Best Clinical Practice in Botulinum Toxin Treatment for Children with Cerebral Palsy
Abstract
:1. Introduction
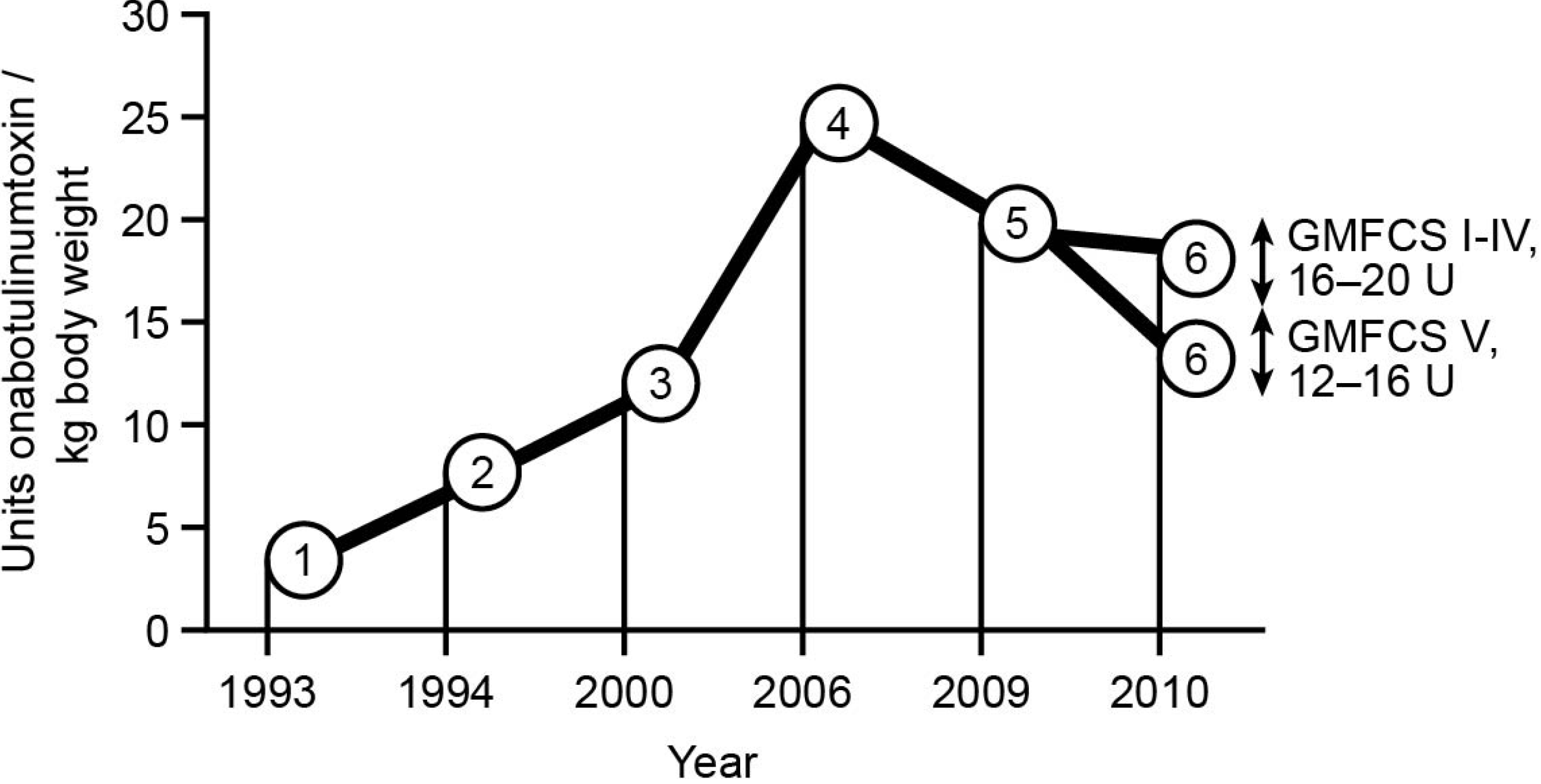
2. Methods
3. Indications for BoNT-A Use
| Localization | Unilateral CP | Bilateral ambulant CP | Bilateral non-ambulant CP |
|---|---|---|---|
| Upper limb | Improved function and aesthetics/appearance | N/A | Pain management |
| Easier caring and positioning | |||
| Functional and/or cosmetic improvement of hand position | |||
| Lower limb | Improved gait | Improved gait | Pain management |
| Easier caring and positioning | |||
| Improvement of weight bearing | |||
| Prevention of hip dislocation | |||
| Spine | N/A | N/A | Postural management |
| Care | |||
| Pain management |
4. What Is an Effective and Successful Treatment?
5. The Importance of Goal Setting, Assessment, and Evaluation
- Improvement of function, including gait.
- Improvement of posture.
- Pain management.
- Facilitation of care.
6. The Importance of an Integrated Approach
- There are limited data on the benefits of combining BoNT-A therapy with orthoses [28], and one study has suggested that orthoses may not be as beneficial as casting when used as part of multimodal treatment involving BoNT-A therapy [29]. Reduced muscle tone may be best treated with stabilising orthoses.
