Membrane-Pore Forming Characteristics of the Bordetella pertussis CyaA-Hemolysin Domain
Abstract
:1. Introduction
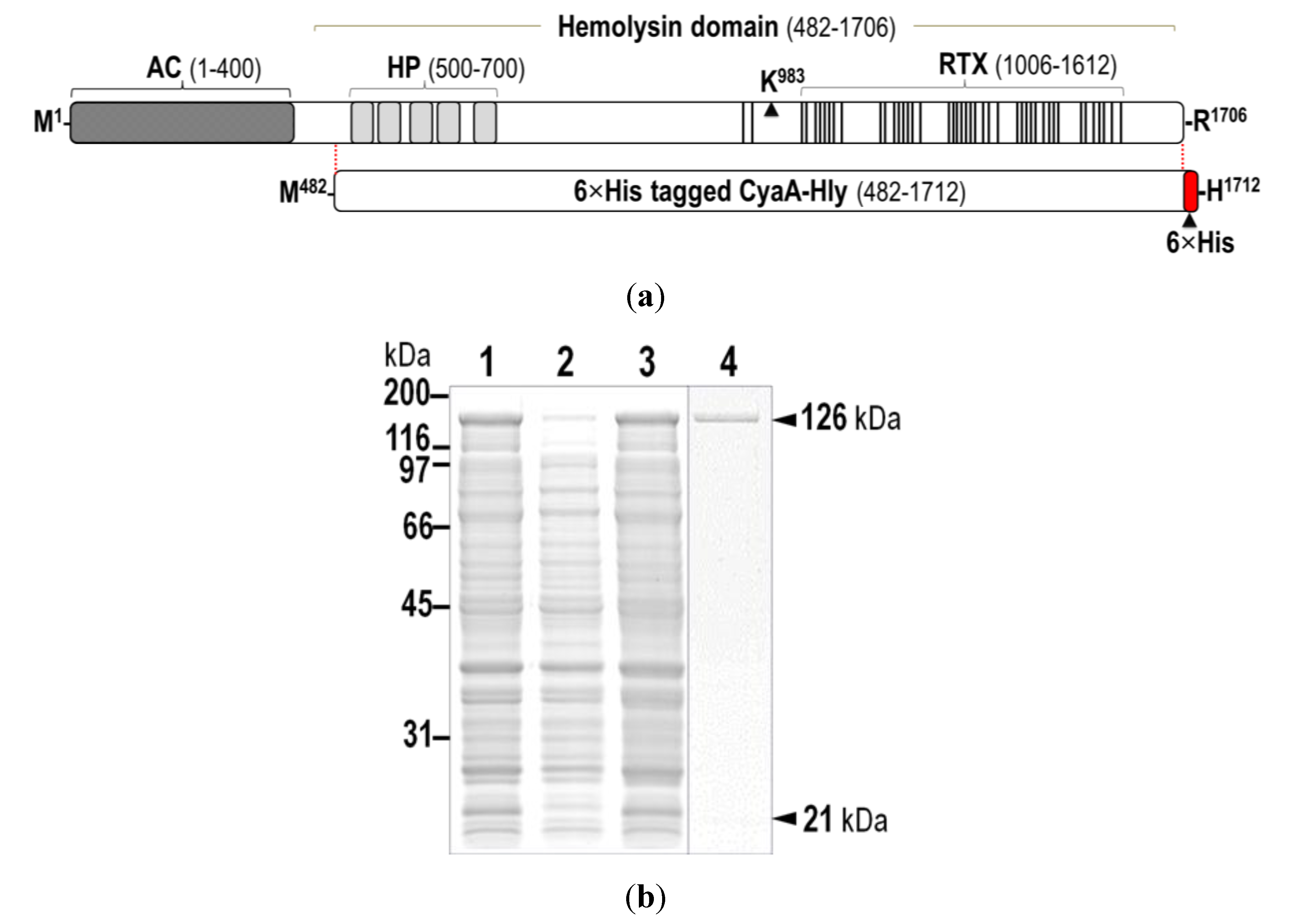
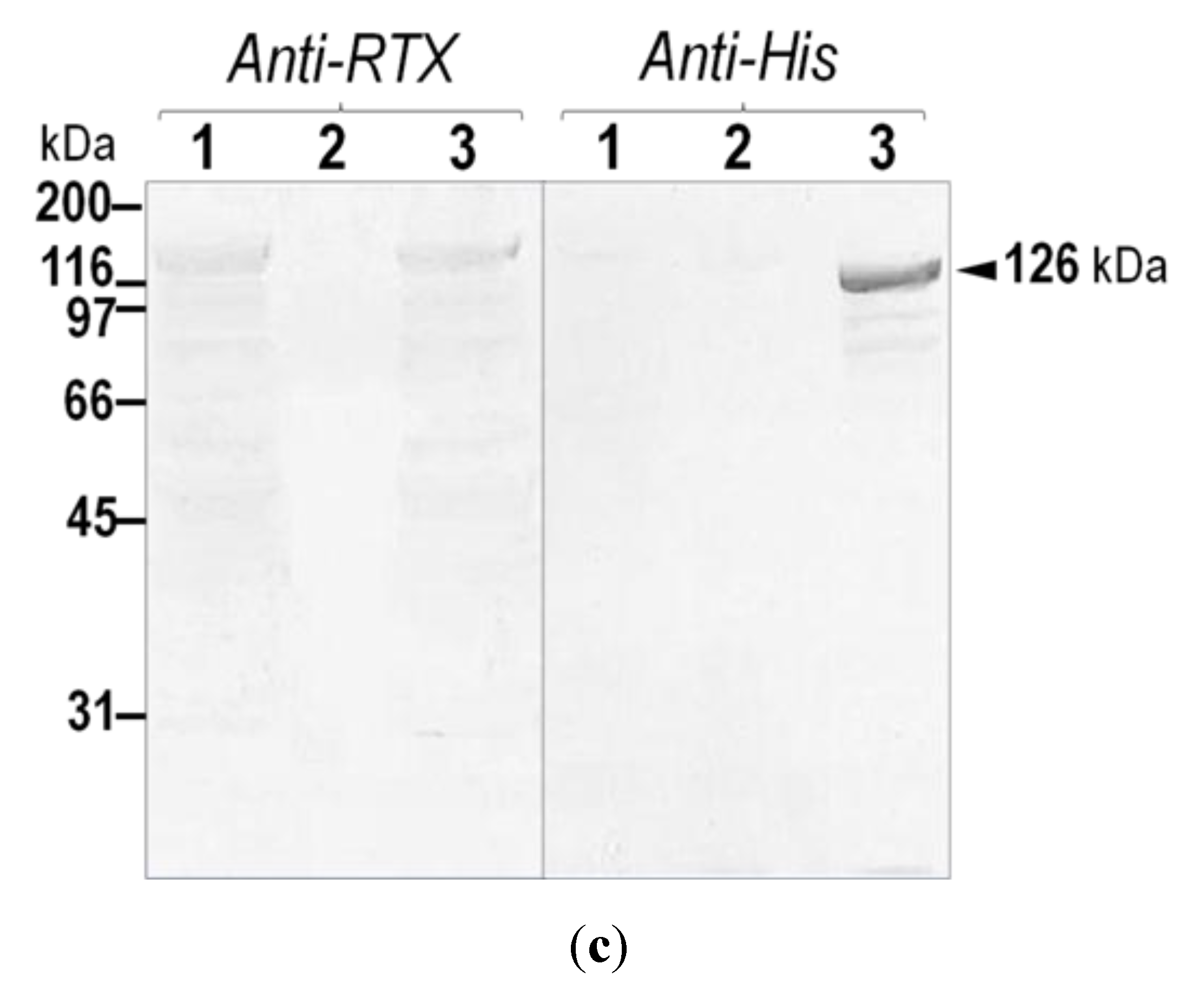
2. Results and Discussion
2.1. Verification of the Expressed His-Tagged CyaA-Hly Domain
| Toxin | Hemolytic activity b (%) ± SEM |
|---|---|
| CyaA-Hly cell lysate a | 63.0 ± 3.0 |
| 6×His-tagged CyaA-Hly cell lysate a | 66.0 ± 3.0 |
2.2. Hemolytic Characteristics of the Soluble His-Tagged CyaA-Hly Toxin
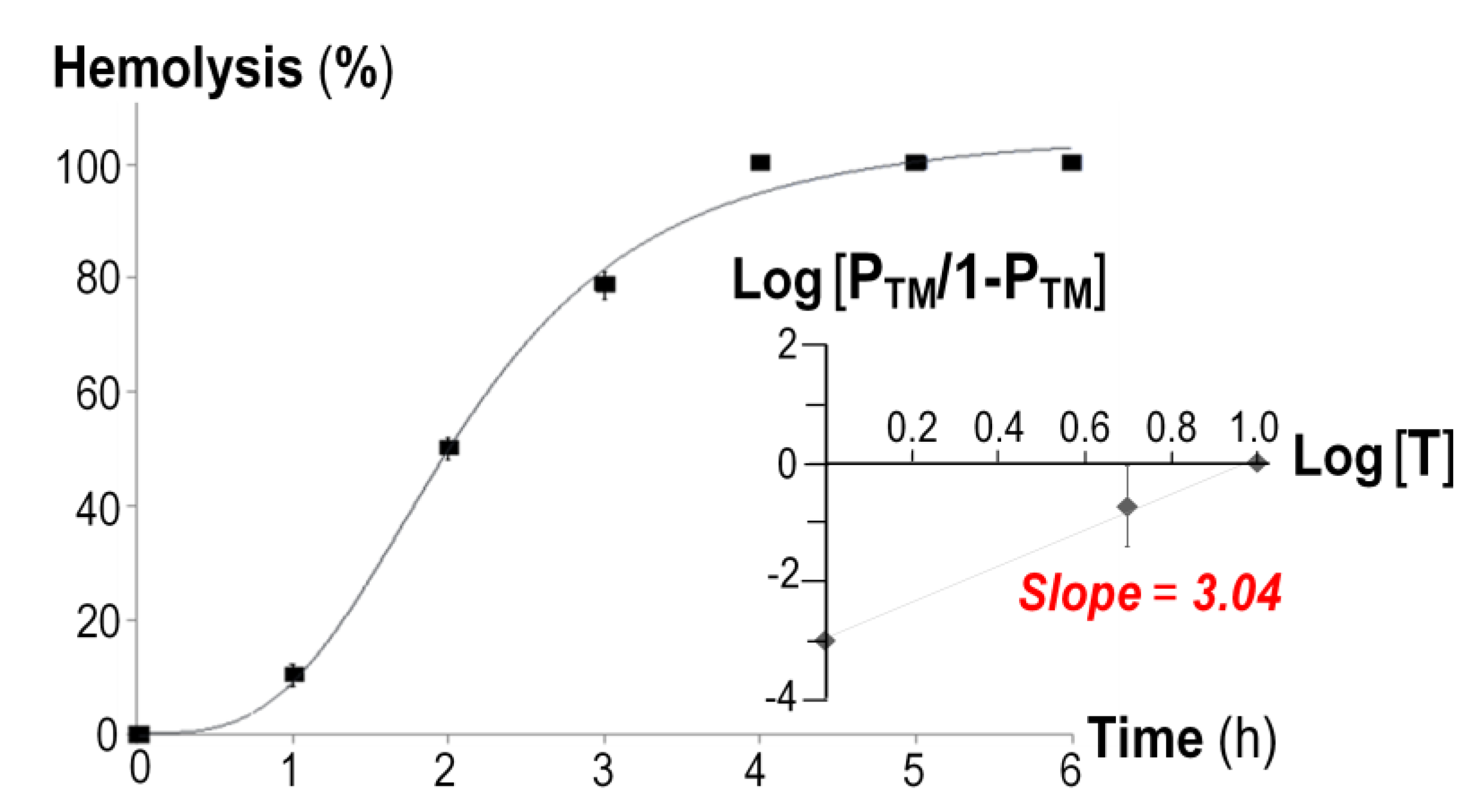
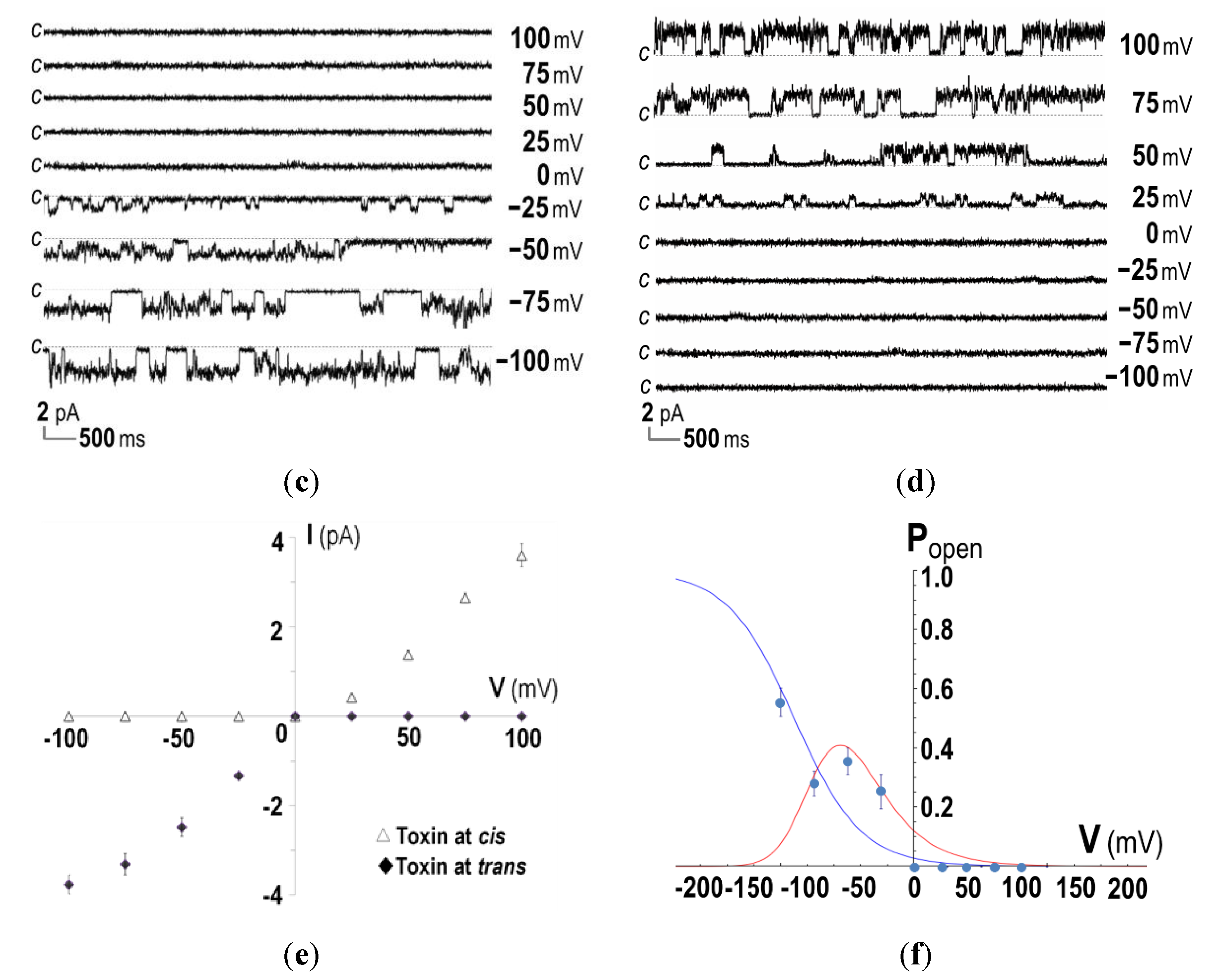
2.3. Ion-Channel Characteristics Formed by His-Tagged CyaA-Hly
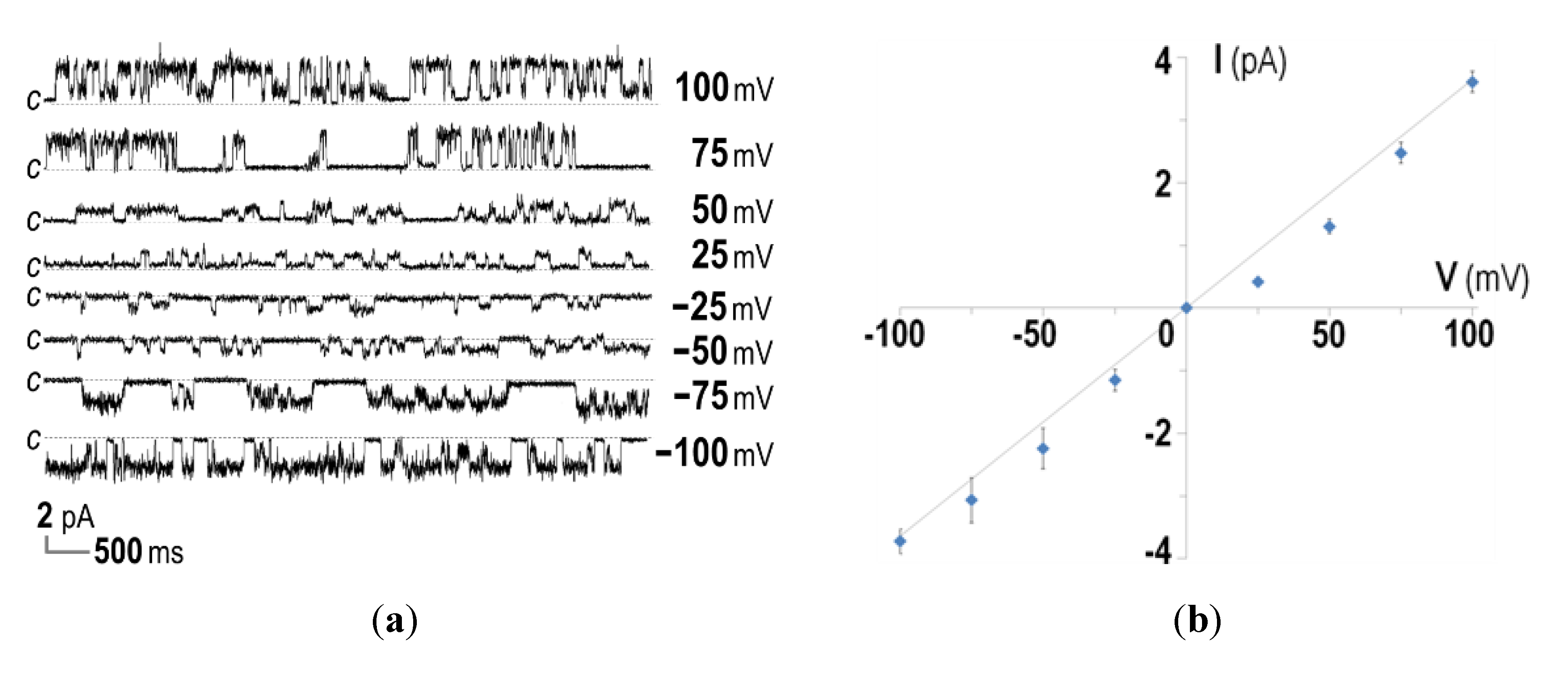
3. Experimental Section
3.1. Construction of Recombinant Plasmid with His-Tagged Fusion
3.2. Protein Expression
3.3. Western Blot Analysis
3.4. Protein Purification
3.5. Hemolytic Activity Assay
3.6. Planar Lipid Bilayers and Single Channel Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Abbreviations
| CyaA | adenylate cyclase-hemolysin toxin |
| DiPhyPC | 1,2-diphytanoyl-sn-glycero-3-phosphocholine |
