1. Introduction
Bradykinin (BK) is a nonapeptide hormone first discovered in 1949 by Rocha et al. upon the basis of its inherent capability to dilate vessels [
1]. It has subsequently been reported that, in mammals, endogenous BK is generated via the effect of kallikrein on kininogen, which was derived mostly from, but not restricted to, liver tissues [
2,
3]. Since the first amphibian canonical BK was reported in the defensive skin secretions systems in 1965 from
Rana temporaria [
4], numerous bradykinin-related peptides (BRPs), with extraordinary diverse primary structure flanked by
C/
N-terminal extension, were isolated and identified from the Ascaphidae, Hylidae, Ranidae and Bombinatatoridae families. Of note is that mammals, reptiles, birds, and even fish were found to produce structurally discrete BRPs [
5]. The research that focused on elucidating the hypervariable primary structures of BRPs across vertebrates was initially conducted by Conlon and his colleagues, and found that the diverse BRPs presented in unique vertebrate taxa all have identical counterparts in certain amphibian species [
3,
6]. Following on from this, the hypothesis was proposed that taxon-specific BRPs might be the most effective ligands for activating taxon-specific receptor mediated signal pathways, and for the improper BRP-ligands, they were believed to act as antagonists of the receptors to suppress the downstream signalling. DNA sequences that encode a wide range of BRPs have been cloned from frog skin secretions of different genus and species, many of which have been proved to have myotropic activities by using diverse mammalian smooth muscle preparations [
5].
BK triggers its effect mainly through two types of receptors, B1 and B2, which all belong to the G protein-coupled receptors family. Compared with B2 receptors, which are generally expressed in multiple tissues, B1 receptors are only constitutively expressed in certain tissues, such as spinal cord, brain, and artery endothelial cells in the aorta and lung [
7]. While upon inflammatory stimulus, B1 receptor expressions in a variety of tissues are upregulated in response to NF-κB (extensively studied transcription factors involved in immune response and infection) activation [
8].
Stimulation or inhibition of B1 or B2 receptors is thought to be related with many pathophysiological disorders. Therefore, development of agonists or antagonists for either B1 or B2 receptors or even both would provide researchers significant implications no matter in the fields of pharmacology or clinical therapy.
Here, we reported a novel BRP, RAP-L1, T6-BK, which was isolated and identified from the skin secretion of the Chinese large odorous frog Odorrana livida by using 3′RACE and 5′RACE “shotgun” cloning technique. The primary sequence was further confirmed via the MS/MS fragmentation sequencing approach. The synthetic replicates of this novel peptide and its analogue RAP- L1, T6, L8-BK, were characterized by multiple organ-bath based ex vivo rat smooth muscle tissue probes. The wild-type RAP-L1, T6-BK was found to stimulate rat bladder, ileum, uterus contractile and tail artery relaxant responses. By contrast, the analogue RAP-L1, T6, L8-BK completely abrogated these functions, and it showed strong inhibition effect upon the BK-induced rat tail artery relaxation. Further pharmacological analysis revealed that BK B2 receptors are highly likely to be involved in the rat tail artery related effects caused by this novel BRP and its analogue.
3. Discussion
Frog skin secretions contain large number of bioactive components, including antimicrobial, protease inhibitory, and pharmacological relevant peptides. As we stated in the introduction, discrete BRP may have unique ligand-receptor specificity and potency, which is vital to provide valuable structure-interaction relationship information for agonistic and antagonistic research. Therefore, we decided to focus on “shotgun cloning” novel BRP by using BK-derived degenerate primer in this project. In addition, BRPs from amphibian secretions and venoms are considered to be homologous counterparts of endogenous mammalian polypeptide hormones. Therefore, study on their physiological and pathological relevance is essential for us to understand metabolic homeostasis in vivo, which could provide us novel insight into cardiac, vascular, renal and inflammatory associated disorders [
10,
11,
12,
13].
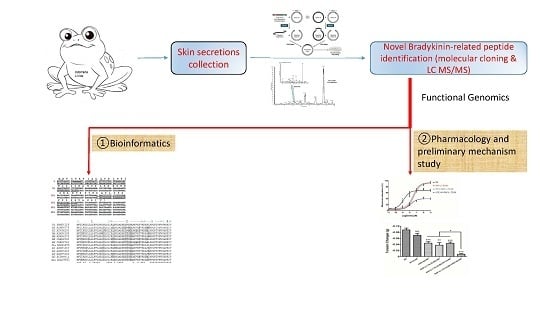
In this study, we describe the structural and functional characterizations of a novel BRP first identified in the skin secretions of Chinese Large frog,
Odorrana livida, through “shotgun” cloning of a biosynthetic precursor-encoding cDNA from a lyophilised skin secretion-derived cDNA library (
Figure 1). Both the open-reading frame nucleotide sequences and amino acid sequences encoding the precursors of BRPs from
Amolops and
Odorrana (
Figure 4) elicited unparalleled similarity (identity: 64%–90%), which suggests a possible evolutionary connection between these two genera of frogs [
14]. The mature novel BRP, RAP-L1, T6-BK, has a three residue
N-terminal extension RAP, and two site-substitutions L1, T6-BK compared with canonical BK. In addition, the T6-BK was found among several species of reptiles such as crocodilians, chelonians, and varanid lizards which contain whole components of the kallikrein-kinin system in mammals [
6]. Threonine 6 is one of the most common site-substitutions of amphibian skin secretion-derived BRPs, which reflects their evolutional adaptations to particular predators [
15]. It is believed that the replacement of Threonine at position 6 of BK is associated with enhanced effect of contracting rat ileum with equivalent potent to relax rat tail artery compared with BK [
16,
17]. Meanwhile,
N-terminal or
C-terminal extended BRPs are commonly identified from amphibian secretions and venoms. This study reported the -RAP- extension at N-terminal end BRP from
Odorrana livida. The BRPs Amolopkinin-W1/W2 isolated from
Amolops wuyiensis exhibited high degree of sequence similarity with RAP-L1, T6-BK, with the
N-terminal extension motif structure -RAA- and -RVA-, respectively. Both the Amolopkinin-W1 and Amolopkinin-W2 exhibited inhibitory effect of BK-induced intestinal smooth muscle contraction, while no direct effects on bladder and uterine smooth muscle preparations were detected [
14]. Despite the fact that the precise functions of diverse
N- or
C-terminal extension of BRPs still remains elusive, they are predicted to be involved in prolonging the interaction time of these peptides as ligands with target receptors, which could be adopted as a defensive strategy by host to survival [
18,
19].
