First Report of Ciguatoxins in Two Starfish Species: Ophidiaster ophidianus and Marthasterias glacialis
Abstract
:1. Introduction
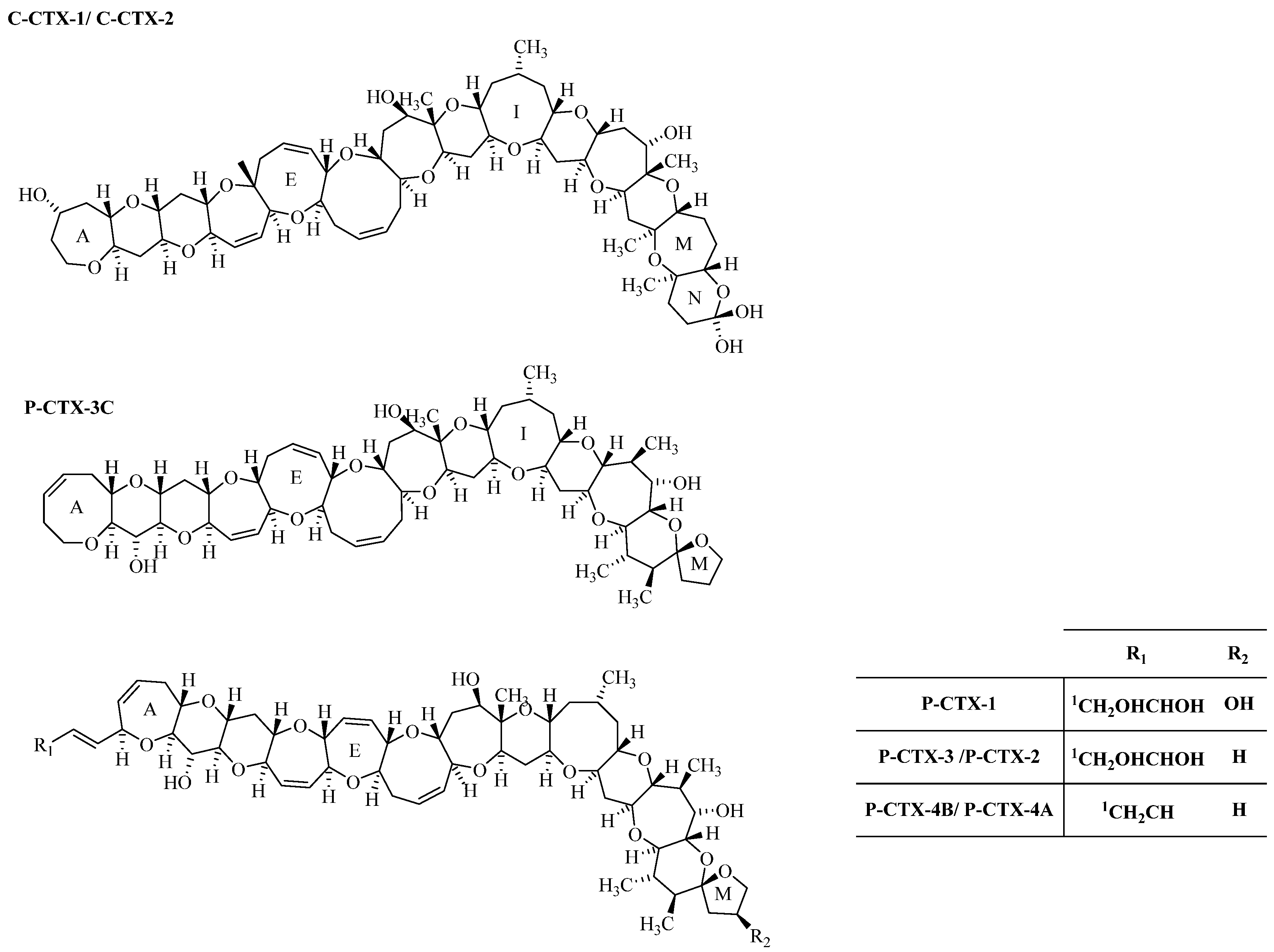
2. Results and Discussion
| Species | Trophic Level | Sampling Site(s) | NrP Samples | AvNr | Edible | Ref. |
|---|---|---|---|---|---|---|
| Aplysia depilans | Grazer | Morocco | 3 | 1 | No | [23] |
| Arbacia lixula | Grazer | Madeira/Azores/Morocco | 9 | 4 | No | [24] |
| Charonia lampas | 3rd level predator | Madeira/Morocco | 3 | 1 | Yes | [25] |
| Cerithium vulgatum | Grazer | Morocco | 1 | 40 | Yes | [26] |
| Diadema africanum | Grazer | Madeira | 2 | 1 | No | [27] |
| Echinaster sepositus | 2nd level predator | Madeira | 1 | 3 | No | [28] |
| Gibbula umbilicalis | Grazer | Morocco | 3 | 100 | Yes | [29] |
| Holothuria(Platyperona)sanctori | Deposit feeder | Morocco | 4 | 1 | Yes | [30,31] |
| Marthasterias glacialis | 2nd level predator | Madeira/Azores/Morocco | 8 | 1 | No | [32] |
| Monodonta lineata | Grazer | Morocco | 5 | 86 | Yes | [29] |
| Mytilus spp. | Filter feeder | Morocco | 4 | 30 | Yes | [33] |
| Onchidella celtica | Grazer | Morocco | 1 | 50 | No | [34] |
| Ophidiaster ophidianus | Detritivorous | Madeira/Azores | 5 | 1 | No | [28] |
| Pattela aspera | Grazer | Madeira | 2 | 15 | Yes | [32] |
| Patella spp. | Grazer | Morocco | 4 | 12 | Yes | [32] |
| Pattela candei | Grazer | Azores | 3 | 10 | Yes | [32] |