| Ni-NTA | nickel-nitrilotriacetic acid |
| PLBs | planar lipid bilayers |
| PMSF | phenylmethylsulfonylfluoride |
| PNT | 1,10-phenanthroline |
| RTX | Repeat in ToXin |
Conflicts of Interest
References
- Van der Ark, A.A.; Hozbor, D.F.; Boog, C.J.; Metz, B.; van den Dobbelsteen, G.P.; van Els, C.A. Resurgence of pertussis calls for re-evaluation of pertussis animal models. Expert Rev. Vaccines 2012, 11, 1121–1137. [Google Scholar]
- Melvin, J.A.; Scheller, E.V.; Miller, J.F.; Cotter, P.A. Bordetella pertussis pathogenesis: Current and future challenges. Nat. Rev. Microbiol. 2014, 12, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.; Fleck, R.A.; Gillett, M.L.; Rigsby, P.R.; Corbel, M.J.; Stacey, G.N.; Xing, D.K. Evaluation of adenyl cyclase toxin constructs from Bordetella pertussis as candidate vaccine components in an in vitro model of complement-dependent intraphagocytic killing. Vaccine 2006, 24, 4794–4803. [Google Scholar] [CrossRef] [PubMed]
- Guermonprez, P.; Khelef, N.; Blouin, E.; Rieu, P.; Ricciardi-Castagnoli, P.; Guiso, N.; Ladant, D.; Leclerc, C. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J. Exp. Med. 2001, 193, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.; Dickinson, P.; Sing, G.; Craigon, M.; Ghazal, P.; Parton, R.; Coote, J.G. Transcriptional responses of murine macrophages to the adenylate cyclase toxin of Bordetella pertussis. Microb. Pathog. 2008, 44, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Bellalou, J.; Sebo, P.; Ladant, D. Bordetella pertussis adenylate cyclase toxin. Structural and functional independence of the catalytic and hemolytic activities. J. Biol. Chem. 1992, 267, 13598–13602. [Google Scholar] [PubMed]
- Basler, M.; Masin, J.; Osicka, R.; Sebo, P. Pore-forming and enzymatic activities of Bordetella pertussis adenylate cyclase toxin synergize in promoting lysis of monocytes. Infect. Immun. 2006, 74, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Powthongchin, B.; Angsuthanasombat, C. High level of soluble expression in Escherichia coli and characterisation of the CyaA pore-forming fragment from a Bordetella pertussis Thai clinical isolate. Arch. Microbiol. 