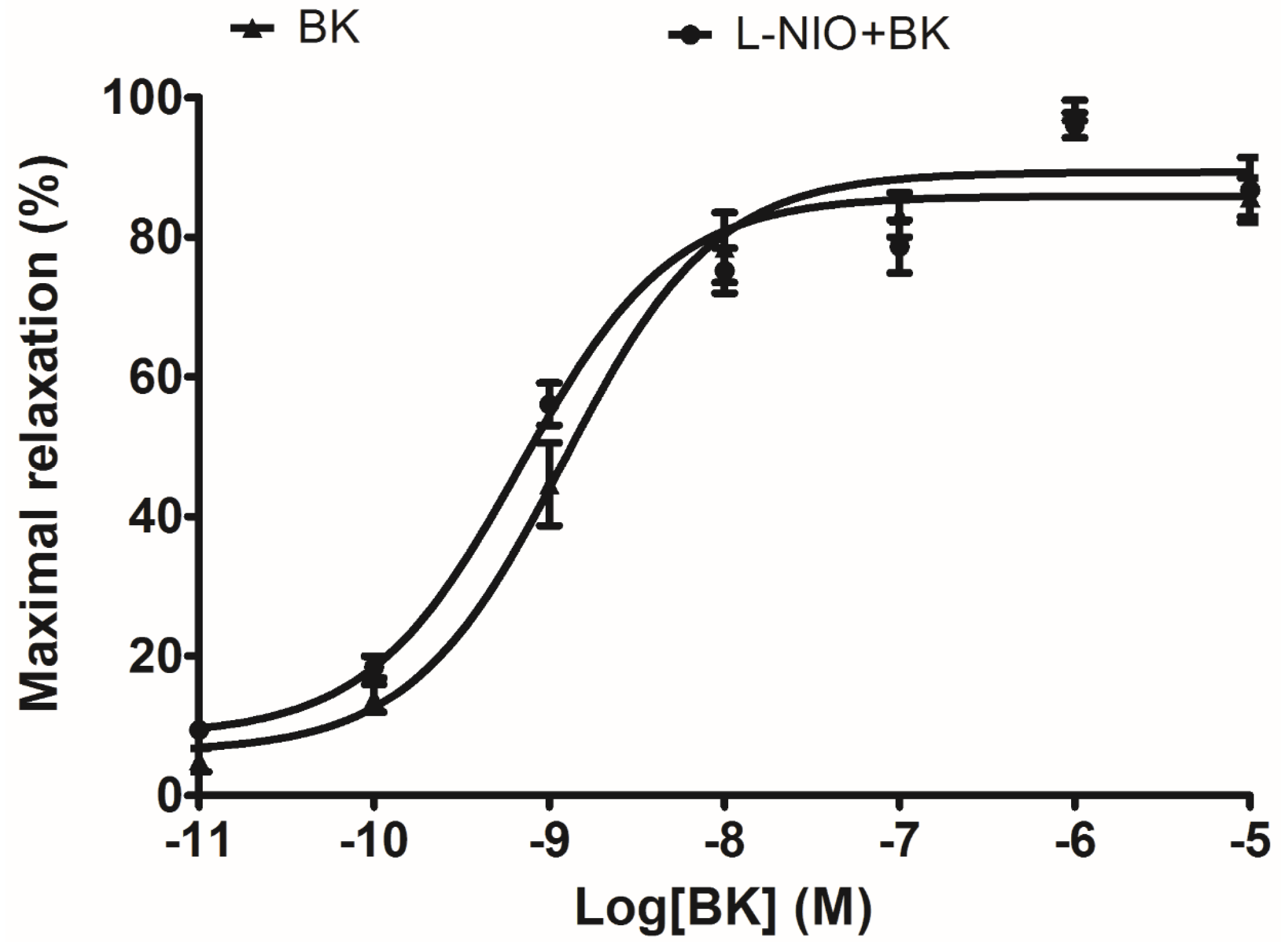
In terms of artery relaxation, considering the fact that a lot of vasodilators were involved in the release of nitric oxide (NO) through a common downstream signalling, and NO is widely accepted as the final substance that induces smooth muscle relaxation and inflammatory status [
20]. The eNOS (NO releaser) inhibitor, L-NIO, was pre-treated in arterial smooth muscle to test whether the well-established vasodilation via BK induction was influenced. Since the result did not show any significant difference, it implied that NO is not possible to be associated with this ex vivo rat tail artery smooth muscle model.
