A Double-Blind Randomized Controlled Trial Investigating the Most Efficacious Dose of Botulinum Toxin-A for Sialorrhea Treatment in Asian Adults with Neurological Diseases
Abstract
:1. Introduction
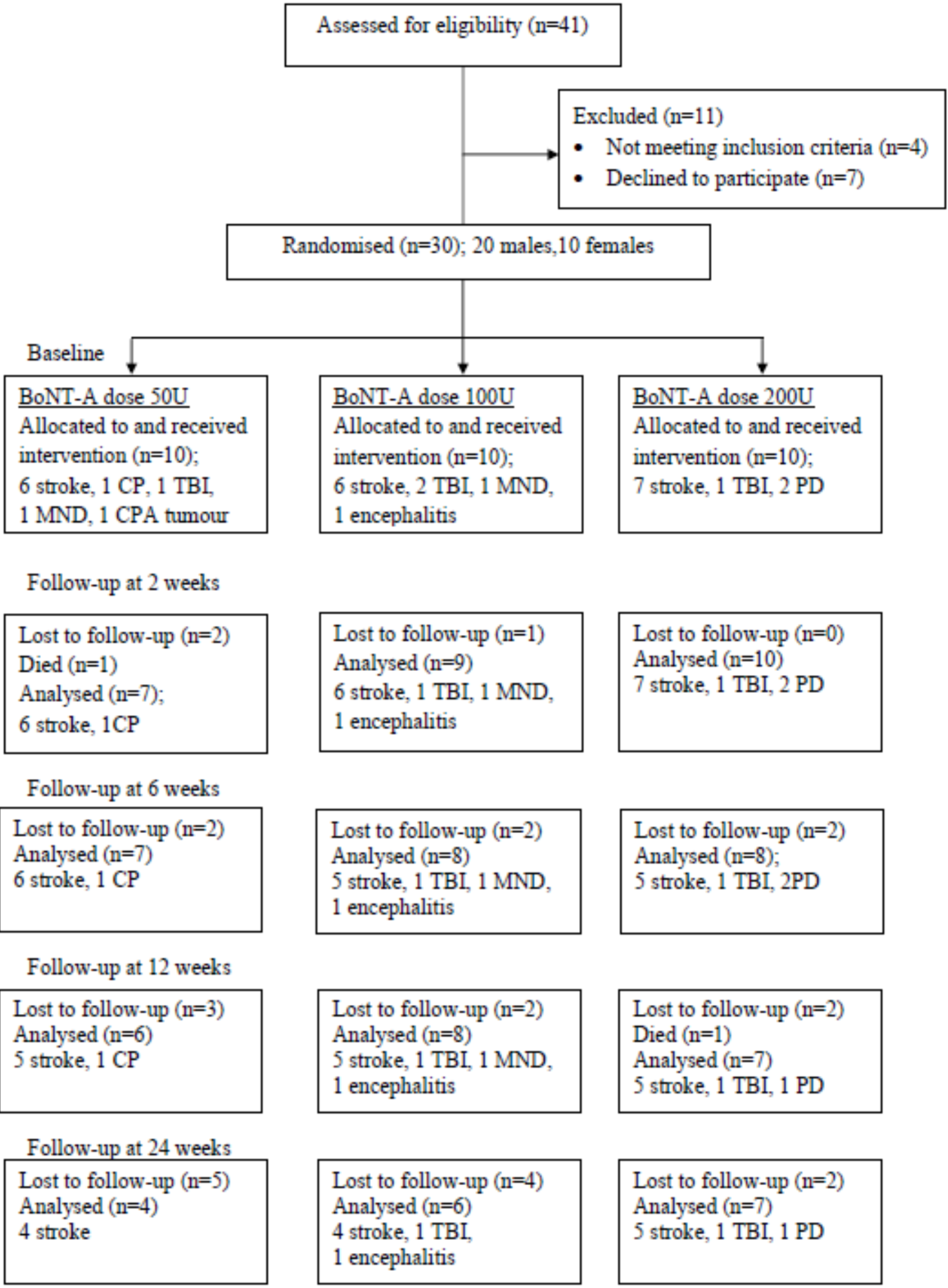
2. Results
2.1. Participants
| No. | Age (Years) | Sex (F/M) | Diagnosis | Duration of Disease (Years) | BoNT-A Dose Allocated |
|---|---|---|---|---|---|
| 1 | 56 | M | Stroke | 4 | 50 |
| 2 | 65 | F | Stroke | 4 | 50 |
| 3 | 56 | M | Stroke | 1 | 50 |
| 4 | 49 | M | Stroke | 2 | 50 |
| 5 | 50 | F | Stroke | 3 | 100 |
| 6 | 56 | M | Stroke | 2 | 100 |
| 7 | 58 | F | Stroke | 3 | 100 |
| 8 | 27 | M | Stroke | 4 | 100 |
| 9 | 23 | M | TBI | 2 | 100 |
| 10 | 39 | M | Encephalitis | 2 | 100 |
| 11 | 55 | F | Stroke | 3 | 200 |
| 12 | 70 | M | Stroke | 3 | 200 |
| 13 | 83 | F | Stroke | 3 | 200 |
| 14 | 73 | M | Stroke | 1 | 200 |
| 15 | 63 | M | Stroke | 1 | 200 |
| 16 | 54 | F | TBI | 3 | 200 |
| 17 | 68 | M | PD | 3 | 200 |
| 18 | 74 | M | Stroke | 9 | 50 |
| 19 | 68 | F | Stroke | 1 | 50 |
| 20 | 19 | M | CP | 3 | 50 |
| 21 | 25 | M | TBI | 5 | 50 |
| 22 | 65 | M | MND | 1 | 50 |
| 23 | 55 | M | CPA Tumour | 1 | 50 |
| 24 | 79 | F | Stroke | 1 | 100 |
| 25 | 56 | M | Stroke | 1 | 100 |
| 26 | 33 | M | TBI | 8 | 100 |
| 27 | 62 | F | MND | 3 | 100 |
| 28 | 79 | M | Stroke | 3 | 200 |
| 29 | 62 | M | Stroke | 1 | 200 |
| 30 | 64 | F | PD | 4 | 200 |
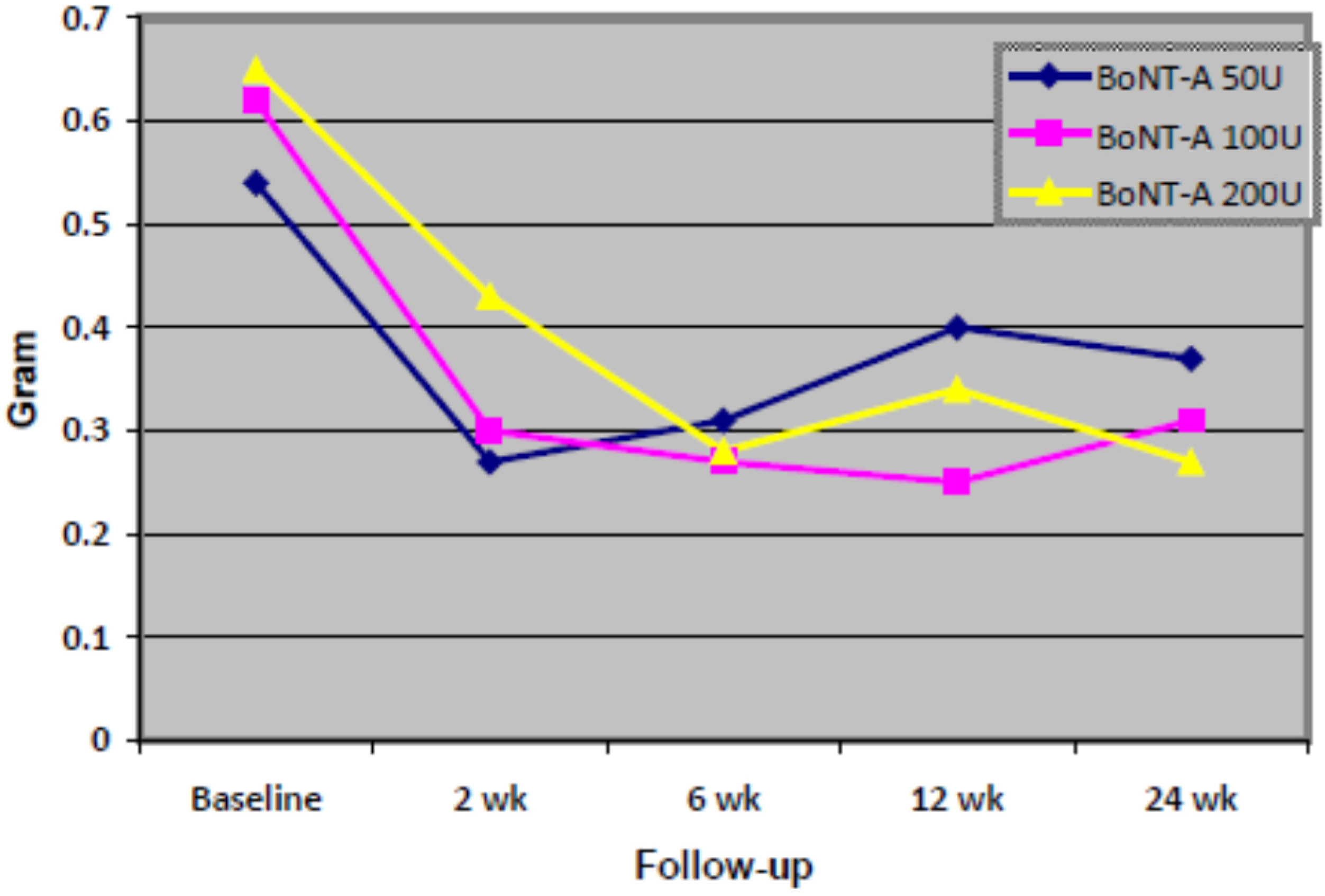
2.2. Efficacy Analysis
| Time | 50 Units, n = 4 | 100 Units, n = 6 | 200 Units, n = 7 | F Statistic (d.f.) a | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Mean, g | (SD) | Mean, g | (SD) | Mean, g | (SD) | |||
| Baseline | 0.54 | (0.603) | 0.62 | (0.323) | 0.65 | (0.824) | 0.62 (3) | 0.626 |
| 2 weeks | 0.27 | (0.267) | 0.30 | (0.105) | 0.43 | (0.591) | ||
| 6 weeks | 0.31 | (0.406) | 0.27 | (0.165) | 0.28 | (0.299) | ||
| 12 weeks | 0.40 | (0.439) | 0.25 | (0.082) | 0.34 | (0.476) | ||
| 24 weeks | 0.37 | (0.342) | 0.31 | (0.193) | 0.27 | (0.271) | ||
| Time | 50 Units, n = 9 | 100 Units, n = 10 | 200 Units, n = 10 | F Statistic (d.f.) a | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Mean, g | (SD) | Mean, g | (SD) | Mean, g | (SD) | |||