| Paracentrotus lividus | Grazer | Madeira/Azores/Morocco | 7 | 1 | Yes | [35] |
| Pollicipes pollicipes | Filter feeder | Morocco | 3 | 35 | Yes | [32] |
| Sphaerechinus granularis | Grazer | Azores | 4 | 1 | Yes | [36] |
| Umbraculum umbraculum | Grazer | Madeira | 1 | 1 | No | [37] |
| Stramonita haemostoma | 2nd level predator | Madeira/Azores/Morocco | 5 | 15 | No | [38] |
| Compound Name | Mass | Polarity |
|---|---|---|
| I-CTX-3 and I-CTX-4 | 1157.6 | Positive |
| Unknown CTX | 1143.6 | Positive |
| Caribean-CTX | 1141.7 | Positive |
| C-CTX-1127 | 1127.6 | Positive |
| CTX-1B | 1111.6 | Positive |
| 54-deoxy-CTX-1B 52-epi-54-deoxy-CTX-1B | 1095.6 | Positive |
| M-CTX-4A and M-CTX-4B | 1079.6 | Positive |
| CTX-4A and CTX-4B | 1061.6 | Positive |
| MTX small | 1060 | Positive |
| 2,3-OH-CTX-3C M-CTX-3C methylacetal | 1055.6 | Positive |
| 2-OH-CTX-3C and M-CTX-3C | 1041.6 | Positive |
| Analogs CTX | 1040.6 | Positive |
| 51-OH-CTX-3C | 1039.6 | Positive |
| 49-epo-CTX-3C and CTX-3C | 1023.6 | Positive |
| Unknown CTX | 1159.6 | Positive |
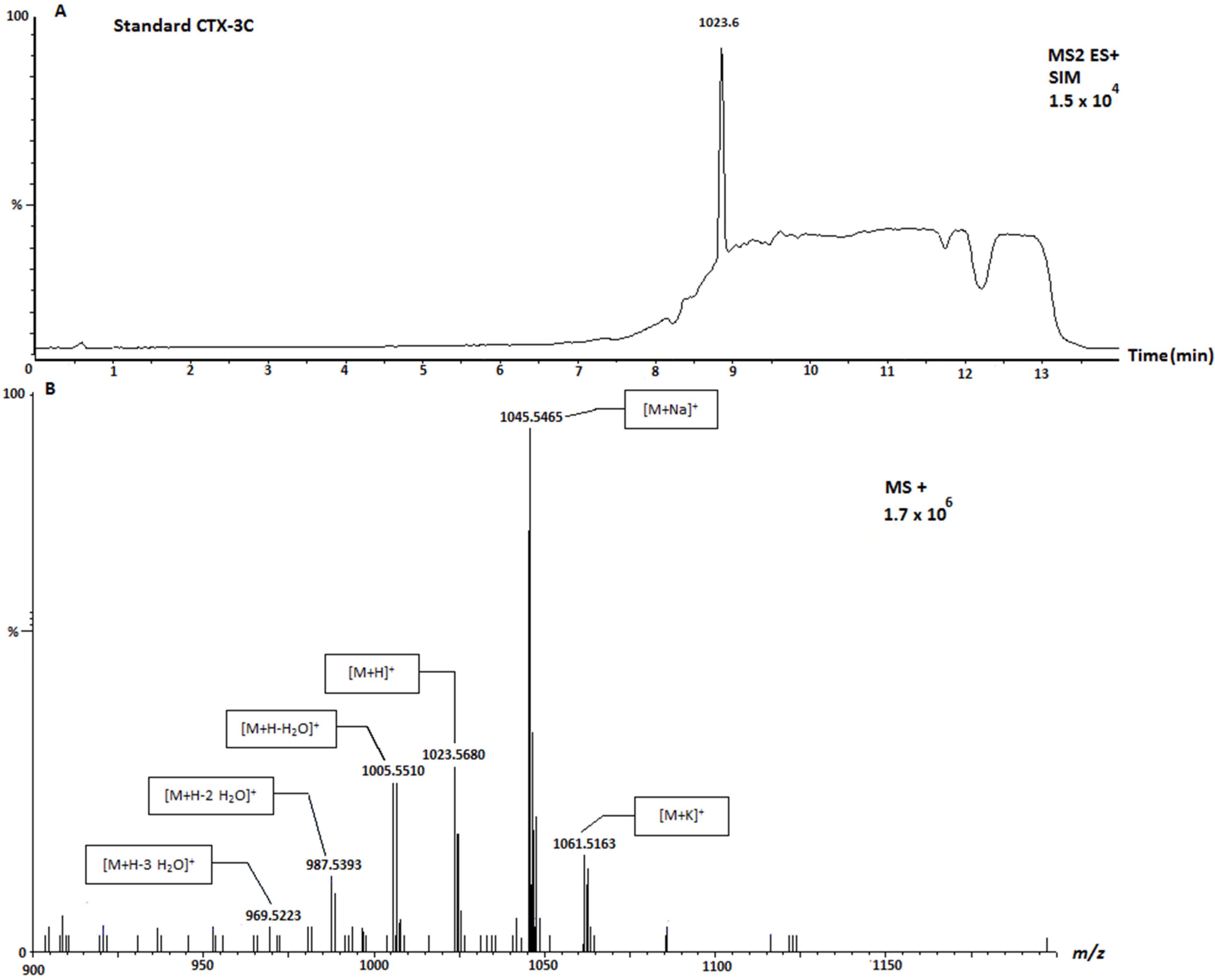
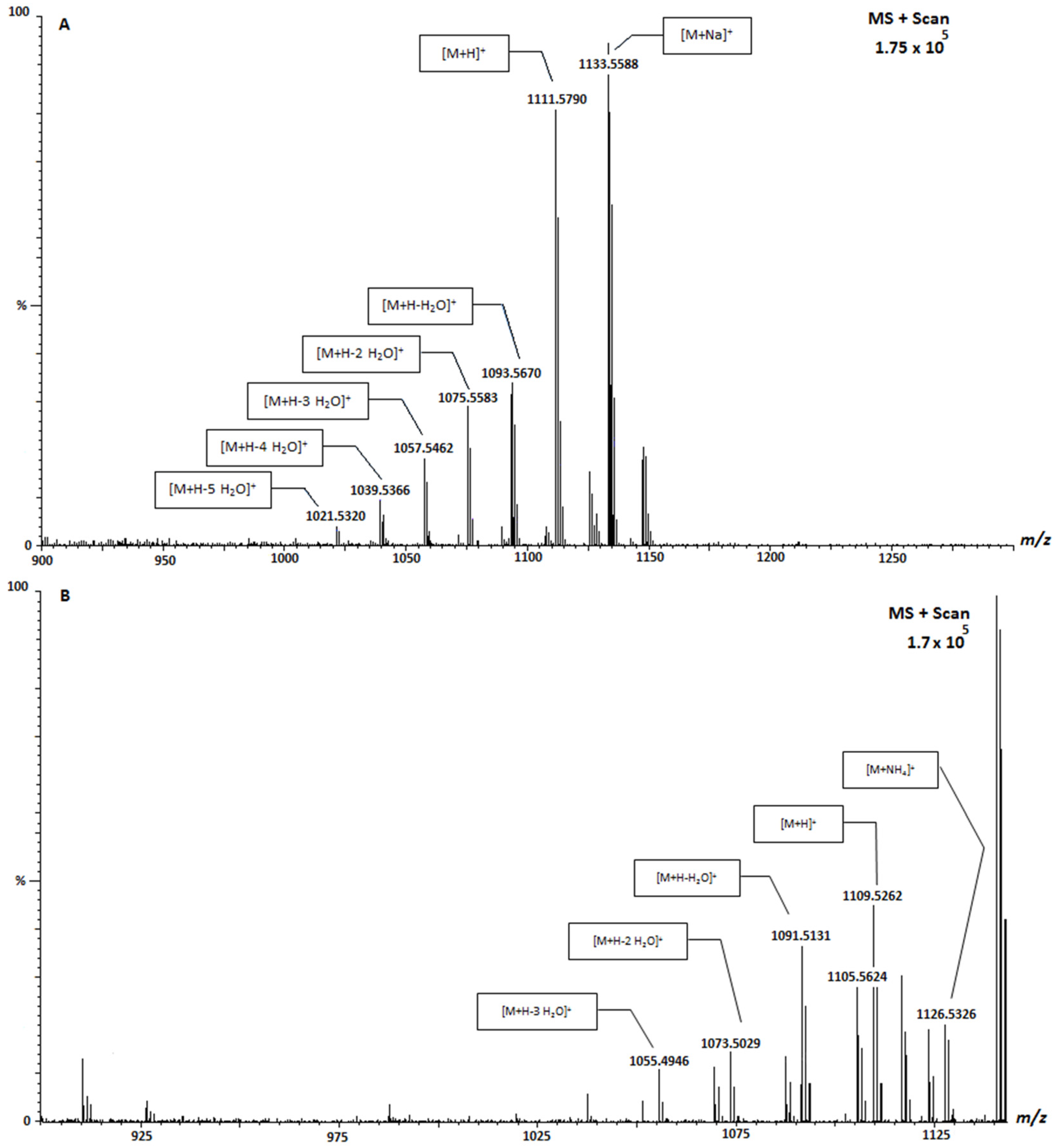

| Sample | Location | Species | 1111 (μg CTX-3C Equivalents/KgFw) | 1123 (μg CTX-3C Equivalents/KgFw) | 1109 (μg CTX-3C Equivalents/KgFw) |
|---|---|---|---|---|---|
| 341 #1 | Madeira | O. ophidianus | 21.55 | 7.24 | |
| 341 #2 | Madeira | O. ophidianus | 46.49 | 29.80 | |
| 411 #2 | Azores | M. glacialis | <LOQ | <LOQ | |