- Orthopaedic surgery has an important role in the treatment of the musculoskeletal deformities and contractures present in the child with CP. The widely accepted principle is the single event multi-level surgery. One of the roles of BoNT-A therapy is to avoid multiple operations in order not to weaken muscles excessively and to protect children from multiple admissions in hospital. The challenge is to time the surgery correctly for the individual child in order to avoid going back. Perioperative BoNT-A injections may help to reduce spasticity-induced post-operative pain and to ease the rehabilitation process. BoNT-A injection may also help to confirm surgical indications: if the patient deteriorates functionally after injecting the target muscles, any planned surgery to these muscles should be approached with caution [36].
- SDR may reduce spasticity in selected individuals. There is a role for BoNT-A therapy in the long-term follow-up of SDR in many children [37].
- In cases of severe generalised spasticity, the combination of oral tone-reducing medications or intrathecal Baclofen treatment and BoNT-A may have a therapeutic effect.
7. Development of Different Treatment Concepts
8. An Integrated Treatment Approach: The Key-Muscle Concept
- AbobotulinumtoxinA: ≤20 units/kg body weight for the first injection and subsequent injections of ≤30 units/kg body weight with a maximum total dose of 1000 units abobotulinumtoxinA, following the European Marketing Authorisation.
- IncobotulinumtoxinA: ≤12 units/kg body weight for the first injection and subsequent injections of ≤15 units/kg body weight with a maximum total dose of 300 units (assuming dose equivalence of 1:1 between onabotulinumtoxinA and incobotulinumtoxinA [63].
8.1. Treatment Goal
8.2. Selection of Key Muscles
8.3. Early Commencement of Treatment
8.4. Long-Term Treatment
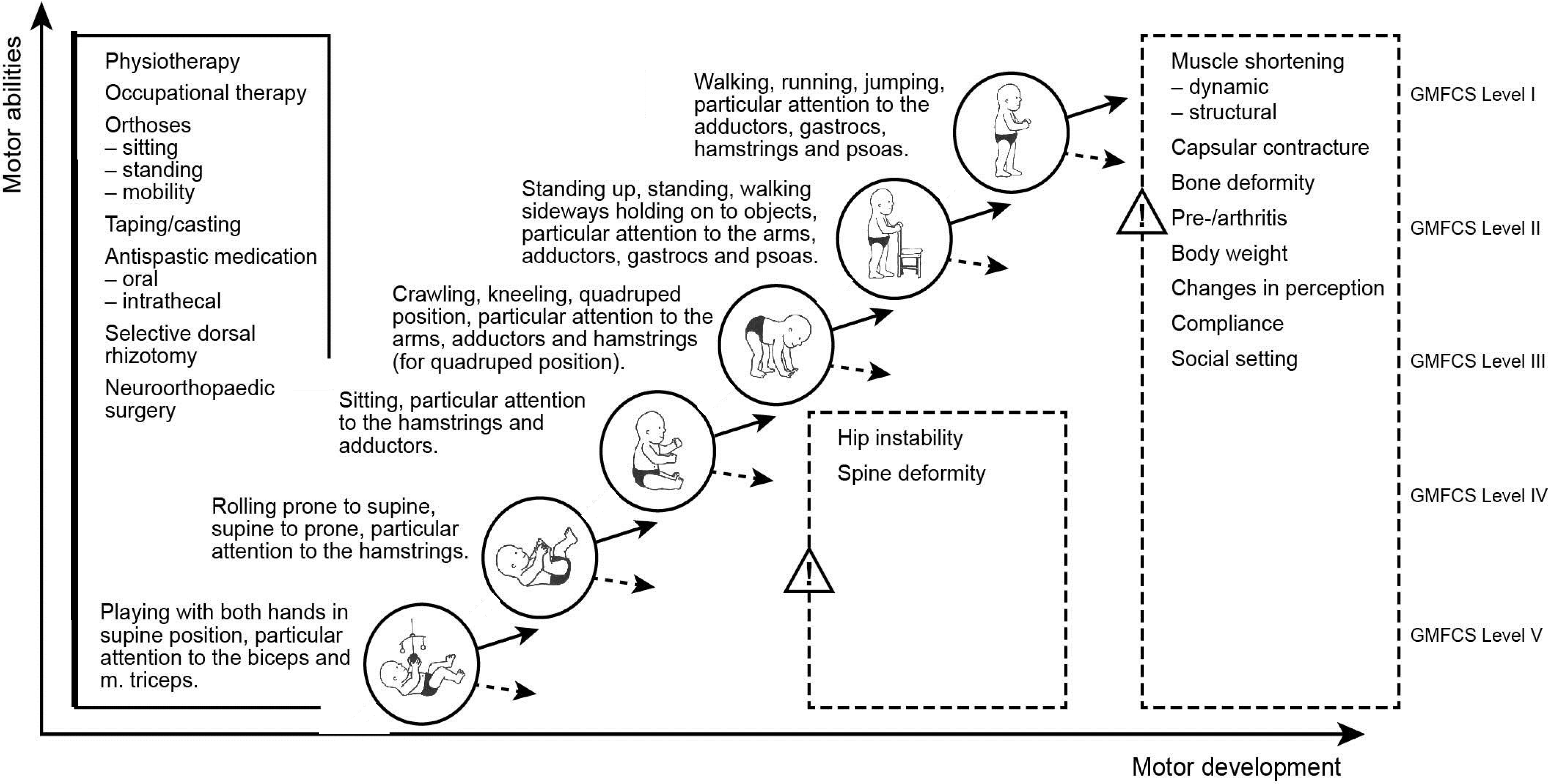
9. Clinical Examination, Assessment, Evaluation and Documentation
- Active and passive range of motion.