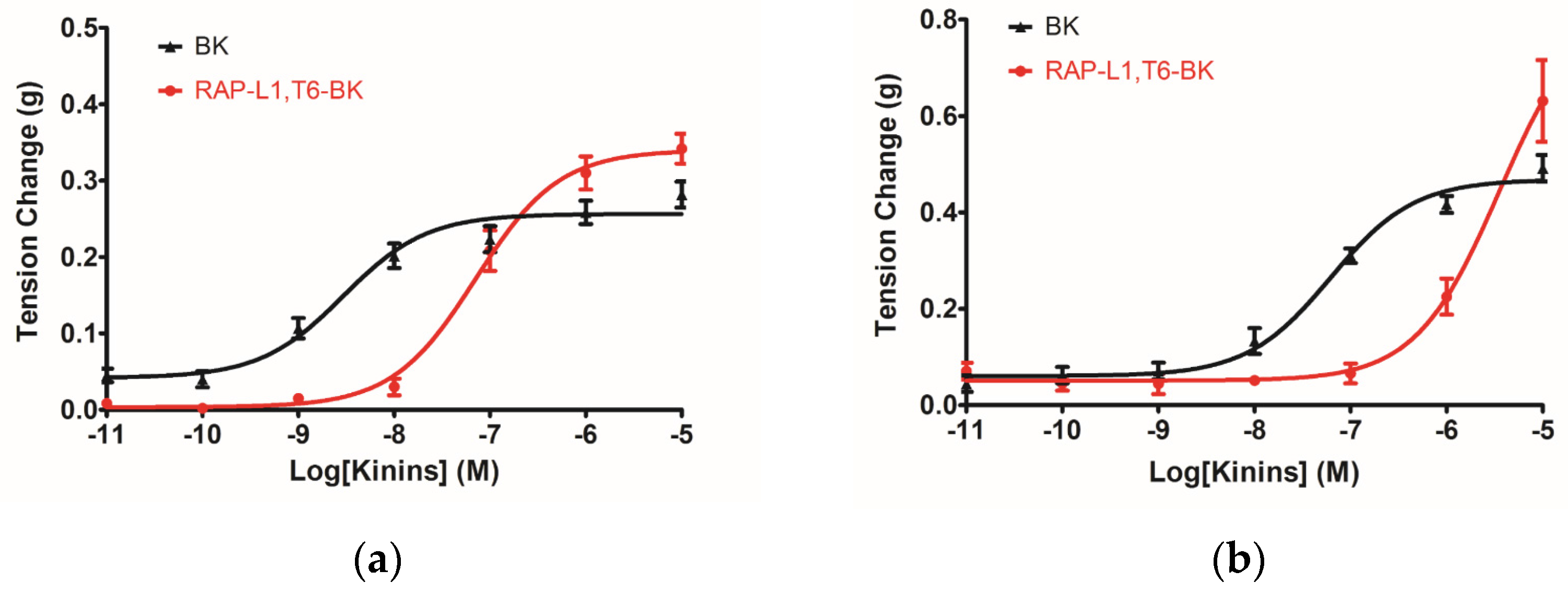
The myotropic potency of this novel identified RAP-L1, T6-BK peptide is relatively weak compared to BK, not only in contraction of rat bladder, ileum and uterus, but also in dilating vessels. Whilst, the novel peptide seemed to exhibited higher efficacy for the receptors compared to BK, which could be deduced from the
Figure 7d that at the concentration of 10
−7 M, RAP-L1, T6-BK have similar vasodilate capacity with BK, and when the concentration raised to 10
−6 M and 10
−5 M, RAP-L1, T6-BK dramatically enhanced the vasodilation on rat tail artery smooth muscle compared to BK, which indicated that the threshold concentration for RAP-L1, T6-BK and BK to induce a significant rat tail artery relax is differentiated, BK is more potency at lower concentration (10
−9 M–10
−7 M), but RAP-L1, T6-BK is more effective at higher concentration (10
−6 M–10
−5 M). In consideration of this, the primary structure similarity between them proposed us the hypothesis that RAP-L1, T6-BK mediates these effects through the cognate G protein-coupled receptors, and thus, we examined the arterial smooth muscle relaxing property modulated by RAP-L1, T6-BK in the presence of HOE140, a well-established BK B2 antagonist and R715, a selective BK B1 antagonist. Surprisingly, we observed a significant attenuation (nearly 60%) in HOE140 treatment condition, while R715 barely antagonize RAP-L1, T6-BK, which suggests a BK B2 receptors-related mechanism. Further experiments are still needed to address this issue thoroughly. Additionally, stimulation of neurokinin receptors in rat bladder, muscarinic receptors in ileal and endothelin receptors in uterine smooth muscle, which leads to intracellular calcium concentration, are reported to be involved in the contractions of these tissues [
21,
22,
23,
24,
25], the relationship between RAP-L1, T6-BK and these receptors are worthy to be investigated in the future.
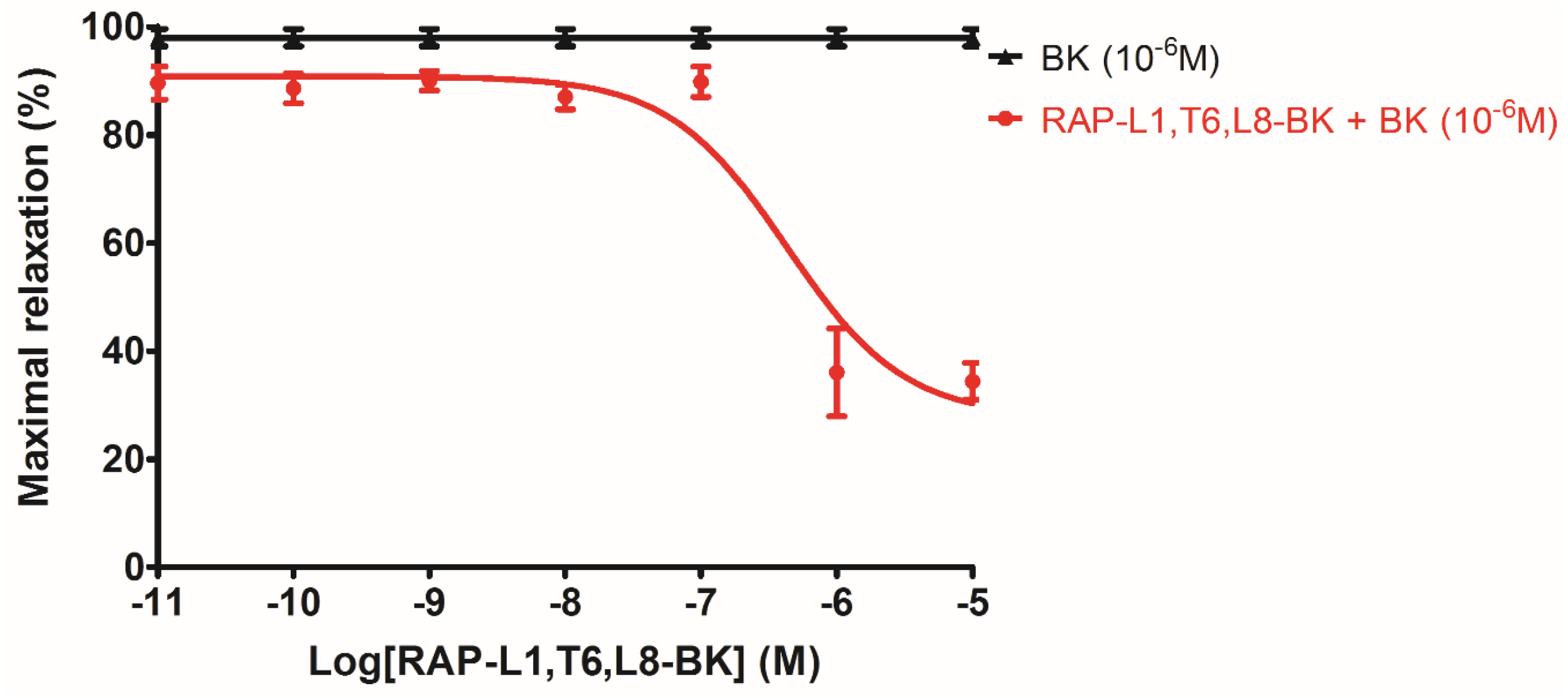
The analogue peptide RAP-L1, T6, L8-BK completely abrogated those bioactivities. Of interest is that only the leucine residue substitution at position 8 of BK would cause such an astonishing function loss. Leucine 1 is a common substitution occurred in BRPs, compared with valine substation at position 1, it normally exhibits moderate agonistic effect of BK [
26]. As the fact that Leu8-BK was reported as a BK receptor antagonist [