| 412 | Azores | O. ophidianus | 19.73 | ||
| 424 | Azores | O. ophidianus | 124.04 | 30.90 | |
| 426 #1 | Azores | M. glacialis | 7.92 | ||
| 435 | Azores | O. ophidianus | 58.78 | 4.40 |
| Analysis of Deviance | ||||
|---|---|---|---|---|
| Model | Factor | χ2 | df | p |
| Binomial Error | Site | 2.9 | 2 | 0.23 |
| Rescaled model coefficients for Site: Intercept (Azores) = 0.36; Madeira = 0.78; Morocco = 4 × 10−8 | ||||
| Gamma Error | Site | 5.5 | 2 | 0.06 |
| Rescaled model coefficients for Site: Intercept (Azores) = 229; Madeira = 1.39; Morocco = 0.004 | ||||
| Analysis of Deviance | ||||
|---|---|---|---|---|
| Model | Factor | χ2 | df | p |
| Binomial Error | Species | 8.5 | 1 | <0.01 |
| Rescaled model coefficients: Intercept (M. glacialis) = 0.11; O. ophidianus = 0.98 | ||||
| Gamma Error | Organism | 9.7 | 1 | <0.01 |
| Rescaled model coefficients: Intercept (M. glacialis) = 9.4; O. ophidianus = 57.7 | ||||
3. Experimental Section
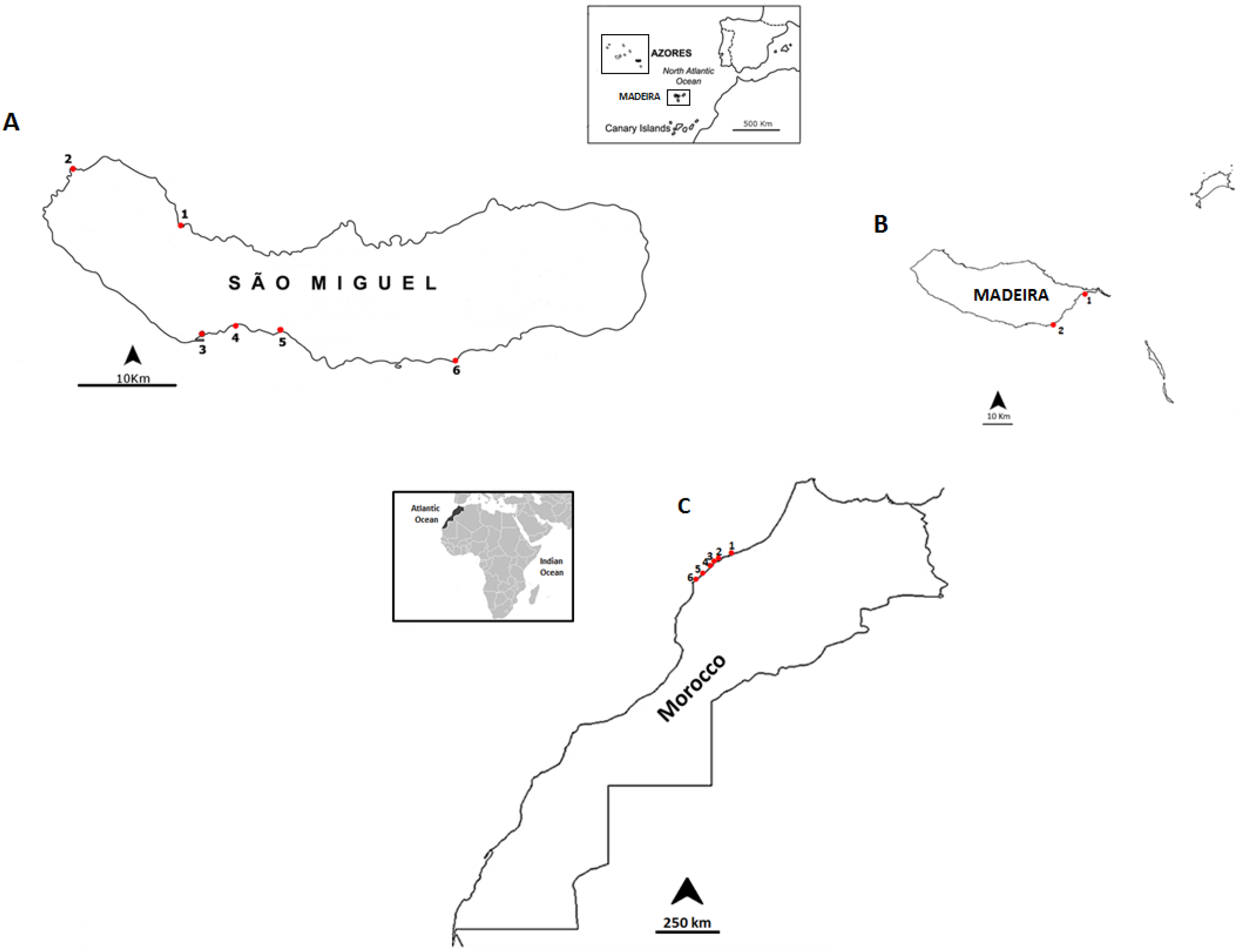
3.1. Selected Species and Sampling Sites
| Date | Location | Sampling Site | Geographic Coordinates |
|---|---|---|---|
| September 2012 | Madeira Island | Reis Magos | 32°39ʹ16.21ʹʹ N; 16°49ʹ05.29ʹʹ W |
| Caniçal | 32°44ʹ20.08ʹʹ N; 16°44ʹ17.55ʹʹ W | ||
| June 2013 | São Miguel Island | Cruzeiro | 37°50ʹ31.19ʹʹ N; 25° 41ʹ33.61ʹʹ W |
| Étar | 37°44ʹ19.31ʹʹ N; 25°39ʹ38.84ʹʹ W | ||
| São Roque | 37°45ʹ15.35ʹʹ N; 25°38ʹ31.60ʹʹ W | ||