- Specified muscle testing e.g., Thomas-test, popliteal angle, Silverskiöld, Duncan-Ely.
- Muscle strength according to Janda, MRC, Oxford-scale.
- Observational gait analysis supported by video documentation [74].
- Activities Scale for Kids (ASK), only applicable to children aged 5–15 years [84].
- Three-dimensional instrumented gait analysis has been invaluable in order to document function before and after BoNT-A injection, which may be used as an objective parameter to assess gait (in cases where gait improvement is the aim). It is also extremely useful in planning surgical management and as an outcome measure in clinical studies [16,85,86].
10. Critical Considerations
11. Application of BoNT
12. Optimising Injection
12.1. Techniques to Guide Injection
12.2. Where to Inject
13. Conclusions
Conflicts of Interest
References
- Koman, L.A.; Smith, B.P.; Shilt, J.S. Cerebral palsy. Lancet 2004, 363, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.N.; Peake, L.J.; Ditchfield, M.R.; Reid, S.M.; Lanigan, A.; Reddihough, D.S. Magnetic resonance imaging findings in a population-based cohort of children with cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bax, M.; Tydeman, C.; Flodmark, O. Clinical and mri correlates of cerebral palsy: The european cerebral palsy study. JAMA: J. Am. Med. Assoc. 2006, 296, 1602–1608. [Google Scholar] [CrossRef]
- Heinen, F.; Desloovere, K.; Schroeder, A.S.; Berweck, S.; Borggraefe, I.; van Campenhout, A.; Andersen, G.L.; Aydin, R.; Becher, J.G.; Bernert, G.; et al. The updated european consensus 2009 on the use of botulinum toxin for children with cerebral palsy. Eur. J. Paediatr. Neurol. EJPN: Off. J. Eur. Paediatr. Neurol. Soc. 2010, 14, 45–66. [Google Scholar] [CrossRef]
- Love, S.C.; Novak, I.; Kentish, M.; Desloovere, K.; Heinen, F.; Molenaers, G.; O'Flaherty, S.; Graham, H.K.; Cerebral Palsy, I. Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: International consensus statement. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2010, 17 (Suppl. 2), 9–37. [Google Scholar]
- Papavasiliou, A.S.; Nikaina, I.; Foska, K.; Bouros, P.; Mitsou, G.; Filiopoulos, C. Safety of botulinum toxin a in children and adolescents with cerebral palsy in a pragmatic setting. Toxins 2013, 5, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Koman, L.A.; Mooney, J.F., 3rd; Smith, B.; Goodman, A.; Mulvaney, T. Management of cerebral palsy with botulinum-a toxin: Preliminary investigation. J. Pediatr. Orthop. 1993, 13, 489–495. [Google Scholar] [CrossRef]
- Cosgrove, A.P.; Corry, I.S.; Graham, H.K. Botulinum toxin in the management of the lower limb in cerebral palsy. Dev. Med. Child Neurol. 1994, 36, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.K.; Aoki, K.R.; Autti-Ramo, I.; Boyd, R.N.; Delgado, M.R.; Gaebler-Spira, D.J.; Gormley, M.E.; Guyer, B.M.; Heinen, F.; Holton, A.F.; et al. Recommendations for the use of botulinum toxin type a in the management of cerebral palsy. Gait Posture 2000, 11, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Molenaers, G.; Fairhurst, C.; Carr, L.J.; Desloovere, K.; Chaleat Valayer, E.; Morel, E.; Papavassiliou, A.S.; Tedroff, K.; Ignacio Pascual-Pascual, S.; et al. European consensus table 2006 on botulinum toxin for children with cerebral palsy. Eur. J. Paediatr. Neurol. EJPN: Off. J. Eur. Paediatr. Neurol. Soc. 2006, 10, 215–225. [Google Scholar] [CrossRef]
- Ward, A.B. Spasticity treatment with botulinum toxins. J. Neural Transm. 2008, 115, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Arner, M.; Himmelmann, K.; Ponten, E.; Stankovic, N.; Hansson, T.; Dahlin, L.B. Upper extremity botulinum toxin treatment in cerebral palsy. Treatment guidelines the first step towards national cooperation. Lakartidningen 2008, 105, 3009–3013. [Google Scholar] [PubMed]
- Garreta-Figuera, R.; Chaler-Vilaseca, J.; Torrequebrada-Gimenez, A. Clinical practice guidelines for the treatment of spasticity with botulinum toxin. Revista Neurol. 2010, 50, 685–699. [Google Scholar]
- Pascual-Pascual, S.I.; Herrera-Galante, A.; Poo, P.; Garcia-Aymerich, V.; Aguilar-Barbera, M.; Bori-Fortuny, I.; Garcia-Ruiz, P.J.; Garreta-Figuera, R.; Lanzas-Melendo, G.; de Miguel-Leon, I.; et al. Guidelines for the treatment of child spasticity using botulinum toxin. Revista Neurol. 2007, 44, 303–309. [Google Scholar]
- DGN Leitlinie “spastik”. Available online: http://www.dgn.org/leitl.shtml (accessed on 30 September 2012).