| Baseline | 0.54 | (0.419) | 0.58 | (0.305) | 0.53 | (0.703) | 0.58 (4) | 0.687 |
| 2 weeks | 0.27 | (0.185) | 0.28 | (0.098) | 0.34 | (0.502) | ||
| 6 weeks | 0.30 | (0.260) | 0.33 | (0.229) | 0.23 | (0.257) | ||
| 12 weeks | 0.33 | (0.287) | 0.30 | (0.237) | 0.28 | (0.403) | ||
| 24 weeks | 0.34 | (0.231) | 0.34 | (0.271) | 0.22 | (0.237) | ||
| BoNT-A Group (Dysport®) | Mean DFS Total Score (Drooling Severity + Frequency) | ||||
|---|---|---|---|---|---|
| Baseline (SD) | Week 2 (SD) | Week 6 (SD) | Week 12 (SD) | Week 24 (SD) | |
| 50 U | 6.2 (0.66) | 4.2 (0.97) | 4.1 * (1.16) | 4.4 (1.40) | 4.7 (1.11) |
| 100 U | 7.2 (1.39) | 5.6 (1.26) | 5.1 * (1.19) | 4.5 * (0.97) | 4.8 * (1.48) |
| 200 U | 7.5 (1.26) | 4.4 * (1.26) | 4.7 * (0.70) | 4.2 * (1.03) | 4.0 * (0.92) |
2.3. Safety
3. Discussion
4. Experimental Section
4.1. Design and Procedure
4.2. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Porta, M.; Gamba, M.; Bertacchi, G.; Vaj, P. Treatment of sialorrhea with ultrasound guided botulinum toxin type A injection in patients with neurological disorders. J. Neurol. Neurosurg. Psychiatry 2001, 70, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Fuster Torres, M.A.; Berini Aytés, L.; Gay Escoda, C. Salivary gland application of botulinum toxin for the treatment of sialorrhea. Med. Oral Patol. Oral Cir. Bucal. 2007, 12, E511–E517. [Google Scholar]
- Blasco, P.A. Surgical management of drooling. Dev. Med. Child Neurol. 1992, 34, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Kalf, J.G.; Smit, A.M.; Bloem, B.R.; Zwarts, M.J.; Mulleners, W.M.; Munneke, M. Botulinum toxin A for drooling in Parkinson’s disease: A pilot study to compare submandibular to parotid gland injections. Parkinsonism Relat. Disord. 2007, 13, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Alrefai, A.H.; Aburahma, S.K.; Khader, Y.S. Treatment of sialorrhea in children with cerebral palsy: A double-blind placebo controlled trial. Clin. Neurol. Neurosurg. 