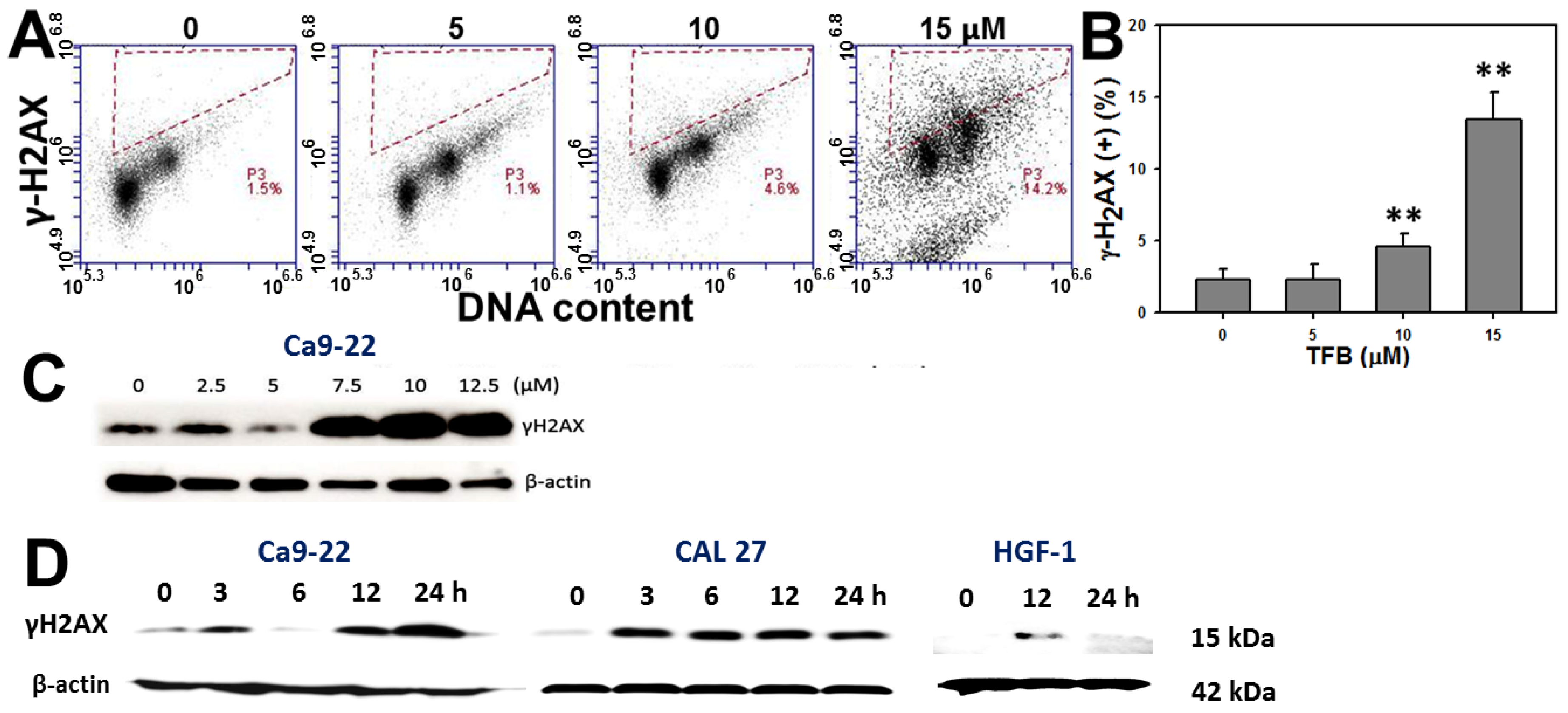
2.7. γH2AX Expression
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Drug Information and Oral Cancer and Normal Cell Lines
5.2. Measurement of Cell Viability—Cellular ATP Content
5.3. Measurement of Cell Cycle Progression
5.4. Measurement of Apoptosis by Annexin V Staining
5.5. Measurement of Apoptosis by Caspase Activity
5.6. Measurement of Intracellular ROS
5.7. Measurement of MitoMP
5.8. Measurement of DNA Damage by γH2AX Expression
5.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.C.; Huang, Y.L.; Lee, C.H.; Chen, M.J.; Lin, L.M.; Tsai, C.C. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J. Oral Pathol. Med. 1995, 24, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Ko, Y.C.; Huang, H.L.; Chao, Y.Y.; Tsai, C.C.; Shieh, T.Y.; Lin, L.M. The precancer risk of betel quid chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in southern Taiwan. Br. J. Cancer 2003, 88, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.L.; Lee, C.P.; Chang, J.G.; Lee, C.H.; Yeh, K.T.; Tsai, Y.S.; Chen, M.K.; Chen, C.H.; Ko, Y.C. Combined effects of differentiation factor 15 and substance use of alcohol, betel quid and cigarette on risk of head and neck cancer. Head Neck Oncol. 2013, 5, 23. [Google Scholar]
- Myoung, H.; Hong, S.P.; Yun, P.Y.; Lee, J.H.; Kim, M.J. Anti-cancer effect of genistein in oral squamous cell carcinoma with respect to angiogenesis and in vitro invasion. Cancer Sci. 2003, 94, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Chang, H.W.; Huang, H.M.; Chong, I.W.; Chen, J.S.; Chen, C.Y.; Wang, H.M. (−)-Anonaine induces DNA damage and inhibits growth and migration of human lung carcinoma h1299 cells. J. Agric. Food Chem. 2011, 59, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Cheng, K.C.; Lin, C.J.; Hsu, S.W.; Fang, W.C.; Hsu, T.F.; Chiu, C.C.; Chang, H.W.; Hsu, C.H.; Lee, A.Y. Obtusilactone A and (−)-sesamin induce apoptosis in human lung cancer cells by inhibiting mitochondrial Lon protease and activating DNA damage checkpoints. Cancer Sci. 2010, 101, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Wu, C.R.; Chang, H.W.; Kumar, K.J.; Lin, M.K.; Chen, C.S.; Cho, H.J.; Huang, C.Y.; Lee, H.Z.; Hsieh, W.T.; et al. Inhibitory effects of Physalis angulata on tumor metastasis and angiogenesis. J. Ethnopharmacol. 2011, 135, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in cancer prevention and therapy: Truth or dare? Toxins 2010, 2, 517–551. [Google Scholar] [CrossRef] [PubMed]
- Melchini, A.; Traka, M.H. Biological profile of erucin: A new promising anticancer agent from cruciferous vegetables. Toxins 2010, 2, 593–612. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef] [PubMed]