- Whelan, M.A.; Delgado, M.R. Practice parameter: Pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): Report of the quality standards subcommittee of the american academy of neurology and the practice committee of the child neurology society. Neurology 2010, 75, 669. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, G.; Andersson, S.; Duppe, H.; Lauge-Pedersen, H.; Nordmark, E.; Westbom, L. Prevention of severe contractures might replace multilevel surgery in cerebral palsy: Results of a population-based health care programme and new techniques to reduce spasticity. J. Pediatr. Orthop. Part B 2005, 14, 269–273. [Google Scholar] [CrossRef]
- Fitoussi, F.; Diop, A.; Maurel, N.; Laasel el, M.; Ilharreborde, B.; Pennecot, G.F. Upper limb motion analysis in children with hemiplegic cerebral palsy: Proximal kinematic changes after distal botulinum toxin or surgical treatments. J. Child. Orthop. 2011, 5, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Molenaers, G.; Desloovere, K.; De Cat, J.; Jonkers, I.; De Borre, L.; Pauwels, P.; Nijs, J.; Fabry, G.; De Cock, P. Single event multilevel botulinum toxin type a treatment and surgery: Similarities and differences. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2001, 8 (Suppl. 5), 88–97. [Google Scholar]
- Turner-Stokes, L. Goal attainment scaling (gas) in rehabilitation: A practical guide. Clin. Rehabil. 2009, 23, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Jasinski, M.; Maciag-Tymecka, I.; Michalowska-Mrozek, J.; Bonikowski, M.; Carr, L.; MacLean, J.; Lin, J.P.; Lynch, B.; Theologis, T.; et al. Botulinum toxin treatment of spasticity in diplegic cerebral palsy: A randomized, double-blind, placebo-controlled, dose-ranging study. Dev. Med. Child Neurol. 2002, 44, 666–675. [Google Scholar] [CrossRef]
- Kanovsky, P.; Bares, M.; Severa, S.; Richardson, A.; Dysport Paediatric Limb Spasticity Study Group. Long-term efficacy and tolerability of 4-monthly versus yearly botulinum toxin type a treatment for lower-limb spasticity in children with cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.; Fatone, S.; Rodda, J.; Olesch, C.; Starr, R.; Cullis, E.; Gallagher, D.; Carlin, J.B.; Nattrass, G.R.; Graham, K. High- or low-technology measurements of energy expenditure in clinical gait analysis? Dev. Med. Child Neurol. 1999, 41, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Desloovere, K.; Molenaers, G.; Jonkers, I.; De Cat, J.; De Borre, L.; Nijs, J.; Eyssen, M.; Pauwels, P.; de Cock, P. A randomized study of combined botulinum toxin type a and casting in the ambulant child with cerebral palsy using objective outcome measures. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2001, 8 (Suppl. 5), 75–87. [Google Scholar]
- Heinen, F.; Schroeder, A.S.; Fietzek, U.; Berweck, S. When it comes to botulinum toxin, children and adults are not the same: Multimuscle option for children with cerebral palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 2029–2030. [Google Scholar] [CrossRef]
- Dumas, H.M.; O'Neil, M.E.; Fragala, M.A. Expert consensus on physical therapist intervention after botulinum toxin a injection for children with cerebral palsy. Pediatr. Phys. Ther. Off. Publ. Sect. Pediatr. Am. Phys. Ther. Assoc. 2001, 13, 122–132. [Google Scholar]
- Mayston, M. Evidence-based physical therapy for the management of children with cerebral palsy. Dev. Med. Child Neurol. 2005, 47, 795. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, E.M.; Ferreira, G.B.; Maia Moreira, R.C.; Kirkwood, R.N.; Fetters, L. Efficacy of ankle-foot orthoses on gait of children with cerebral palsy: Systematic review of literature. Pediatr. Phys. Ther. Off. Publ. Sect. Pediatr. Am. Phys. Ther. Assoc. 2008, 20, 207–223. [Google Scholar]
- Hoare, B.J.; Wallen, M.A.; Imms, C.; Villanueva, E.; Rawicki, H.B.; Carey, L. Botulinum toxin a as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy (update). Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Bottos, M.; Benedetti, M.G.; Salucci, P.; Gasparroni, V.; Giannini, S. Botulinum toxin with and without casting in ambulant children with spastic diplegia: A clinical and functional assessment. Dev. Med. Child Neurol. 2003, 45, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Ackman, J.D.; Russman, B.S.; Thomas, S.S.; Buckon, C.E.; Sussman, M.D.; Masso, P.; Sanders, J.; D’Astous, J.; Aiona, M.D.; Shriners Hospitals BTX-A Study Group. Comparing botulinum toxin a with casting for treatment of dynamic equinus in children with cerebral palsy. Dev. Med. Child Neurol. 2005, 47, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Glanzman, A.M.; Kim, H.; Swaminathan, K.; Beck, T. Efficacy of botulinum toxin a, serial casting, and combined treatment for spastic equinus: A retrospective analysis. Dev. Med. Child Neurol. 2004, 46, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.M.; Rethlefsen, S.A.; Fern-Buneo, A.; Wren, T.A.; Skaggs, D.L. Botulinum toxin as an adjunct to serial casting treatment in children with cerebral palsy. J. Bone Joint Surg. Am. Volume 2004, 86-A, 2377–2384. [Google Scholar]
