Anti-Fibrotic Effect of Natural Toxin Bee Venom on Animal Model of Unilateral Ureteral Obstruction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Histological Examination of the UUO Mice with or without Treatment with Bee Venom

2.2. Bee Venom Suppresses Pro-Inflammatory Cytokines in the Kidneys of UUO
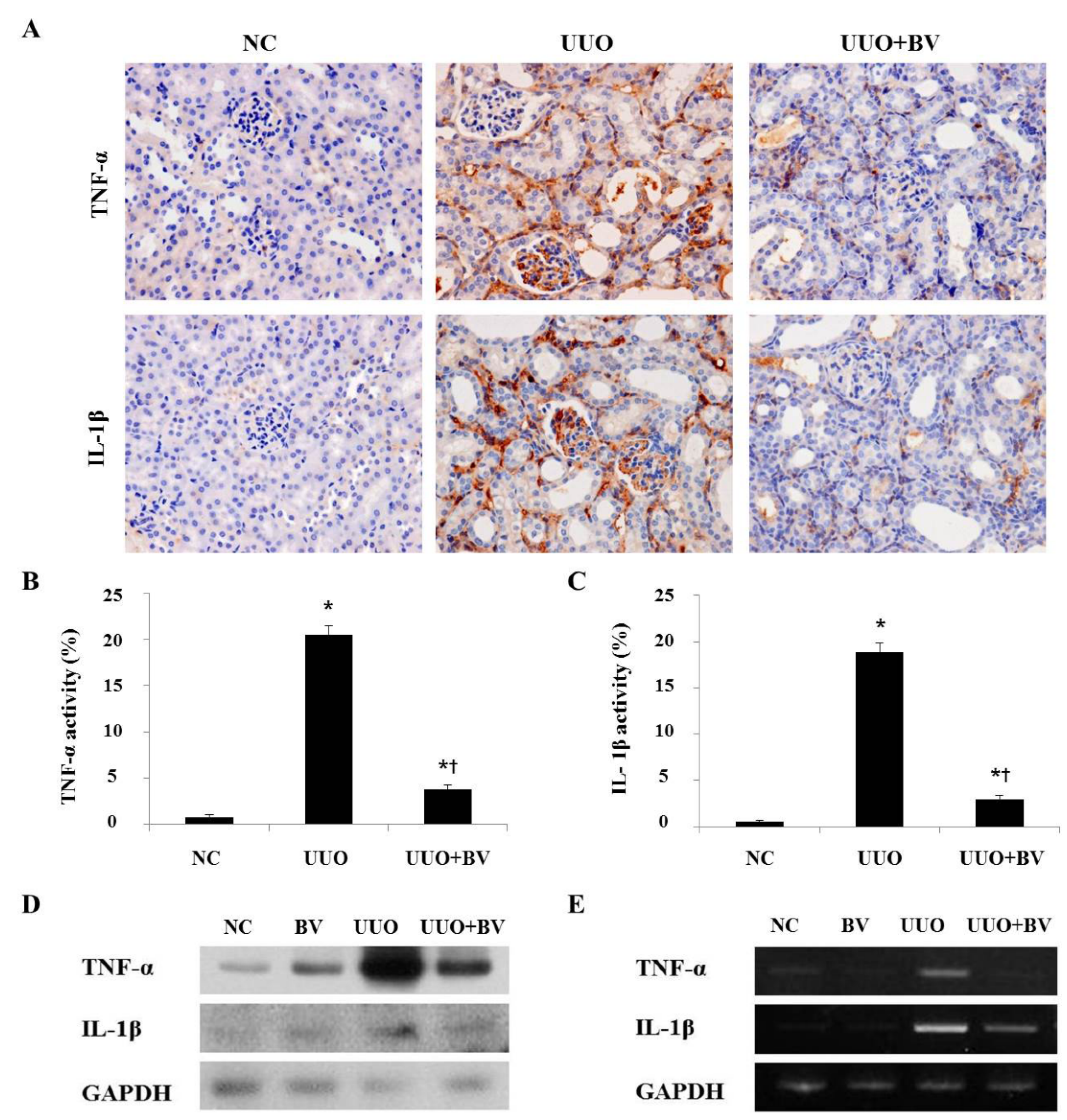
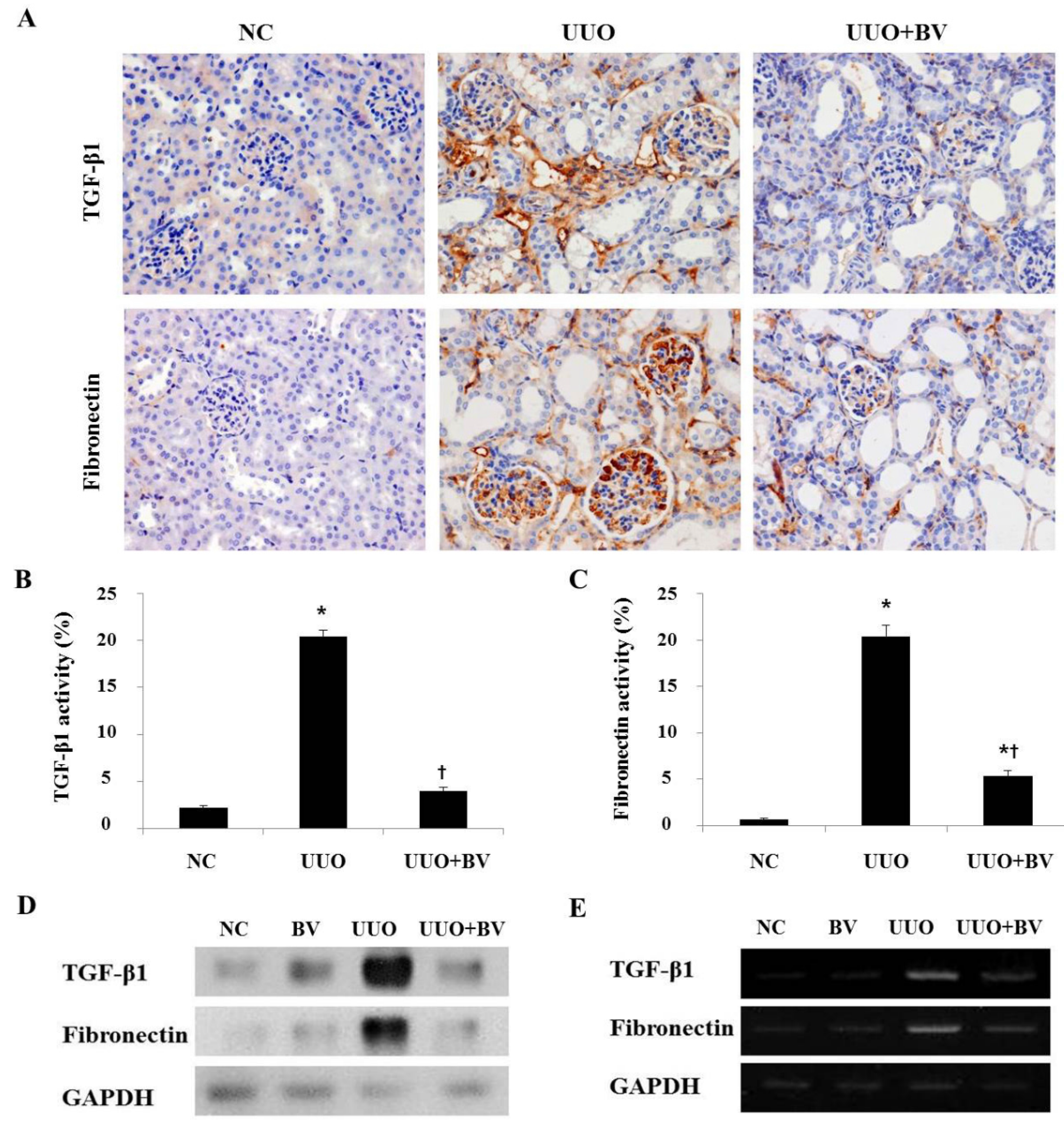
2.3. Bee Venom Inhibits the Fibrotic Gene Expression in an Animal Model of UUO
2.4. UUO-Induced Renal Myofibroblast Activation is Suppressed in Bee Venom Treated Mice
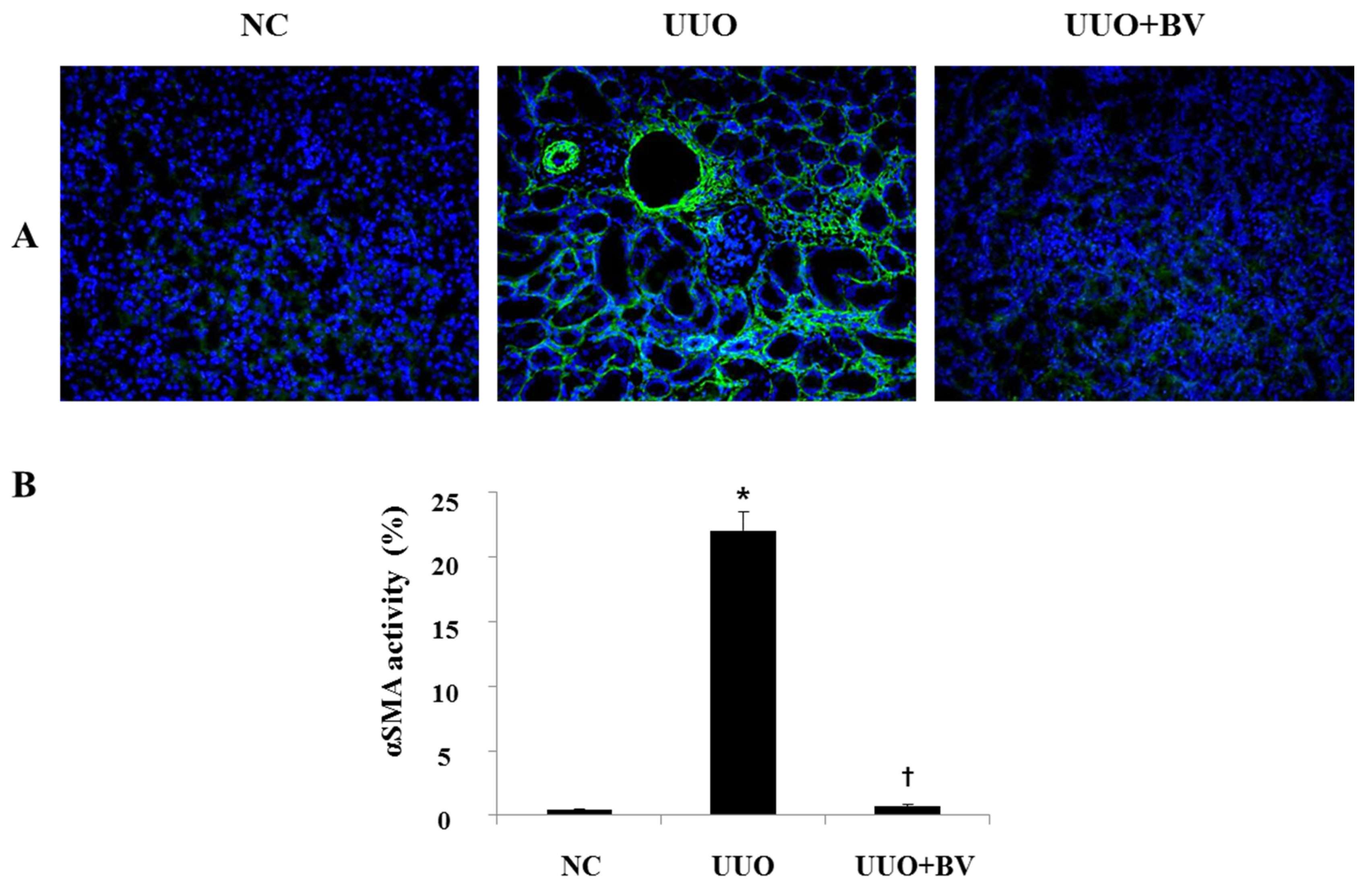
2.5. Discussion
3. Experimental Section
3.1. Collection of Bee Venom
3.2. Animal Model
3.3. Western Blot Analysis
3.4. Histological Analysis
3.5. Immunohistochemical Staining
3.6. Immunofluorescent Staining
3.7. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anders, H.J.; Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011, 80, 915–925. [Google Scholar] [PubMed]
- Harris, R.C.; Neilson, E.G. Toward a unified theory of renal progression. Annu. Rev. Med. 2006, 57, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A. Molecular insights into renal interstitial fibrosis. J. Am. Soc. Nephrol. 1996, 7, 2495–2508. [Google Scholar] [PubMed]
- Liu, N.; Guo, J.K.; Pang, M.; Tolbert, E.; Ponnusamy, M.; Gong, R.; Bayliss, G.; Dworkin, L.D.; Yan, H.; Zhuang, S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Pradere, J.P.; Gonzalez, J.; Klein, J.; Valet, P.; Gres, S.; Salant, D.; Bascands, J.L.; Saulnier-Blache, J.S.; Schanstra, J.P. Lysophosphatidic