- Cabello, C.M.; Bair, W.B., 3rd; Lamore, S.D.; Ley, S.; Bause, A.S.; Azimian, S.; Wondrak, G.T. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic. Biol. Med. 2009, 46, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Wei, X.; Xue, Y.; Li, W.; Zhang, J.; Xiang, M.; Zhang, M.; Luo, Z.; Li, Y.; Yao, G.; et al. Wilsonols A-L, megastigmane sesquiterpenoids from the leaves of Cinnamomum wilsonii. J. Nat. Prod. 2013, 76, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.H.; Lo, Y.C.; Wu, B.N.; Wang, H.M.; Lo, W.L.; Yen, C.M.; Lin, R.J. Anticancer activity of isoobtusilactone A from Cinnamomum kotoense: Involvement of apoptosis, cell-cycle dysregulation, mitochondria regulation, and reactive oxygen species. J. Nat. Prod. 2008, 71, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, T.Z.; Chen, C.H.; Wu, C.C.; Cheng, J.T.; Yiin, S.J.; Shih, M.K.; Wu, M.J.; Chern, C.L. Isoobtusilactone A-induced apoptosis in human hepatoma Hep G2 cells is mediated via increased NADPH oxidase-derived reactive oxygen species (ROS) production and the mitochondria-associated apoptotic mechanisms. Food Chem. Toxicol. 2007, 45, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hsu, Y.L.; Chen, Y.Y.; Hung, J.Y.; Huang, M.S.; Kuo, P.L. Isokotomolide A, a new butanolide extracted from the leaves of Cinnamomum kotoense, arrests cell cycle progression and induces apoptosis through the induction of p53/p21 and the initiation of mitochondrial system in human non-small cell lung cancer A549 cells. Eur. J. Pharmacol. 2007, 574, 94–102. [Google Scholar] [PubMed]
- Yang, S.S.; Hou, W.C.; Huang, L.W.; Lee, T.H. A new gamma-lactone from the leaves of Cinnamomum kotoense. Nat. Prod. Res. 2006, 20, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.J.; Lo, W.L.; Wang, Y.D.; Chen, C.Y. A novel cytotoxic monoterpenoid from the leaves of Cinnamomum subavenium. Nat. Prod. Res. 2008, 22, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Wang, H.M.; Wu, T.W.; Chen, Y.J.; Shieh, J.J.; Lin, J.H.; Ho, T.F.; Luo, R.J.; Chen, C.Y.; Chang, C.C. Subamolide B isolated from medicinal plant Cinnamomum subavenium induces cytotoxicity in human cutaneous squamous cell carcinoma cells through mitochondrial and CHOP-dependent cell death pathways. Evid Based Complement. Altern. Med. 2013, 2013, 630415. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Chen, C.Y.; Huang, A.M.; Li, J.H. Subamolide A, a component isolated from Cinnamomum subavenium, induces apoptosis mediated by mitochondria-dependent, p53 and ERK1/2 pathways in human urothelial carcinoma cell line NTUB1. J. Ethnopharmacol. 