- Hayek, S.; Gershon, A.; Wientroub, S.; Yizhar, Z. The effect of injections of botulinum toxin type a combined with casting on the equinus gait of children with cerebral palsy. J. Bone Joint Surg. Br. Volume 2010, 92, 1152–1159. [Google Scholar] [CrossRef]
- Park, E.S.; Rha, D.W.; Yoo, J.K.; Kim, S.M.; Chang, W.H.; Song, S.H. Short-term effects of combined serial casting and botulinum toxin injection for spastic equinus in ambulatory children with cerebral palsy. Yonsei Med. J. 2010, 51, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Rutz, E.; Hofmann, E.; Brunner, R. Preoperative botulinum toxin test injections before muscle lengthening in cerebral palsy. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2010, 15, 647–653. [Google Scholar]
- van Schie, P.E.; Schothorst, M.; Dallmeijer, A.J.; Vermeulen, R.J.; van Ouwerkerk, W.J.; Strijers, R.L.; Becher, J.G. Short- and long-term effects of selective dorsal rhizotomy on gross motor function in ambulatory children with spastic diplegia. J. Neurosurg. Pediatr. 2011, 7, 557–562. [Google Scholar] [PubMed]
- Scott, A.B. Botulinum toxin injection of eye muscles to correct strabismus. Trans. Am. Ophthalmol. Soc. 1981, 79, 734–770. [Google Scholar] [PubMed]
- Scott, A.B.; Rosenbaum, A.; Collins, C.C. Pharmacologic weakening of extraocular muscles. Investig. Ophthalmol. 1973, 12, 924–927. [Google Scholar]
- Ward, A.B.; Molenaers, G.; Colosimo, C.; Berardelli, A. Clinical value of botulinum toxin in neurological indications. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2006, 13 (Suppl. 4), 20–26. [Google Scholar]
- Fehlings, D.; Rang, M.; Glazier, J.; Steele, C. Botulinum toxin type a injections in the spastic upper extremity of children with hemiplegia: Child characteristics that predict a positive outcome. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2001, 8 (Suppl. 5), 145–149. [Google Scholar]
- Goldberg, M.J. Botulinum toxin type a improved ankle function in children with cerebral palsy and dynamic equinus foot deformity. J. Bone Joint Surg. Am. Volume 2000, 82, 874. [Google Scholar]
- Love, S.C.; Valentine, J.P.; Blair, E.M.; Price, C.J.; Cole, J.H.; Chauvel, P.J. The effect of botulinum toxin type a on the functional ability of the child with spastic hemiplegia a randomized controlled trial. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2001, 8 (Suppl. 5), 50–58. [Google Scholar]
- Metaxiotis, D.; Siebel, A.; Doederlein, L. Repeated botulinum toxin a injections in the treatment of spastic equinus foot. Clin. Orthop. Relat. Res. 2002, 394, 177–185. [Google Scholar] [CrossRef]
- Ubhi, T.; Bhakta, B.B.; Ives, H.L.; Allgar, V.; Roussounis, S.H. Randomised double blind placebo controlled trial of the effect of botulinum toxin on walking in cerebral palsy. Arch. Dis. Child. 2000, 83, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Heinen, F.; Schenkel, A.; Doll, B.; Ebersbach, G.; Muller, J.; Poewe, W. Botulinum toxin a in the management of spastic gait disorders in children and young adults with cerebral palsy: A randomized, double-blind study of “high-dose” versus “low-dose” treatment. Neuropediatrics 1999, 30, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Fazzi, E.; Maraucci, I.; Torrielli, S.; Motta, F.; Lanzi, G. Factors predicting the efficacy of botulinum toxin-a treatment of the lower limb in children with cerebral palsy. J. Child Neurol. 2005, 20, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Gough, M.; Fairhurst, C.; Shortland, A.P. Botulinum toxin and cerebral palsy: Time for reflection? Dev. Med. Child Neurol. 2005, 47, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, G.; Andersson, S.; Duppe, H.; Lauge-Pedersen, H.; Nordmark, E.; Westbom, L. Prevention of dislocation of the hip in children with cerebral palsy. The first ten years of a population-based prevention programme. J. Bone Joint Surg. Br. Volume 2005, 87, 95–101. [Google Scholar]
- Molenaers, G.; Schorkhuber, V.; Fagard, K.; Van Campenhout, A.; de Cat, J.; Pauwels, P.; Ortibus, E.; De Cock, P.; Desloovere, K. Long-term use of botulinum toxin type a in children with cerebral palsy: Treatment consistency. Eur. J. Paediatr. Neurol. EJPN: Off. J. Eur. Paediatr. Neurol. Soc. 2009, 13, 421–429. [Google Scholar] [CrossRef]
- Naumann, M.; Albanese, A.; Heinen, F.; Molenaers, G.; Relja, M. Safety and efficacy of botulinum toxin type a following long-term use. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2006, 13 (Suppl. 4), 35–40. [Google Scholar]