acid and renal fibrosis. Biochim. Biophys. Acta 2008, 1781, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; He, S.; Tolbert, E.; Gong, R.; Bayliss, G.; Zhuang, S. Suramin alleviates glomerular injury and inflammation in the remnant kidney. PLoS ONE 2012, 7, e36194. [Google Scholar] [CrossRef] [PubMed]
- Boor, P.; Sebekova, K.; Ostendorf, T.; Floege, J. Treatment targets in renal fibrosis. Nephrol. Dial. Transplant. 2007, 22, 3391–3407. [Google Scholar] [CrossRef] [PubMed]
- Billingham, M.E.; Morley, J.; Hanson, J.M.; Shipolini, R.A.; Vernon, C.A. Letter: An anti-inflammatory peptide from bee venom. Nature 1973, 245, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Bascands, J.L.; Schanstra, J.P. Obstructive nephropathy: Insights from genetically engineered animals. Kidney Int. 2005, 68, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Huang, X.R.; Wang, W.; Li, J.H.; Heuchel, R.L.; Chung, A.C.; Lan, H.Y. The protective role of Smad7 in diabetic kidney disease: Mechanism and therapeutic potential. Diabetes 2011, 60, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, J.H.; Kim, K.H.; Lee, W.R.; Chang, Y.C.; Park, K.K.; Lee, K.G.; Han, S.M.; Yeo, J.H.; Pak, S.C. Bee venom inhibits hepatic fibrosis through suppression of pro-fibrogenic cytokine expression. Am. J. Chin. Med. 2010, 38, 921–935. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Lee, W.R.; Kim, K.H.; Kim, J.Y.; Lee, S.J.; Han, S.M.; Lee, K.G.; Lee, C.K.; Park, K.K. Inhibitory effects of bee venom on Propionibacterium acnes-induced inflammatory skin disease in an