2009, 111, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Jongerius, P.H.; van den Hoogen, F.J.; van Limbeek, J.; Gabreels, F.J.; van Hulst, K.; Rotteveel, J.J. Effect of botulinum toxin in the treatment of drooling: A controlled clinical trial. Pediatrics 2004, 114, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.; Bahroo, L.; Pagan, F. An update on the use of botulinum toxin therapy in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2015, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Vashishta, R.; Nguyen, S.A.; White, D.R.; Gillespie, M.B. Botulinum toxin for the treatment of sialorrhea: A meta-analysis. Otolaryngol. Head Neck Surg. 2013, 148, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Guidubaldi, A.; Fasano, A.; Ialongo, T.; Piano, C.; Pompili, M.; Mascianà, R.; Siciliani, L.; Sabatelli, M.; Bentivoglio, A.R. Botulinum toxin A versus B in sialorrhea: A prospective, randomized, double-blind, crossover pilot study inpatients with amyotrophic lateral sclerosis or Parkinson’s disease. Mov. Disord. 2011, 26, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Zangaglia, R.; Cristina, S.; Sommaruga, M.G.; Martignoni, E.; Nappi, G.; Pacchetti, C. Double-blind, placebo-controlled study to evaluate the efficacy and safety of botulinum toxin A in the treatment of drooling in parkinsonism. Mov. Disord. 2003, 18, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.L.; Bigalke, H.; Dressler, D. Pharmacology of botulinum toxin: Differences between type A preparations. Eur. J. Neurol. 2006, 13 (Suppl. S1), 2–10. [Google Scholar] [CrossRef] [PubMed]
- Reddihough, D.; Erasmus, C.E.; Johnson, H.; McKellar, G.M.W.; Jongerius, P.H.; Cerebral Palsy Institute. Botulinum toxin assessment, intervention and aftercare for pediatric and adult drooling; international consensus statement. Eur. J. Neurol. 2010, 17, 109–121. [Google Scholar] [CrossRef]
- Bakheit, A.M.; Zakine, B.; Maisonobe, P.; Aymard, C.; Fhedoroff, K.; Hefter, H.; Jacinto, J.; Jost, W.H.; Molteni, F.; Stam, H.; et al. The profile of patients and current practice of treatment of upper limb muscle spasticity with botulinum toxin type A: An international survey. Int. J. Rehabil. Res. 2010, 33, 199–204. [Google Scholar] [CrossRef]