- Willis, A.W.; Crowner, B.; Brunstrom, J.E.; Kissel, A.; Racette, B.A. High dose botulinum toxin a for the treatment of lower extremity hypertonicity in children with cerebral palsy. Dev. Med. Child Neurol. 2007, 49, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Crowner, B.E.; Brunstrom, J.E.; Racette, B.A. Iatrogenic botulism due to therapeutic botulinum toxin a injection in a pediatric patient. Clin. Neuropharmacol. 2007, 30, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.; Selber, P.; Graham, H.K.; Reddihough, D. Botulinum neurotoxin a: An unusual systemic effect. J. Paediatr. Child Health 2007, 43, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.; Smith, K.; Sheedy, M.; Adair, B.; Yu, X.; Graham, H.K. Systemic adverse events following botulinum toxin a therapy in children with cerebral palsy. Dev. Med. Child Neurol. 2010, 52, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, A.M. The use of botulinum toxin for the treatment of muscle spasticity in the first 2 years of life. Int. J. Rehabil. Res. Int. Zeitschrift fur Rehabilitationsforschung. Revue Int. Rech. Readapt. 2010, 33, 104–108. [Google Scholar]
- Graham, K. Safety of botulinum toxin a in cerebral palsy. Toxicon: Off. J. Int. Soc. Toxinol. 2008, 51, 1–54. [Google Scholar]
- Palisano, R.J.; Hanna, S.E.; Rosenbaum, P.L.; Russell, D.J.; Walter, S.D.; Wood, E.P.; Raina, P.S.; Galuppi, B.E. Validation of a model of gross motor function for children with cerebral palsy. Phys. Ther. 2000, 80, 974–985. [Google Scholar] [PubMed]
- Ryll, U.; Bastiaenen, C.; De Bie, R.; Staal, B. Effects of leg muscle botulinum toxin a injections on walking in children with spasticity-related cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2011, 53, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Placzek, R. Botulinum toxin a in children with infantile cerebral palsy: Indications and treatment concepts. Der. Orthop. 2010, 39, 23–30. [Google Scholar] [CrossRef]
- Placzek, R.; Siebold, D.; Funk, J.F. Development of treatment concepts for the use of botulinum toxin a in children with cerebral palsy. Toxins 2010, 2, 2258–2271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, B. A dose-response relationship research on botulinum toxin type a local intramuscular injections of lower extremity spasticity in children with cerebral palsy. Child’s Nervous Syst. ChNS: Off. J. Int. Soc. Pediatr. Neurosurg. 2008, 24, 545–547. [Google Scholar] [CrossRef]
- Wohlfarth, K.; Muller, C.; Sassin, I.; Comes, G.; Grafe, S. Neurophysiological double-blind trial of a botulinum neurotoxin type a free of complexing proteins. Clin. Neuropharmacol. 2007, 30, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.; Cotton, E. The Petö System and Its Evolution in Britain; Acorn Foundation: London, UK, 1994. [Google Scholar]
- Russell, D.J.; Avery, L.M.; Rosenbaum, P.L.; Raina, P.S.; Walter, S.D.; Palisano, R.J. Improved scaling of the gross motor function measure for children with cerebral palsy: Evidence of reliability and validity. Phys. Ther. 2000, 80, 873–885. [Google Scholar] [PubMed]
- Wijnhoven, T.M.; de Onis, M.; Onyango, A.W.; Wang, T.; Bjoerneboe, G.E.; Bhandari, N.; Lartey, A.; Rashidi, B. Assessment of gross motor development in the who multicentre growth reference study. Food Nutr. Bull. 2004, 25, S37–S45. [Google Scholar] [PubMed]
- Kargo, W.J.; Nitz, D.A. Early skill learning is expressed through selection and tuning of cortically represented muscle synergies. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 11255–11269. [Google Scholar]
- Hikosaka, O.; Nakamura, K.; Sakai, K.; Nakahara, H. Central mechanisms of motor skill learning. Curr.Opin. Neurobiol. 2002, 12, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.A.; Armand, J.; Kirkwood, P.A.; Yang, H.W.; Davis, J.N.; Lemon, R.N. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: An anatomical and electrophysiological study. Cereb. Cortex 2002, 12, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Pidcock, F.S.; Fish, D.E.; Johnson-Greene, D.; Borras, I.; McGready, J.; Silberstein, C.E. Hip migration percentage in children with cerebral palsy treated with botulinum toxin type a. Arch. Phys. Med. Rehabil. 2005, 86, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Druschel, C.; Althuizes, H.C.; Funk, J.F.; Placzek, R. Off label use of botulinum toxin in children under two years of age: A systematic review. Toxins 2013, 5, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Pascual, S.I.; Pascual-Castroviejo, I. Safety of botulinum toxin type a in children younger than 2 years. Eur. J. Paediatr. Neurol. EJPN: Off. J. Eur. Paediatr. Neurol. Soc. 2009, 13, 511–515. [Google Scholar] [CrossRef]
- Siebold, D.; Rickensdorf, S. Neurologische rehabilitation von kindern mit hirnschädigung im ersten und zweiten lebensjahr—Berliner modell. Praxis Ergotherapie 2009. Praxis der Kinder-Reha 4–10.