animal model. Int. J. Mol. Med. 2014, 34, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Misseri, R.; Meldrum, K.K. Mediators of fibrosis and apoptosis in obstructive uropathies. Curr. Urol. Rep. 2005, 6, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Dorf, M.E.; Proudfoot, A.; Salant, D.J.; Gutierrez-Ramos, J.C. Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J. Leukoc. Biol. 1997, 62, 676–680. [Google Scholar] [PubMed]
- Misseri, R.; Rink, R.C.; Meldrum, D.R.; Meldrum, K.K. Inflammatory mediators and growth factors in obstructive renal injury. J. Surg. Res. 2004, 119, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Morrissey, J.; McCracken, R.; Tolley, T.; Liapis, H.; Klahr, S. Contributions of angiotensin II and tumor necrosis factor-alpha to the development of renal fibrosis. Am. J. Physiol. Ren. Physiol. 2001, 280, F777–F785. [Google Scholar]
- Meldrum, K.K.; Misseri, R.; Metcalfe, P.; Dinarello, C.A.; Hile, K.L.; Meldrum, D.R. TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1456–R1464. [Google Scholar] [CrossRef] [PubMed]
- Nikolic-Paterson, D.J.; Main, I.W.; Tesch, G.H.; Lan, H.Y.; Atkins, R.C. Interleukin-1 in renal fibrosis. Kidney Int. Suppl. 1996, 54, S88–S90. [Google Scholar] [PubMed]
- Garcia-Sanchez, O.; Lopez-Hernandez, F.J.; Lopez-Novoa, J.M. An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney Int. 2010, 77, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.S.; Burrows, C.; Shanks, J.H.; Venning, M.; McWilliam, L.J. Interstitial myofibroblasts: Predictors of progression in membranous nephropathy. J. Clin. Pathol. 