| Mosteiros | 37°53ʹ25.57ʹʹ N; 25°49ʹ14.72ʹʹ W | ||
| Lagoa | 37°44ʹ42.38ʹʹ N; 25°19ʹ.47ʹʹ W | ||
| Caloura | 37°42ʹ49.34ʹʹ N; 25°29ʹ54.54ʹʹ W | ||
| July 2013 | Morocco Coast | Casablanca corniche | 33°36ʹ01.2ʹʹ N; 7°39ʹ57.5ʹʹ W |
| El Jadida Haras | 33°14ʹ42.0ʹʹ N; 8°28ʹ37.5ʹʹ W | ||
| El Jadida Sâada | 33°14ʹ42.4ʹʹ N; 8°32ʹ26.9ʹʹ W | ||
| Sidi Bouzid | 33°13ʹ57.1ʹʹ N; 8°33ʹ20.9ʹʹ W | ||
| Mrizika | 32°57ʹ21.8ʹʹ N; 8°46ʹ53.2ʹʹ W | ||
| Oualidia | 32°43ʹ55.8ʹʹ N; 9°02ʹ57.6ʹʹ W |
3.2. Reagents
3.3. Sample Extraction
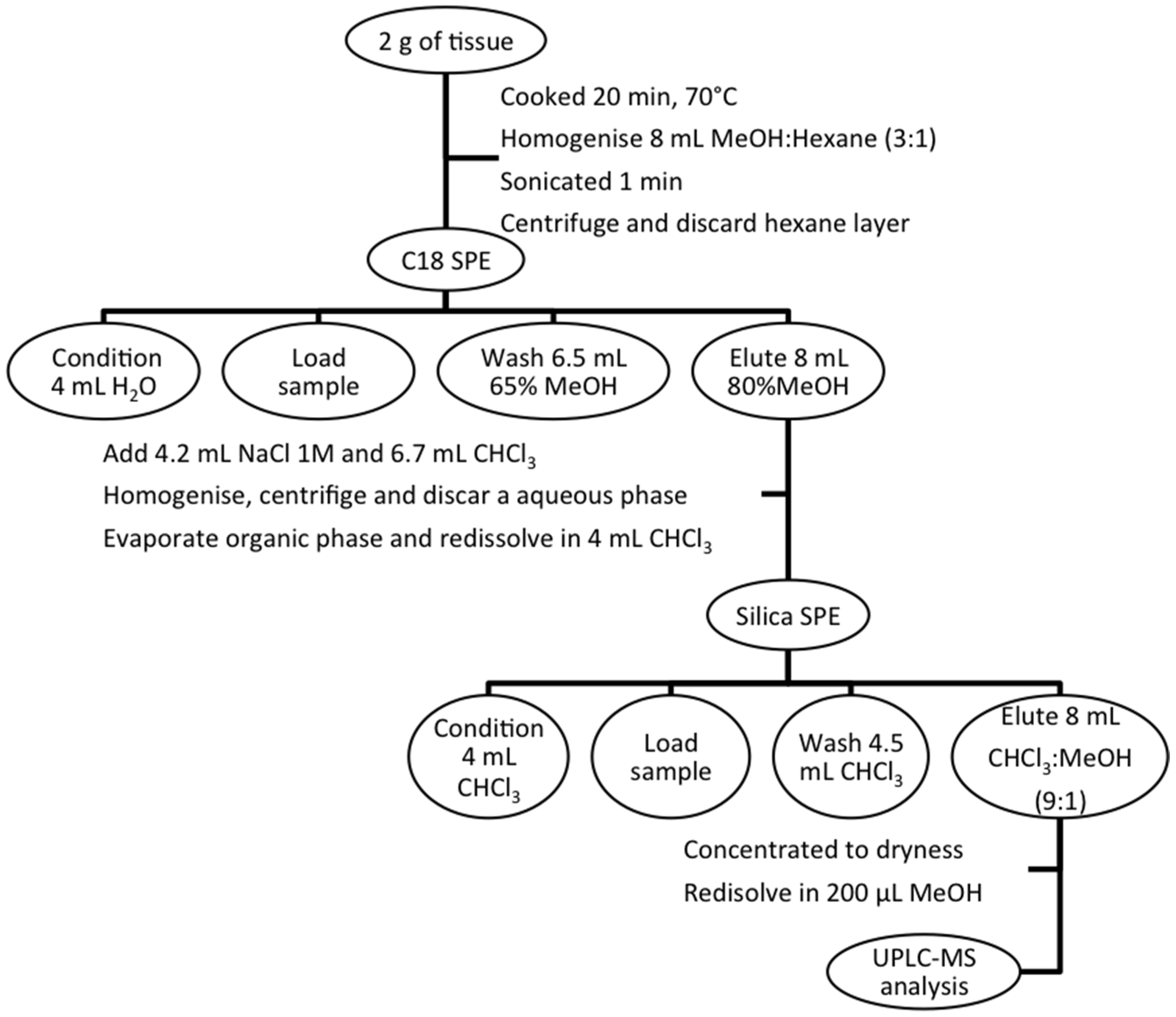
3.4. UPLC-MS Conditions
3.5. UPLC-MS-IT-TOF Conditions
3.6. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.P.; di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. Efsa panel on contaminants in the food chain; scientific opinion on marine biotoxins in shellfish—Emerging toxins: Ciguatoxin group. EFSA J. 2010, 1627, 1–38. [Google Scholar]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Yasumoto, T. Structures of ciguatoxin and its congener. J. Am. Chem. Soc. 1989, 111, 8929–8931. [Google Scholar] [CrossRef]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structures and configurations of ciguatoxin from the Moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990, 112, 4380–4386. [Google Scholar] [CrossRef]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Lewis, R.J.; Sellin, M.; Poli, M.A.; Norton, R.S.; MacLeod, J.K.; Sheil, M.M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef]