2011, 137, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Huang, Y.H.; Lin, J.J.; Liau, B.C.; Wang, S.Y.; Wu, Y.C.; Jong, T.T. Cytotoxic lignan esters from Cinnamomum osmophloeum. Planta Med. 2010, 76, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.J.; Cheng, M.J.; Huang, J.C.; Lo, W.L.; Yeh, Y.T.; Yen, C.M.; Lu, C.M.; Chen, C.Y. Cytotoxic compounds from the stems of Cinnamomum tenuifolium. J. Nat. Prod. 2009, 72, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Kuo, S.Y.; Li, Y.P.; Kang, Y.F.; Yeh, Y.T.; Huang, J.C.; Chen, C.Y. A new benzodioxocinone from the leaves of Cinnamomum tenuifolium. Nat. Prod. Res. 2012, 26, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.F.; Ng, T.Y.; Liu, S.S.; Tsang, P.C.; Kwong, P.W.; Ngan, H.Y. Potential application of the ATP cell viability assay in the measurement of intrinsic radiosensitivity in cervical cancer. Gynecol. Oncol. 2005, 96, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Errington, J.; Chen, V.J.; Curtin, N.J.; Boddy, A.V.; Newell, D.R. Cellular ATP depletion by LY309887 as a predictor of growth inhibition in human tumor cell lines. Clin. Cancer Res. 2000, 6, 271–277. [Google Scholar] [PubMed]
- Dong, H.P.; Wu, H.M.; Chen, S.J.; Chen, C.Y. The effect of butanolides from Cinnamomum tenuifolium on platelet aggregation. Molecules 2013, 18, 11836–11841. [Google Scholar] [CrossRef] [PubMed]
- Daker, M.; Lin, V.Y.; Akowuah, G.A.; Yam, M.F.; Ahmad, M. Inhibitory effects of Cinnamomum burmannii Blume stem bark extract and trans-cinnamaldehyde on nasopharyngeal carcinoma cells; synergism with cisplatin. Exp. Ther. Med. 2013, 5, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Huang, C.Y.; Li, K.T.; Li, R.N.; Liaw, C.C.; Wu, S.H.; Liu, J.R.; Sheu, J.H.; Chang, H.W. Sinuleptolide inhibits proliferation of oral cancer Ca9–22 cells involving apoptosis, oxidative stress, and DNA damage. Arch. Oral Biol. 2016, 66, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.H.; Farooqi, A.A.; Li, K.T.; Butt, G.; Tang, J.Y.; Wu, C.Y.; Cheng, Y.B.; Hou, M.F.; Chang, H.W. Methanolic extracts of Solieria robusta inhibits proliferation of oral cancer Ca9–22 cells via apoptosis and oxidative stress. Molecules 2014, 19, 18721–18732. [Google Scholar] [CrossRef] [PubMed]
- Thuret, G.; Chiquet, C.; Herrag, S.; Dumollard, J.M.; Boudard, D.; Bednarz, J.; Campos, L.; Gain, P. Mechanisms of staurosporine induced apoptosis in a human corneal endothelial cell line. Br. J. Ophthalmol. 2003, 87, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Nicolier, M.; Decrion-Barthod, A.Z.; Launay, S.; Pretet, J.L.; Mougin, C. Spatiotemporal activation of caspase-dependent and -independent pathways in staurosporine-induced apoptosis of p53wt and p53mt human cervical carcinoma cells. Biol. Cell 2009, 101, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.C.; Yang, J.I.; Lee, J.C.; Tseng, C.N.; Chan, Y.C.; Hseu, Y.C.; Tang, J.Y.; Chuang, L.Y.; Huang, H.W.; Chang, F.R.; et al. Anti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complement. Altern. Med. 