27], we performed further experiments to examine whether RAP-L1, T6-BK and its analogue RAP-L1, T6, L8-BK could inhibit BK-induced relax on rat tail artery smooth muscle, the results revealed that, as expected, RAP-L1, T6-BK did not show BK inhibition property (
Figure S1), but RAP-L1, T6, L8-BK significantly reduced BK relaxation activity on rat tail artery nearly 50% in the concentration range 10
−8 M–10
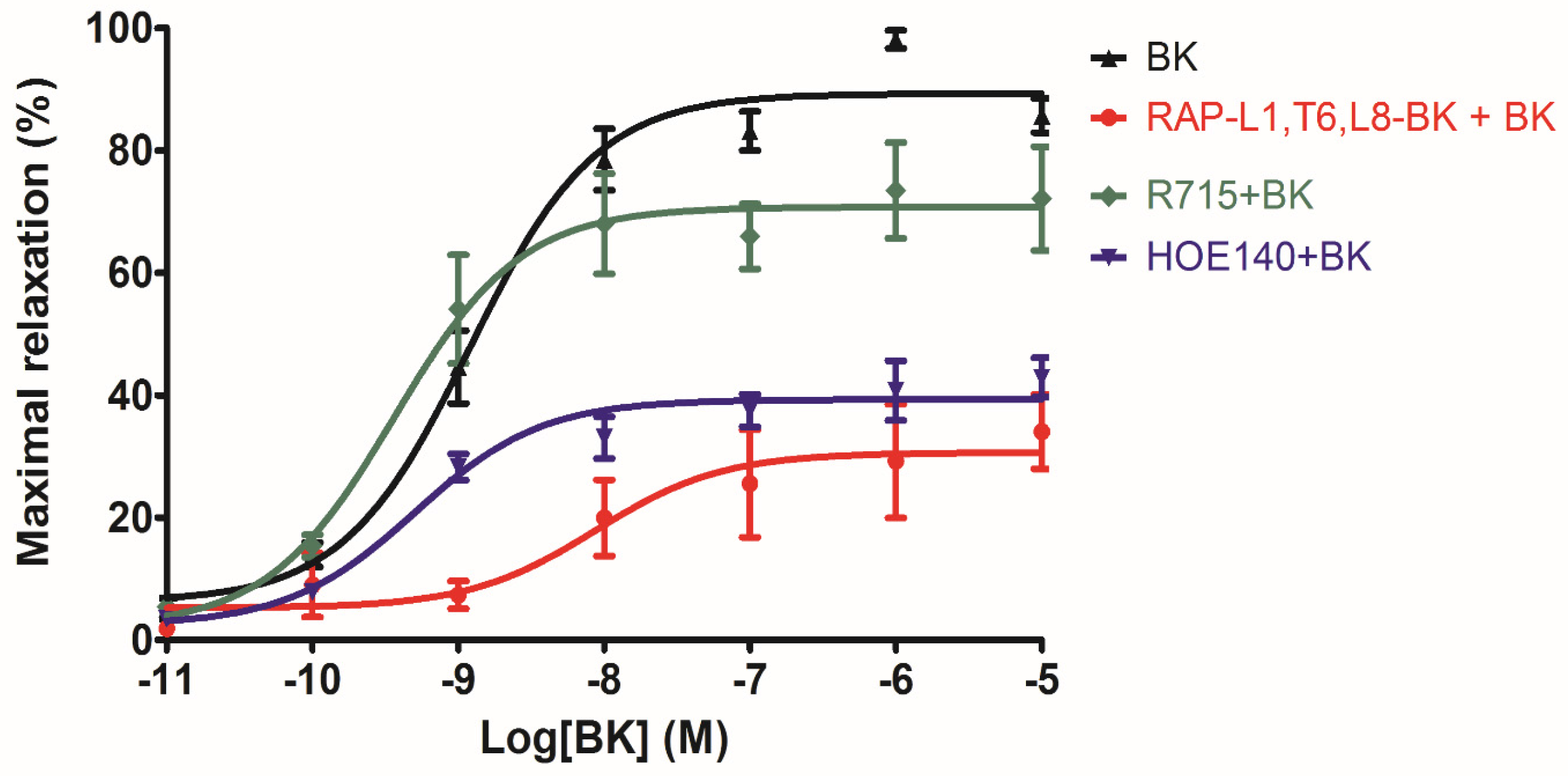
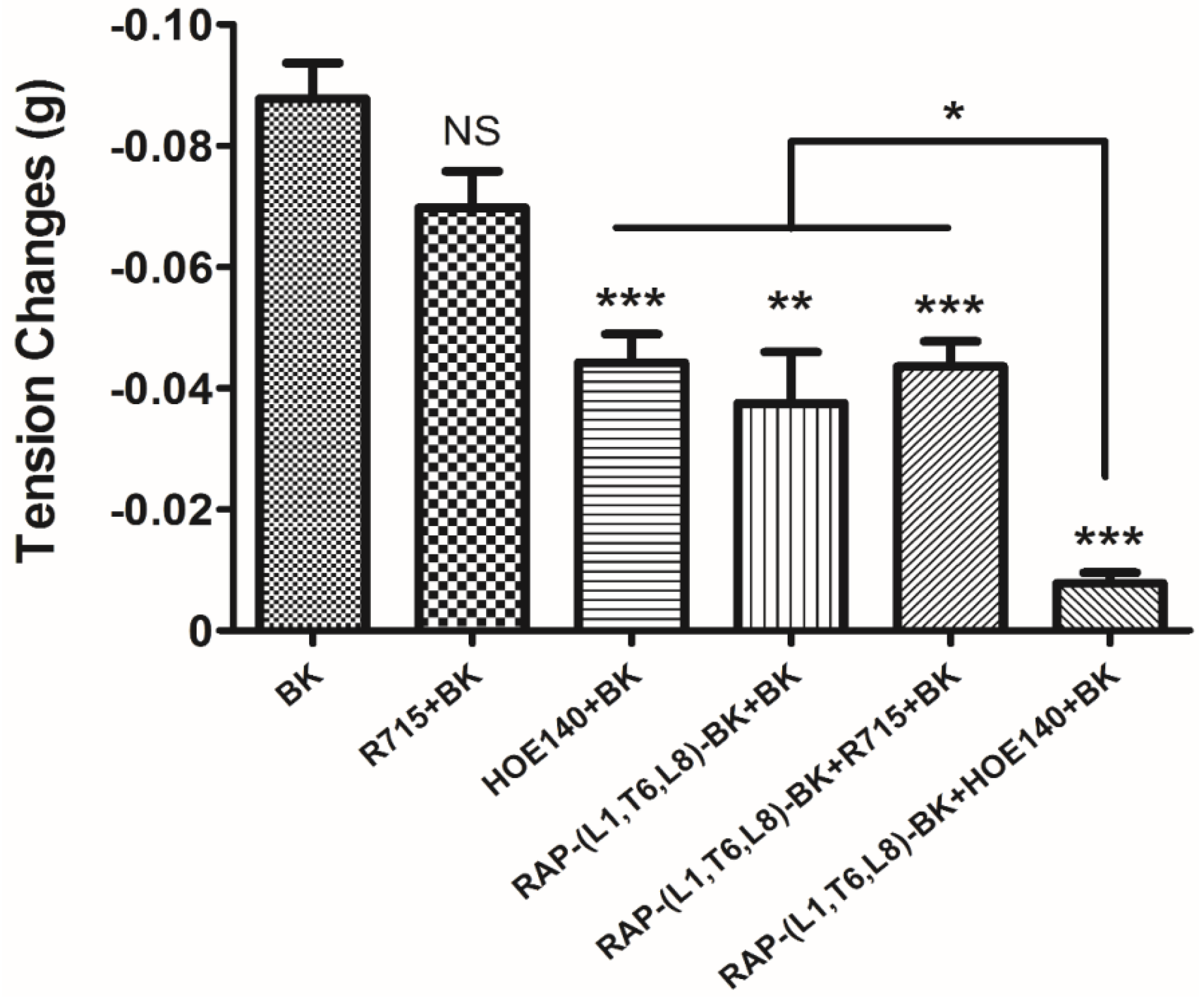
−5 M. These data suggested that although this novel BRP has a three residues -RAP- extension and leucine 1, threonine 6 substitutions, the leucine 8 still plays the essential and dominant role in reversing the BK’s effect. To investigate the precise manner how RAP-L1, T6, L8-BK exert BK antagonistic effect, again, we pre-treated rat tail artery smooth muscle with specific BK B1 receptors and/or B2 receptors inhibitors. When the tissues pre-treated with R157, BK-induced artery relaxation has a trend to be attenuated, but no statistical differences were observed. In contrast, when the tissues were pre-treated with HOE140, BK-induced artery relaxation was largely impaired. This probably due to that B1 receptors are rare expressed in rat tail artery or B2 receptor-mediated signalling is mainly responsible for BK-induced relaxation in rat tail artery, further examination by pre-treating the tissues with RAP-L1, T6, L8-BK along or together with R157 showed similar BK inhibition potency, which implies that the peptide might exert the effect in a B2 receptors-dependent manner. While RAP-L1, T6, L8-BK pre-treatment along with HOE140 almost abolished BK-induced relaxation suggested a novel synergistic inhibition mechanism via the BK B2 receptors might be involved compared with HOE140. In terms of this additive effect, others receptors, rather than BK receptors, activated by RAP-L1, T6, L8-BK is also a feasible option. Therefore, the primary structure initiated ligands-receptor interaction study of BRPs could provide researchers valuable information for developing novel molecular therapeutics.
Taken together, due to the inherent characteristics of the physiopathological significance of diverse BRPs for their potential to treat multiple vascular, cardiac diseases, they have been focused on for many years. In this project, we reported a novel BRP and its analogue isolated from Chinese large frog Odorrana livida, which presented distinct bio-functionalities, have unique structure/activity relationship, and continuously provides virtues for drug design.