- Lewis, R.J.; Vernoux, J.P.; Brereton, I.M. Structure of caribbean ciguatoxin isolated from Caranx latus. J. Am. Chem. Soc. 1998, 120, 5914–5920. [Google Scholar] [CrossRef]
- Pottier, I.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Characterization of multiple caribbean ciguatoxins and congeners in individual specimens of horse-eye jack (Caranx latus) by high performance liquid chromatography/mass spectrometry. Toxicon 2002, 40, 929–939. [Google Scholar] [CrossRef]
- Vernoux, J.P.; Lewis, R.J. Isolation and characterisation of caribbean ciguatoxins from the horse-eye jack (Caranx latus). Toxicon 1997, 35, 889–900. [Google Scholar] [CrossRef]
- Satake, M.; Fukui, M.; Legrand, A.M.; Cruchet, P.; Yasumoto, T. Isolation and structures of new ciguatoxin analogs, 2,3-dihydroxyctx3c and 51-hydroxyctx3c, accumulated in tropical reef fish. Tetrahedron Lett. 1998, 39, 1197–1198. [Google Scholar] [CrossRef]
- Satake, M.; Ishibashi, Y.; Legrand, A.M.; Yasumoto, T. Isolation and structure of ciguatoxin-4a, a new ciguatoxin precursos, from cultures of dinoflagellate Gambierdiscus toxicus and parrotfish Scarus gibbus. Biosci. Biotechnol. Biochem. 1997, 60, 2103–2105. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Murata, M.; Yasumoto, T. Gambierol: A new toxic polyether compound isolated from the marine dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1993, 115, 361–362. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. The structure of CTX3C, a ciguatoxin congener isolated from cultured Gambierdsicus toxicus. Tetrahedron Lett. 1993, 34, 1975–1978. [Google Scholar] [CrossRef]