2012, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.Y.; Chiu, C.C.; Haung, R.W.; Yeh, C.C.; Huang, K.J.; Chang, K.F.; Hseu, Y.C.; Chang, F.R.; Chang, H.W.; Wu, Y.C. Antiproliferative effects of goniothalamin on Ca9–22 oral cancer cells through apoptosis, DNA damage and ROS induction. Mutat. Res. 2012, 747, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Haung, J.W.; Chang, F.R.; Huang, K.J.; Huang, H.M.; Huang, H.W.; Chou, C.K.; Wu, Y.C.; Chang, H.W. Golden berry-derived 4beta-hydroxywithanolide E for selectively killing oral cancer cells by generating ROS, DNA damage, and apoptotic pathways. PLoS ONE 2013, 8, e64739. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, W.; Hao, W.; Ren, G.; Lu, J.; Chen, X. Cucurbitacin B induces DNA damage, G2/M phase arrest, and apoptosis mediated by reactive oxygen species (ROS) in leukemia K562 cells. Anticancer Agents Med. Chem. 2014, 14, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.H.; Yu, C.S.; Lu, H.F.; Yang, J.S.; Huang, H.Y.; Chen, P.Y.; Wu, S.H.; Ip, S.W.; Chiang, S.Y.; Lin, J.G.; et al. Chrysophanol-induced cell death (necrosis) in human lung cancer A549 cells is mediated through increasing reactive oxygen species and decreasing the level of mitochondrial membrane potential. Environ. Toxicol. 2014, 29, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Lee, M.S.; Wu, C.R.; Cho, H.J.; Lin, K.Y.; Lai, G.H.; Wang, S.Y.; Kuo, Y.H.; Kumar, K.J.; Yang, H.L. The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAPK signaling pathway. J. Agric. Food Chem. 2012, 60, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.J.; Hsuuw, Y.D.; Chan, W.H. Characterization of apoptosis induced by emodin and related regulatory mechanisms in human neuroblastoma cells. Int. J. Mol. Sci. 2013, 14, 20139–20156. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.C.; El-Shazly, M.; Juan, Y.S.; Chang, C.Y.; Su, J.H.; Chen, Y.C.; Shih, S.P.; Chen, H.M.; Wu, Y.C.; Lu, M.C. Cracking the cytotoxicity code: Apoptotic induction of 10-acetylirciformonin B is mediated through ROS generation and mitochondrial dysfunction. Mar. Drugs 2014, 12, 3072–3090. [Google Scholar] [CrossRef] [PubMed]
- Thangam, R.; Senthilkumar, D.; Suresh, V.; Sathuvan, M.; Sivasubramanian, S.; Pazhanichamy, K.; Gorlagunta, P.K.; Kannan, S.; Gunasekaran, P.; Rengasamy, R.; et al. Induction of ROS-dependent mitochondria-mediated intrinsic apoptosis in MDA-MB-231 cells by glycoprotein from Codium decorticatum. J. Agric. Food Chem. 2014, 62, 3410–3421. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.C.; Tseng, C.N.; Yang, J.I.; Huang, H.W.; Fang, Y.; Tang, J.Y.; Chang, F.R.; Chang, H.W. Antiproliferation and induction of apoptosis in Ca9–22 oral cancer cells by ethanolic extract of Gracilaria tenuistipitata. Molecules 2012, 17, 10916–10927. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ji, N.; Zhou, Y.; Li, J.; Liu, X.; Wang, Z.; Chen, Q.; Zeng, X. CAL 27 is an oral adenosquamous carcinoma cell line. Oral Oncol. 2009, 45, e204–e207. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Stebbins, J.L.; Kitada, S.; Dash, R.; Zhai, D.; Placzek, W.J.; Wu, B.; Rega, M.F.; Zhang, Z.; Barile, E.; et al. An optically pure apogossypolone derivative as potent pan-active inhibitor of anti-apoptotic bcl-2 family proteins. Front. Oncol. 2011, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Chang, H.W.; Chuang, D.W.; Chang, F.R.; Chang, Y.C.; Cheng, Y.S.; Tsai, M.T.; Chen, W.Y.; Lee, S.S.; Wang, C.K.; et al. Fern plant-derived protoapigenone leads to DNA damage, apoptosis, and G(2)/m arrest in lung cancer cell line H1299. DNA Cell Biol. 2009, 28, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Liu, P.L.; Huang, K.J.; Wang, H.M.; Chang, K.F.; Chou, C.K.; Chang, F.R.; Chong, I.W.; Fang, K.; Chen, J.S.; et al. Goniothalamin inhibits growth of human lung cancer cells through DNA damage, apoptosis, and reduced migration ability. J. Agric. Food Chem. 2011, 59, 4288–4293. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Lee, S.H.; Meng, X.W.; Loegering, D.A.; Kottke, T.J.; Henzing, A.J.; Ruchaud, S.; Samejima, K.; Earnshaw, W.C. Apoptosis-associated caspase activation assays. Methods 2008, 44, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.Y.; Hou, M.F.; Yang, Z.W.; Tang, J.Y.; Li, K.T.; Huang, H.W.; Huang, Y.H.; Lee, S.Y.; Fu, T.F.; Hsieh, C.Y.; et al. Concentration effects of grape seed extracts in anti-oral cancer cells involving differential apoptosis, oxidative stress, and DNA damage. BMC Complement. Altern. Med. 2015, 15, 94. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Yen, C.-Y.; Wang, H.-R.; Yang, H.-P.; Tang, J.-Y.; Huang, H.-W.; Hsu, S.-H.; Chang, H.-W. Tenuifolide B from Cinnamomum tenuifolium Stem Selectively Inhibits Proliferation of Oral Cancer Cells via Apoptosis, ROS Generation, Mitochondrial Depolarization, and DNA Damage. Toxins 2016, 8, 319. https://doi.org/10.3390/toxins8110319
Chen C-Y, Yen C-Y, Wang H-R, Yang H-P, Tang J-Y, Huang H-W, Hsu S-H, Chang H-W. Tenuifolide B from Cinnamomum tenuifolium Stem Selectively Inhibits Proliferation of Oral Cancer Cells via Apoptosis, ROS Generation, Mitochondrial Depolarization, and DNA Damage. Toxins. 2016; 8(11):319. https://doi.org/10.3390/toxins8110319
Chicago/Turabian StyleChen, Chung-Yi, Ching-Yu Yen, Hui-Ru Wang, Hui-Ping Yang, Jen-Yang Tang, Hurng-Wern Huang, Shih-Hsien Hsu, and Hsueh-Wei Chang. 2016. "Tenuifolide B from Cinnamomum tenuifolium Stem Selectively Inhibits Proliferation of Oral Cancer Cells via Apoptosis, ROS Generation, Mitochondrial Depolarization, and DNA Damage" Toxins 8, no. 11: 319. https://doi.org/10.3390/toxins8110319