4. Materials and Methods
4.1. Specimen Preparation and Secretion Harvesting
Adult Chinese Large Odorous frogs, Odorrana livida (n = 3, the length from snout to vent was 8–10 cm) were obtained from the mountain areas of Fujian province, People’s Republic of China. The gentle electrical stimulation (5 V, 50 Hz, 4 ms plus width) (C.F. Palmer, London, UK) was performed to induce the secretion from the glands of the frog dorsal surface. After secretion harvesting, the frogs were released. This method was non-lethal to frogs. The distilled deionized water was used to rinse the frog skin surface for secretions collection. The secretions were then subjected to snap frozen with liquid nitrogen, lyophilized and stored at −20 °C before use. Sampling of skin secretion was performed by Mei Zhou under UK Animal (Scientific Procedures) Act 1986, project license PPL 2694, issued by the Department of Health, Social Services and Public Safety, Northern Ireland. Procedures had been vetted by the IACUC of Queen’s University Belfast, and approved on 1 March 2011.
4.2. “Shotgun” Cloning of a Novel BRP Encoded cDNA Library
Five milligrams of lyophilized Odorrana livida skin secretion was dissolved in lysis/Binding Buffer. The extraction of polyadenylated mRNA was performed by using Dynabeads® mRNA DIRECT™ Kit (Dynal Biotech, Liverpool, UK). The cDNA library was obtained in reverse transcription reaction. Both 3′-RACE and 5′-RACE PCRs were employed to obtain the novel full-length prepro-BRP nucleic acid sequence by using SMART-RACE kit (Clontech, Oxford, UK). Briefly, the 3′-RACE PCR reaction employed a specific sense primer (5′-CCRVCNGGGTTYASSCCWTTY-3′) (R = A/G; V = A/C/G; N = A/C/T/G; Y = C/T; S = C/G; W = A/T) that was complementary to the nucleic acid sequence encoding the common -PPGFSPF- and -PPGFTPF- internal amino acid sequences of ranid frog skin BRPs and a UPM primer (supplied with the kit). The 3′-RACE reactions were purified and cloned into pGEM®-T Easy Vector (Promega Corporation, Southampton, UK) and sequenced using an ABI 3100 automated capillary sequencer. The 5′-RACE PCR employed an antisense primer (AS: 5′-CCGTATGTCATTTAGCTAAATGATGA-3′), which was designed to a highly conserved domain located within the 3′ non-translated region of the obtained 3′-RACE transcripts, and a UPM primer. In addition, the 5′-RACE products were gel-purified, cloned, and sequenced.