- Kerr Graham, H.; Selber, P. Musculoskeletal aspects of cerebral palsy. J. Bone Joint Surg. Br. Volume 2003, 85, 157–166. [Google Scholar] [CrossRef]
- Steenbeek, D.; Ketelaar, M.; Galama, K.; Gorter, J.W. Goal attainment scaling in paediatric rehabilitation: A critical review of the literature. Dev. Med. Child Neurol. 2007, 49, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Steenbeek, D.; Meester-Delver, A.; Becher, J.G.; Lankhorst, G.J. The effect of botulinum toxin type a treatment of the lower extremity on the level of functional abilities in children with cerebral palsy: Evaluation with goal attainment scaling. Clin. Rehabil. 2005, 19, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [PubMed]
- Scholtes, V.A.; Becher, J.G.; Beelen, A.; Lankhorst, G.J. Clinical assessment of spasticity in children with cerebral palsy: A critical review of available instruments. Dev. Med. Child Neurol. 2006, 48, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Alhusaini, A.A.; Dean, C.M.; Crosbie, J.; Shepherd, R.B.; Lewis, J. Evaluation of spasticity in children with cerebral palsy using ashworth and tardieu scales compared with laboratory measures. J. Child Neurol. 2010, 25, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; Burke, K.; Clegg, N.J.; Browne, R.; Rushing, C.; Fehlings, D.; Matthews, D.; Tilton, A.; Delgado, M.R. Reliability of the tardieu scale for assessing spasticity in children with cerebral palsy. Arch. Phys. Med. Rehabil. 2010, 91, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Bourke-Taylor, H. Melbourne assessment of unilateral upper limb function: Construct validity and correlation with the pediatric evaluation of disability inventory. Dev. Med. Child Neurol. 2003, 45, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.M.; Randall, M.J.; Reddihough, D.S.; Oke, L.E.; Byrt, T.A.; Bach, T.M. Development of a clinical assessment of quality of movement for unilateral upper-limb function. Dev. Med. Child Neurol. 1994, 36, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Krumlinde-Sundholm, L.; Holmefur, M.; Kottorp, A.; Eliasson, A.C. The assisting hand assessment: Current evidence of validity, reliability, and responsiveness to change. Dev. Med. Child Neurol. 2007, 49, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Plint, A.C.; Gaboury, I.; Owen, J.; Young, N.L. Activities scale for kids: An analysis of normals. J. Pediatr. Orthop. 2003, 23, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Cimolin, V.; Galli, M.; Piccinini, L.; Berti, M.; Crivellini, M.; Turconi, A.C. Quantitative analysis of gait pattern and energy consumption in children with cerebral palsy. J. Appl. Biomater. Biomechan. JABB 2007, 5, 28–33. [Google Scholar]
- Scholtes, V.A.; Dallmeijer, A.J.; Knol, D.L.; Speth, L.A.; Maathuis, C.G.; Jongerius, P.H.; Becher, J.G. The combined effect of lower-limb multilevel botulinum toxin type a and comprehensive rehabilitation on mobility in children with cerebral palsy: A randomized clinical trial. Arch. Phys. Med. Rehabil. 2006, 87, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Viehweger, E.; Robitail, S.; Rohon, M.A.; Jacquemier, M.; Jouve, J.L.; Bollini, G.; Simeoni, M.C. Measuring quality of life in cerebral palsy children. Ann. Readapt. Med. Phys.: Revue Sci. Soc. Francaise Reeduc. Fonct. Readapt. Med. Phys. 2008, 51, 119–137. [Google Scholar]
- Vinson, J.; Shank, L.; Thomas, P.D.; Warschausky, S. Self-generated domains of quality of life in children with and without cerebral palsy. J. Dev. Phys. Disabil. 2010, 22, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Ramstad, K.; Jahnsen, R.; Skjeldal, O.H.; Diseth, T.H. Mental health, health related quality of life and recurrent musculoskeletal pain in children with cerebral palsy 8‒18 years old. Disabil. Rehabil. 2012, 34, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, K.; Romkes, J.; Brunner, R. The association between premature plantarflexor muscle activity, muscle strength, and equinus gait in patients with various pathologies. Res. Dev. Disabil. 2013, 34, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, K.; Romkes, J.; Coslovsky, M.; Brunner, R. The influence of muscle strength on the gait profile score (gps) across different patients. Gait Posture 2014, 39, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kranz, G.; Haubenberger, D.; Voller, B.; Posch, M.; Schnider, P.; Auff, E.; Sycha, T. Respective potencies of botox and dysport in a human skin model: A randomized, double-blind study. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 231–236. [Google Scholar] [CrossRef]