- Holland, W.C.; Litaker, R.W.; Tomas, C.R.; Kibler, S.R.; Place, A.R.; Davenport, E.D.; Tester, P.A. Differences in the toxicity of six gambierdiscus (dinophyceae) species measured using an in vitro human erythrocyte lysis assay. Toxicon 2013, 65, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Z.; Deckey, R.; Jiao, G.L.; Ren, H.F.; Li, M. Caribbean maitotoxin elevates [Ca(2+)]i and activates non-selective cation channels in HIT-T15 cells. World J. Diabetes 2013, 4, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Pottier, I.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Analysis of toxin profiles in three different fish species causing ciguatera fish poisoning in guadalupe, french west indies. Food Addit. Contam. 2002, 19, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Fleming, L.E.; Fernandez, M.; Bienfang, P.; Schrank, K.; Dickey, R.; Bottein, M.Y.; Backer, L.; Ayyar, R.; Weisman, R.; et al. Ciguatera fish poisoning: Treatment, prevention and management. Mar. Drugs 2008, 6, 456–479. [Google Scholar] [CrossRef] [PubMed]
- De Fouw, J.C.; van Egmond, H.P.; Speijers, G.J.A. Ciguatera Fish Poisoning: A Review. Available online: http://www.rivm.nl/bibliotheek/rapporten/388802021.pdf (accessed on 17 December 2010).