4.3. Identification and Structure Characterization of a Novel BRP from the Odorrana livida Skin Secretion
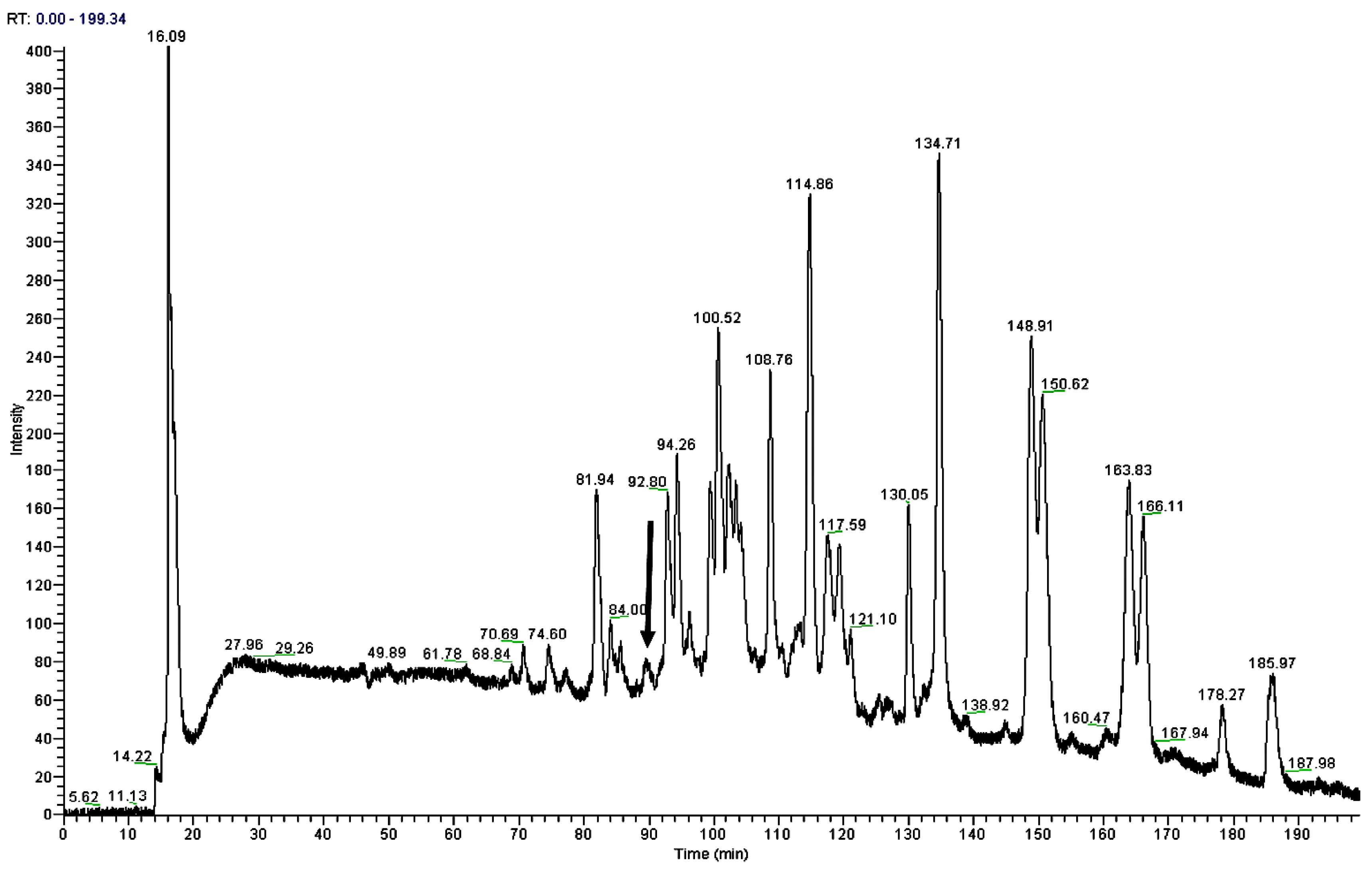
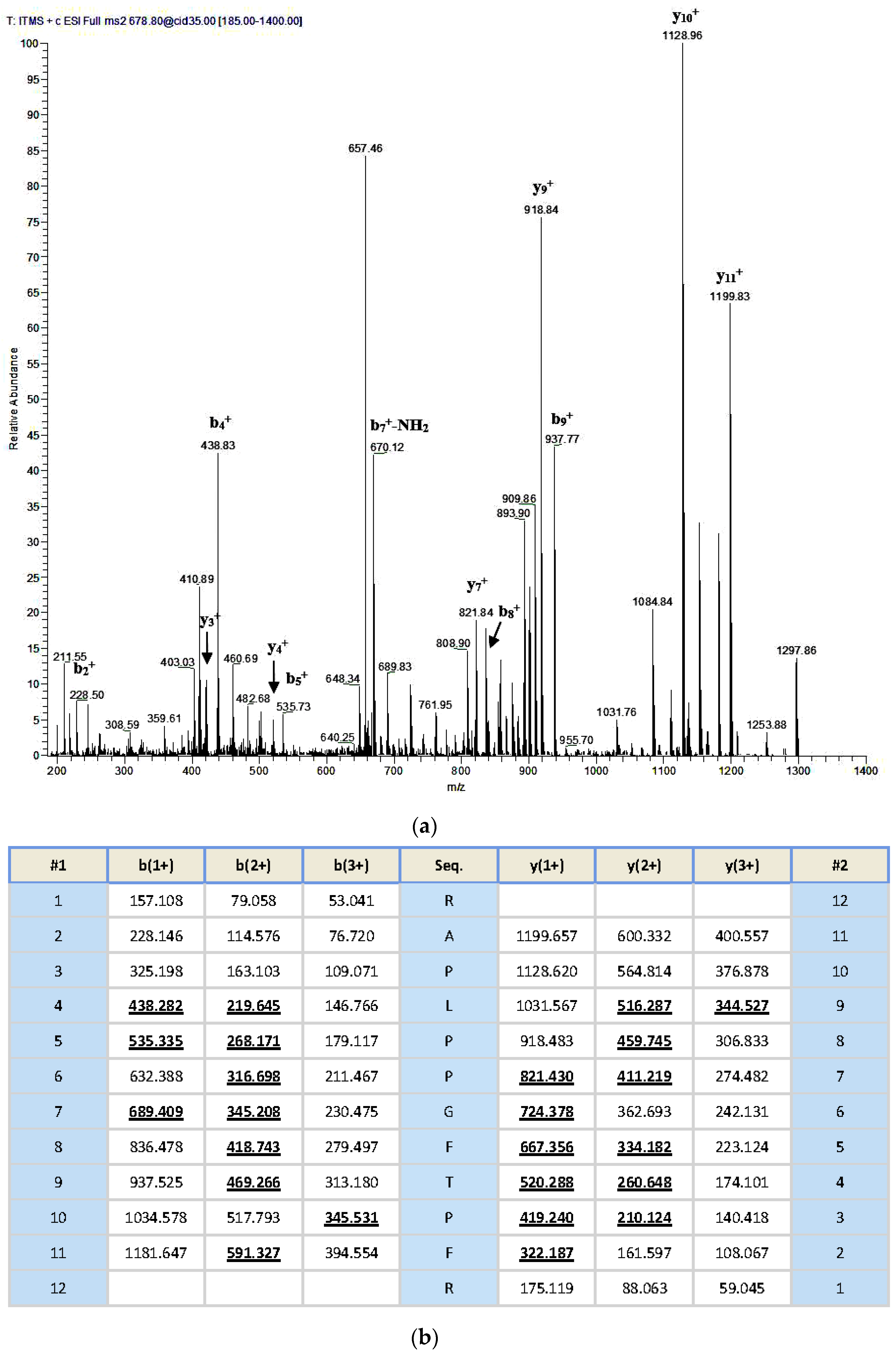
An aliquoted sample of five milligrams lyophilized Odorrana livida skin secretion was dissolved in 1 mL trifluoroacetic acid (TFA)/water (0.05/99.95, v/v), followed by centrifugation to clarify the sample solution. The clear supernatant was decanted and pumped into an analytical reverse phase HPLC column (pheomenex C-18, 250 × 10 mm) for fractionation by using a Cecil Adept 4200 HPLC system (Amersham Biosciences, Buckinghamshire, UK). The linear gradient elute method was used from TFA/water (0.05/99.95, v/v) to TFA/water/Acetonitrile (0.05/19.95/80.00, v/v/v) over 240 min at a flow rate of 1 mL/min. The fractions were collected automatically at one-minute intervals; the effluent absorbance was continuously monitored at 214 nm. Fractions were lyophilized and stored at −20 °C prior to being subjected to the smooth muscle assay. Meanwhile, the molecular masses of polypeptides in the collected fractions were analysed through matrix-assisted, laser desorption/ionization, time-of-flight mass spectrometry (MALDI-TOF MS) on a linear time-of-flight Voyager DE mass spectrometer (PerSeptive Biosystems, Framingham, MA, USA). The sample was analysed in positive detection mode using α-cyano-4-hydroxycinnamic acid as the matrix. Internal instrument calibration was performed by using standard peptides of established molecular masses, which provides the determined accuracy of ±0.1%. Fractions with the masses coincidence with those deduced mature peptides from the molecular cloning were subjected to MS/MS fragmentation sequencing analysis by using a Thermoquest gradient reversed phase HPLC system (Thermo Fisher Scientific, San Francisco, CA, USA), fitted with an analytical column (C-18), and interfaced with a Thermoquest LCQ™ Deca electrospray ion-trap mass spectrometer (Thermo Fisher Scientific, San Francisco, CA, USA).
4.4. Chemical Synthesis of Novel BRP (RAP-L1, T6-BK) and Its Modified Analogue RAP-L1, T6, L8-BK