1997, 50, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Goumenos, D.S.; Brown, C.B.; Shortland, J.; el Nahas, A.M. Myofibroblasts, predictors of progression of mesangial IgA nephropathy? Nephrol. Dial. Transplant. 1994, 9, 1418–1425. [Google Scholar] [PubMed]
- Hewitson, T.D.; Wu, H.L.; Becker, G.J. Interstitial myofibroblasts in experimental renal infection and scarring. Am. J. Nephrol. 1995, 15, 411–417. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, H.J.; Kim, K.H.; Lee, W.R.; Kim, J.Y.; Lee, S.J.; Pak, S.C.; Han, S.M.; Park, K.K. Anti-Fibrotic Effect of Natural Toxin Bee Venom on Animal Model of Unilateral Ureteral Obstruction. Toxins 2015, 7, 1917-1928. https://doi.org/10.3390/toxins7061917
An HJ, Kim KH, Lee WR, Kim JY, Lee SJ, Pak SC, Han SM, Park KK. Anti-Fibrotic Effect of Natural Toxin Bee Venom on Animal Model of Unilateral Ureteral Obstruction. Toxins. 2015; 7(6):1917-1928. https://doi.org/10.3390/toxins7061917
Chicago/Turabian StyleAn, Hyun Jin, Kyung Hyun Kim, Woo Ram Lee, Jung Yeon Kim, Sun Jae Lee, Sok Cheon Pak, Sang Mi Han, and Kwan Kyu Park. 2015. "Anti-Fibrotic Effect of Natural Toxin Bee Venom on Animal Model of Unilateral Ureteral Obstruction" Toxins 7, no. 6: 1917-1928. https://doi.org/10.3390/toxins7061917
APA StyleAn, H. J., Kim, K. H., Lee, W. R., Kim, J. Y., Lee, S. J., Pak, S. C., Han, S. M., & Park, K. K. (2015). Anti-Fibrotic Effect of Natural Toxin Bee Venom on Animal Model of Unilateral Ureteral Obstruction. Toxins, 7(6), 1917-1928. https://doi.org/10.3390/toxins7061917