- Thomas-Stonell, N.; Greenberg, J. Three treatment approaches and clinical factors in the reduction of drooling. Dysphagia 1988, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lakraj, A.A.; Moghimi, N.; Jabbari, B. Sialorrhea: Anatomy, pathophyisology and treatment with emphasis on the role of botulinum toxins. Toxins 2013, 5, 1010–1031. [Google Scholar] [CrossRef] [PubMed]
- Basciani, M.; di Rienzo, F.; Fontana, A.; Copetti, M.; Pellegrini, F.; Intiso, D. Botulinum toxin type B for sialorrhea in children with cerebral palsy: A randomized trial comparing three doses. Dev. Med. Child Neurol. 2011, 53, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Møller, E.; Daugaard, D.; Holm, O.; Winge, K.; Bardow, A.; Lykkeaa, J.; Belhage, B.; Bakke, M. Repeated treatments of drooling with botulinum toxin B in neurology. Acta Neurol. Scand. 2015, 131, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Squires, N.; Humberstone, M.; Wills, A.; Arthur, A. The use of botulinum toxin injections to manage drooling in amyotrophic lateral sclerosis/motor neurone disease: A systematic review. Dysphagia 2014, 29, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Ney, J.P.; Joseph, K.R. Neurologic uses of botulinum neurotoxin type A. Neuropsychiatr. Dis. Treat. 2007, 3, 785–798. [Google Scholar] [PubMed]
- Porte, M.; Chaléat-Valayer, E.; Patte, K.; D’Anjou, M.C.; Boulay, C.; Laffont, I. Relevance of intraglandular injections of Botulinum toxin for the treatment of sialorrhea in children with cerebral palsy: A review. Eur. J. Paediatr. Neurol. 2014, 18, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Giess, R.; Naumann, N.; Wener, E.; Riemann, R.; Beck, M.; Puls, I.; Reiners, C.; Toyka, K.V. Injections of botulinum toxin A into the salivary glands improve sialorrhea in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2000, 69, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Tighe, D.; Gok, G.; Moody, A.; Howlett, D. Dysphagia as a complication of botulinum toxin injection to treat drooling. Br. J. Oral Maxillofac. Surg. 2014, 52, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H. The option of sonographic guidance in Botulinum toxin injection for drooling in Parkinson’s disease. J. Neural. Transm. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lipp, A.; Trottenberg, T.; Schink, T.; Kupsch, A.; Arnold, G. A randomized trial of botulinum toxin A for treatment of drooling. Neurology 2003, 61, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Young, C.A.; Ellis, C.; Johnson, J.; Sathasivam, S.; Pih, N. Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis. Cochrane Database Syst. Rev. 2011, 11, CD006981. [Google Scholar] [CrossRef]
- Srivanitchapoom, P.; Pandey, S.; Hallett, M. Drooling in Parkinson’s disease: A review. Parkinsonism Relat. Disord. 2014, 20, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazlan, M.; Rajasegaran, S.; Engkasan, J.P.; Nawawi, O.; Goh, K.-J.; Freddy, S.J. A Double-Blind Randomized Controlled Trial Investigating the Most Efficacious Dose of Botulinum Toxin-A for Sialorrhea Treatment in Asian Adults with Neurological Diseases. Toxins 2015, 7, 3758-3770. https://doi.org/10.3390/toxins7093758
Mazlan M, Rajasegaran S, Engkasan JP, Nawawi O, Goh K-J, Freddy SJ. A Double-Blind Randomized Controlled Trial Investigating the Most Efficacious Dose of Botulinum Toxin-A for Sialorrhea Treatment in Asian Adults with Neurological Diseases. Toxins. 2015; 7(9):3758-3770. https://doi.org/10.3390/toxins7093758
Chicago/Turabian StyleMazlan, Mazlina, Shivani Rajasegaran, Julia Patrick Engkasan, Ouzreiah Nawawi, Khean-Jin Goh, and Saini Jeffery Freddy. 2015. "A Double-Blind Randomized Controlled Trial Investigating the Most Efficacious Dose of Botulinum Toxin-A for Sialorrhea Treatment in Asian Adults with Neurological Diseases" Toxins 7, no. 9: 3758-3770. https://doi.org/10.3390/toxins7093758
APA StyleMazlan, M., Rajasegaran, S., Engkasan, J. P., Nawawi, O., Goh, K.-J., & Freddy, S. J. (2015). A Double-Blind Randomized Controlled Trial Investigating the Most Efficacious Dose of Botulinum Toxin-A for Sialorrhea Treatment in Asian Adults with Neurological Diseases. Toxins, 7(9), 3758-3770. https://doi.org/10.3390/toxins7093758