Once the unequivocal primary structure of the novel bradykinin-related peptide had been determined through MS/MS fragmentation sequencing and molecular cloning of its biosynthetic precursor-encoding cDNA, Fmoc chemistry based solid phase peptide synthesis was performed to produced RAP-L1, T6-BK peptide replicates by using Tribute® Peptide Synthesizer (Protein Technologies, Inc., Tucson, AZ, USA). The analogue RAP-L1, T6, L8-BK was also synthesized with the same method. All synthetic peptides were purified with reverse phase HPLC. The authenticity of their primary structures and purity were confirmed via MALDI-TOF MS and LCQ MS/MS fragmentation sequencing.
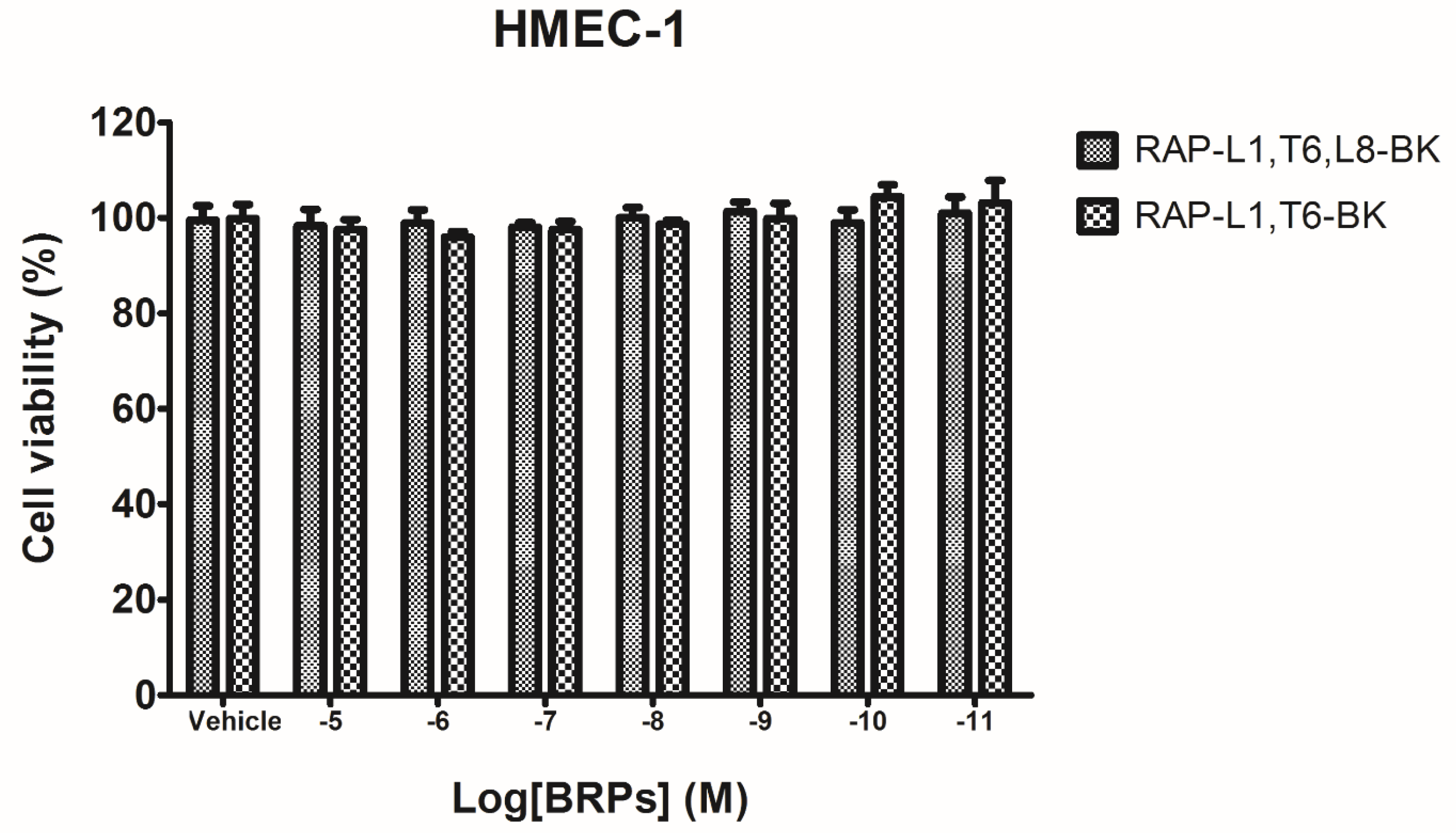
4.5. Preliminary Study of Inherent Cytotoxicity of the Novel BRPs
Prior to the smooth muscle assay for evaluating the pharmacological actions of BRPs, the MTT cell viability assay was performed. Cultured human microvessel endothelial cells (HMECs) were used to assess the possible influence of inherent cytotoxicity caused by the synthetic novel BRPs. In detail, the HMECs were seeded in 96-well plates with the density 5000 cells/well. After overnight incubation, HMECs were subjected to 6 h starvation prior to being treated with synthetic BRPs (RAP-L1, T6-BK and RAP-L1, T6, L8-BK) ranging from 10
−11 M to 10
−5 M for 24 h. Cell viabilities were assessed by MTT assay, as described previously [
28].
4.6. Ex Vivo Rat Bladder, Ileum, Uterus and Tail Artery Smooth Muscle Pharmacology
Wistar rats (200–250 g) were euthanized by asphyxiation with carbon dioxide in accordance with institutional animal experimentation ethics and UK animal research guidelines. The rats were placed on their dorsal surfaces, an incision was made along the mid ventral line towards the bottom of the abdomen and subcutaneous fat was carefully removed. The smooth muscle tissues of bladder, ileum, uterus, and tail artery were dissected gently followed by being placed in ice-cold Krebs’ solution immediately (118 mM NaCl, 4.7 mM KCl, 25 mM NaHCO
3, 1.15 mM NaH
2PO
4, 2.5 mM CaCl
2, 1.1 mM MgCl
2 and 5.6 mM glucose), and equilibrated with a mixed gas (95% CO
2 and 5% O
2). The tissues were dissected and mounted as shown in the table to obtain maximum viability and to achieve the best response (
Table 1).
After dissection, small strips of particular tissues were connected to a triangular hook. Then, the removed tissues were mounted to the force transducers in a 2 mL organ bath which were perfused with Krebs’ solution (118 mM NaCl, 4.7 mM KCl, 25 mM NaHCO3, 1.15 mM NaH2PO4, 2.5 mM CaCl2, 1.1 mM MgCl2 and 5.6 mM glucose) at 37 °C and constantly bubbling 95% O2 and 5% CO2 gas mixture. The prepared tissues were attached to the force transducer in order to detect the