- Bentur, Y.; Spanier, E. Ciguatoxin-like substances in edible fish on the eastern mediterranean. Clin. Toxicol. 2007, 45, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Hurbungs, M.; Jones, A.; Lewis, R.J. Multiple ciguatoxins present in indian ocean reef fish. Toxicon 2002, 40, 1347–1353. [Google Scholar] [CrossRef]
- Fda. Fish and Fishery Products Hazards and Controls Guidance. 2011. Available online: http://www.Fda.Gov/downloads/food/guidancecomplianceregulatoryinformation/guidancedocuments/seafood/ucm251970.Pdf (accessed on 12 March 2013). [Google Scholar]
- Regulation (ec) No 854/2004 of the european parliament and of the council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Oj l 139, 30.4.2004. pp. 206–320, EURO-LEX; Access to European Law. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32007R1246 (accessed on 18 June 2015).
- Cdc (centers for disease control and prevention), 2009. Cluster of ciguatera fish poisoning-north carolina, 2007. Morbidity and mortality weekly report (mmwr). 58, pp. 283–285. Available from http://www.Cdc.Gov/mmwr/pdf/wk/mm5811.Pdf.Centers of Disease Control and Prevention. Available online: http://www.cdc.gov/ (accessed on 18 June 2015).
- Carefoot, T.H. Aplysia: Its biology and ecology. Oceanogr. Mar. Biol. Ann. Rev. 1987, 25, 167–284. [Google Scholar]
- Bulleri, F.; Benedetti-Cecchi, L.; Cinelli, F. Grazing by the sea urchins Arbacia lixula l. And Paracentrotus lividus lam. in the northwest mediterranean. J. Exp. Mar. Biol. Ecol. 1999, 241, 81–85. [Google Scholar] [CrossRef]
- Lin, S.J.; Hwang, D.F. Possible source of tetrodotoxin in the starfish astropecten scoparius. Toxicon 2001, 39, 573–579. [Google Scholar] [CrossRef]
- Nicolaidou, A.; Nott, J.A. The role of the marine gastropod Cerithium vulgatum in the biogeochemical cycling of metals. Biogeochem. Cycl. Sediment Ecol. 1999, 59, 137–146. [Google Scholar]
- Rodríguez, A.; Hernández, J.C.; Clemente, S.; Coppard, S.E. A new species of diadema (echinodermata:Echinoidea:Diadematidae) from the eastern atlantic ocean and a neotype designation of Diadema antillarum (philippi, 1845). Zootaxa 2013, 3636, 144–170. [Google Scholar] [CrossRef]
- Ferguson, J.C. Feeding activity in echinaster and its induction with dissolved nutrients. Biol. Bull. 1969, 136, 374–384. [Google Scholar] [CrossRef]
- Crothers, J.H. Common topshells: An introduction to the biology of Osilinus lineatus with notes on other species in the genus. Field Stud. 2001, 10, 115–160. [Google Scholar]
- Navarro, P.G.; García-Sanz, S.; Barrio, J.M.; Tuya, F. Feeding and movement patterns of the sea cucumber Holothuria sanctori. Mar. Biol. 2013, 160, 2957–2966. [Google Scholar] [CrossRef]
- Toral-Granda, V.; Lovatelli, A.; Vasconcellos, M.; Scientific committee composed of Conand C., Hamel J.F., Mercier A., Purcell S. and Uthicke S. Proceedings of the International Workshop on the Sustainable Use and Management of Sea Cucumber Fisheries, SPC Beche-deMer Information Bulletin, Puerto Ayora, Galapagos Islands, Ecuador, 19–23 November 2008.