- Marchetti, A.; Magar, R.; Findley, L.; Larsen, J.P.; Pirtosek, Z.; Ruzicka, E.; Jech, R.; Slawek, J.; Ahmed, F. Retrospective evaluation of the dose of dysport and botox in the management of cervical dystonia and blepharospasm: The real dose study. Mov. Disord. Off. J. Mov. Disord. Soc. 2005, 20, 937–944. [Google Scholar] [CrossRef]
- Wohlfarth, K.; Sycha, T.; Ranoux, D.; Naver, H.; Caird, D. Dose equivalence of two commercial preparations of botulinum neurotoxin type a: Time for a reassessment? Curr. Med. Res. Opin. 2009, 25, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Shannon, K.M.; Bennett, J.P., Jr.; Friedman, J.H. Efficacy of pramipexole, a novel dopamine agonist, as monotherapy in mild to moderate parkinson’s disease. The pramipexole study group. Neurology 1997, 49, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, R.; Vaz, M.A.; Youssef, A.R.; Longino, D.; Herzog, W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (botox). J. Biomechan. 2011, 44, 39–44. [Google Scholar] [CrossRef]
- Park, C.; Park, K.; Kim, J. Growth effects of botulinum toxin type a injected unilaterally into the masseter muscle of developing rats. J. Zhejiang Univ. Sci. B 2015, 16, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Koman, L.A.; Goodman, A.; Smith, B.P. Botulinum Toxin Type a in the Management of Cerebral Palsy; Wake Forest University Press: Winston-Salem, NC, USA, 2002. [Google Scholar]
- Chin, T.Y.; Nattrass, G.R.; Selber, P.; Graham, H.K. Accuracy of intramuscular injection of botulinum toxin a in juvenile cerebral palsy: A comparison between manual needle placement and placement guided by electrical stimulation. J. Pediatr. Orthop. 2005, 25, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Molloy, F.M.; Shill, H.A.; Kaelin-Lang, A.; Karp, B.I. Accuracy of muscle localization without emg: Implications for treatment of limb dystonia. Neurology 2002, 58, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, B.; Seller, K.; Wild, A.; Jaeger, M.; Krauspe, R. Ultrasound-guided botulinum toxin injection technique for the iliopsoas muscle. Dev. Med. Child Neurol. 2003, 45, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, M.J.; Shilt, J.S.; Smith, B.P.; Estrada, R.L.; Castle, J.A.; Koman, L.A. Technique for iliopsoas ultrasound-guided active electromyography-directed botulinum a toxin injection in cerebral palsy. J. Pediatr. Orthop. 2002, 22, 165–168. [Google Scholar] [PubMed]
- Py, A.G.; Zein Addeen, G.; Perrier, Y.; Carlier, R.Y.; Picard, A. Evaluation of the effectiveness of botulinum toxin injections in the lower limb muscles of children with cerebral palsy. Preliminary prospective study of the advantages of ultrasound guidance. Ann. Phys. Rehabil. Med. 2009, 52, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Childers, M.K.; Kornegay, J.N.; Aoki, R.; Otaviani, L.; Bogan, D.J.; Petroski, G. Evaluating motor end-plate-targeted injections of botulinum toxin type a in a canine model. Muscle Nerve 1998, 21, 653–655. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strobl, W.; Theologis, T.; Brunner, R.; Kocer, S.; Viehweger, E.; Pascual-Pascual, I.; Placzek, R. Best Clinical Practice in Botulinum Toxin Treatment for Children with Cerebral Palsy. Toxins 2015, 7, 1629-1648. https://doi.org/10.3390/toxins7051629
Strobl W, Theologis T, Brunner R, Kocer S, Viehweger E, Pascual-Pascual I, Placzek R. Best Clinical Practice in Botulinum Toxin Treatment for Children with Cerebral Palsy. Toxins. 2015; 7(5):1629-1648. https://doi.org/10.3390/toxins7051629
Chicago/Turabian StyleStrobl, Walter, Tim Theologis, Reinald Brunner, Serdar Kocer, Elke Viehweger, Ignacio Pascual-Pascual, and Richard Placzek. 2015. "Best Clinical Practice in Botulinum Toxin Treatment for Children with Cerebral Palsy" Toxins 7, no. 5: 1629-1648. https://doi.org/10.3390/toxins7051629