tension changes. The responses of tension change were recorded and amplified through pressure transducers connected to a PowerLab System (AD Instruments Pty Ltd., Oxford, UK). The peptide solutions of RAP-L1, T6-BK and RAP-L1, T6, L8-BK were prepared in the range of 10−11 M to 10−5 M with fresh Krebs’ solutions to construct the dose–response curves. Each concentration of individual peptide was applied to a minimum of six muscle strips.
For the rat tail artery preparations, endothelial linings were essentially removed from the dissected smooth muscle, a basal tension of 0.5 g was applied to the organ bath incubated tail artery before adding phenylephrine (1 × 10−5 M) for 10 min to achieve constriction plateaux. To further exclude the possible vasorelaxant effect caused by endothelial-derived nitric oxide (NO), tail artery smooth muscles were pre-treated with/without 0.5 µM eNOS (endothelium nitric oxide synthase) inhibitor N5-(1-Iminoethyl)-l-ornithine dihydrochloride (L-NIO, Tocris Biosciences, San Diego, CA, USA) for 20 min, prior to performing a BK sequential concentration (10−11 M to 10−5 M) -induced vasodilation.
For urinary bladder and ileum smooth muscle preparations, the bladder and ileum strips were gradually exposed to increasing tension until 0.5 g was reached and maintained. The preparations were then exposed to peptides as described above and relative changes in tension were recorded.
For uterus smooth muscle preparations, female Wistar rats were euthanized by carbon dioxide. The dissected uterine horns were mounted in each organ bath, incubated with 37 °C Krebs’ solution for 10 min with no tension. The uterus strips were gradually exposed to increasing tension until 0.5 g was reached and maintained. The uterus smooth muscle preparations were exposed to peptides as described previously and, changes in spontaneous contraction frequencies, instead of tension change, were recorded.
To examine what type of BK receptors in rat tail artery smooth muscle were targeted by BRPs, a single 10−6 M dose of BK B1 receptor antagonist R715 and/or the BK B2 receptor antagonist, HOE140 (Sigma Aldrich, Irvine, UK) were applied to artery smooth muscle preparations prior to peptide treatment.
Data from this study were analysed by one-way ANOVA with Bonferroni’s post-test and two-way ANOVA using GraphPad Prism software (version 5.01, San Diego, CA, USA). A p value less than 0.05 was considered significant.