- Knox, G.A. Hard shores. In The Ecology of Seashores; Kennish, M.J., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 20–86. [Google Scholar]
- Buschbaum, C.; Dittmann, S.; Hong, J.S.; Hwang, I.; Strasser, M.; Thiel, M.; Valdivia, N.; Yoon, S.; Reise, K. Mytilid mussels: Global habitat engineers in coastal sediments. Helgol. Mar. Res. 2008, 63, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Dayrat, B. Review of the current knowledge of the systematics of onchidiidae (mollusca:Gastropoda:Pulmonata) with a checklist of nominal species. Zootaxa 2009, 2068, 1–26. [Google Scholar]
- Lemée, R.; Boudouresque, C.F.; Gobert, J.; Malestroit, P.; Mari, X.; Meinesz, A.; Menager, V.; Ruitton, S. Feeding behaviour of paracentrotus lividus in the presence of caulerpa taxifolia introduced in the mediterranean sea. Oceanol. Acta 1995, 19, 245–253. [Google Scholar]
- Martinez-Pita, I.; Sanches-Espana, A.; Garcia, F.J. Gonadal growth and reproduction in the sea urchin Sphaerechinus granularis (lamarck 1816) in southern spain. Sci. Mar. 2008, 72, 603–611. [Google Scholar]
- Valdes, A. On the publication data, authorship, and type species of Umbraculum and Tylodina gastropoda; opisthobranchia; tylodinoidea. Nautilus 2001, 115, 29–34. [Google Scholar]
- Ramírez, R.; Tuya, F.; Haroun, R.J. Spatial patterns in the population structure of the whelk Stramonita haemastoma (linnaeus, 1766) (gastropoda: Muricidae) in the canarian archipelago (eastern atlantic). Sci. Mar. 2009, 73, 431–437. [Google Scholar] [CrossRef]
- Llewellyn, L.E. Revisiting the association between sea surface temperature and the epidemiology of fish poisoning in the south pacific: Reassessing the link between ciguatera and climate change. Toxicon 2010, 56, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Germain, M.; Deparis, X.; Pauillac, S.; Legrand, A.M. Seasonal abundance and toxicity of the dinoflagellate Gambierdiscus spp. (dinophyceae), the causative agent of ciguatera in tahiti, french polynesia. Mar. Biol. 1999, 135, 259–267. [Google Scholar] [CrossRef]
- Ashton, M.; Tosteson, T.; Tosteson, C. The effect of elevated temperature on the toxicity of the laboratory cultured dinoflagellate Ostreopsis lenticularis (dinophyceae). J. Trop. Biol. 2003, 51, 1–6. [Google Scholar]
- Matsumoto, T.; Nagashima, Y.; Kusuhara, H.; Sugiyama, Y.; Ishizaki, S.; Shimakura, K.; Shiomi, K. Involvement of carrier-mediated transport system in uptake of tetrodotoxin into liver tissue slices of puffer fish Takifugu rubripes. Toxicon 2007, 50, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Hirama, M.; Oishi, T.; Uehara, H.; Inoue, M.; Maruyama, M.; Oguri, H.; Satake, M. Total synthesis of ciguatoxin CTX3C. Science 2001, 294, 1904–1907. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hirama, M. Evolution of a practical total synthesis of ciguatoxin CTX3C. Account. Chem. Res. 2004, 37, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Lopez, S.P.; Alfonso, A.; Vale, C.; Rodríguez, P.; Gouveia, N.N.; Gouveia, N.; Delgado, J.; Vale, P.; Hirama, M.; et al. First toxin profile of ciguateric fish in madeira arquipelago (europe). Anal. Chem. 2010, 82, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Yang, A.; Jones, A. Rapid extraction combined with lc-tandem mass spectrometry (crem-lc/ms/ms) for the determination of ciguatoxins in ciguateric fish flesh. Toxicon 2009, 54, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science: New York, NY, USA, 2009. [Google Scholar]
- Frees, E.W. Regression Modeling with Actuarial and Financial Applications; Cambridge University Press: New York, NY, USA, 2011. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2014. Available online: http://www.R-project.org/ (accessed on 2 June 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.; Rodriguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. First Report of Ciguatoxins in Two Starfish Species: Ophidiaster ophidianus and Marthasterias glacialis. Toxins 2015, 7, 3740-3757. https://doi.org/10.3390/toxins7093740
Silva M, Rodriguez I, Barreiro A, Kaufmann M, Neto AI, Hassouani M, Sabour B, Alfonso A, Botana LM, Vasconcelos V. First Report of Ciguatoxins in Two Starfish Species: Ophidiaster ophidianus and Marthasterias glacialis. Toxins. 2015; 7(9):3740-3757. https://doi.org/10.3390/toxins7093740
Chicago/Turabian StyleSilva, Marisa, Inés Rodriguez, Aldo Barreiro, Manfred Kaufmann, Ana Isabel Neto, Meryem Hassouani, Brahim Sabour, Amparo Alfonso, Luis M. Botana, and Vitor Vasconcelos. 2015. "First Report of Ciguatoxins in Two Starfish Species: Ophidiaster ophidianus and Marthasterias glacialis" Toxins 7, no. 9: 3740-3757. https://doi.org/10.3390/toxins7093740
APA StyleSilva, M., Rodriguez, I., Barreiro, A., Kaufmann, M., Neto, A. I., Hassouani, M., Sabour, B., Alfonso, A., Botana, L. M., & Vasconcelos, V. (2015). First Report of Ciguatoxins in Two Starfish Species: Ophidiaster ophidianus and Marthasterias glacialis. Toxins, 7(9), 3740-3757. https://doi.org/10.3390/toxins7093740