2008, 189, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Linhartova, I.; Bumba, L.; Masin, J.; Basler, M.; Osicka, R.; Kamanova, J.; Prochazkova, K.; Adkins, I.; Hejnova-Holubova, J.; Sadilkova, L.; et al. RTX proteins: A highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 2010, 34, 1076–1112. [Google Scholar] [PubMed]
- Welch, R.A. RTX toxin structure and function: A story of numerous anomalies and few analogies in toxin biology. Cur. Top. Microbiol. Immunol. 2001, 257, 85–111. [Google Scholar]
- Welch, R.A. Pore-forming cytolysins of gram-negative bacteria. Mol. Microbiol. 1991, 5, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Chenal, A.; Guijarro, J.I.; Raynal, B.; Delepierre, M.; Ladant, D. RTX calcium binding motifs are intrinsically disordered in the absence of calcium: Implication for protein secretion. J. Biol. Chem. 2009, 284, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Pojanapotha, P.; Thamwiriyasati, N.; Powthongchin, B.; Katzenmeier, G.; Angsuthanasombat, C. Bordetella pertussis CyaA-RTX subdomain requires calcium ions for structural stability against proteolytic degradation. Protein Expres. Purif. 2011, 75, 127–132. [Google Scholar] [CrossRef]
- Frey, J. The role of RTX toxins in host specificity of animal pathogenic Pasteurellaceae. Vet. Microbiol. 2011, 153, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Schmid, A.; Benz, R.; Goebel, W. Mutations affecting pore formation by haemolysin from Escherichia coli. Mol. Gen. Genet. 1991, 226, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Powthongchin, B.; Angsuthanasombat, C. Effects on haemolytic activity of single proline substitutions in the Bordetella pertussis CyaA pore-forming fragment. Arch. Microbiol. 2009, 191, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kurehong, C.; Powthongchin, B.; Thamwiriyasati, N.; Angsuthanasombat, C. Functional significance of the highly conserved Glu570 in the putative pore-forming helix 3 of the Bordetella pertussis haemolysin toxin. Toxicon 2011, 57, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Gray, M.C.; Hewlett, E.L. Adenylate cyclase toxin from Bordetella pertussis produces ion conductance across artificial lipid bilayers in a calcium- and polarity-dependent manner. J. Biol. Chem. 1994, 269, 22496–22499. [Google Scholar] [PubMed]
- Vojtova-Vodolanova, J.; Basler, M.; Osicka, R.; Knapp, O.; Maier, E.; Cerny, J.; Benada, O.; Benz, R.; Sebo, P. Oligomerization is involved in pore formation by Bordetella adenylate cyclase toxin. FASEB J. 2009, 23, 2831–2843. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of Staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Olson, R. Crystal structure of the Vibrio cholerae cytolysin heptamer reveals common features among disparate pore-forming toxins. Proc. Natl. Acad. Sci. USA 2011, 108, 7385–7390. [Google Scholar] [CrossRef] [PubMed]
- Benz, R.; Maier, E.; Ladant, D.; Ullmann, A.; Sebo, P. Adenylate cyclase toxin (CyaA) of Bordetella pertussis. Evidence for the formation of small ion-permeable channels and comparison with HlyA of Escherichia coli. J. Biol. Chem. 1994, 269, 27231–27139. [Google Scholar] [PubMed]
- Juntadech, T.; Kanintronkul, Y.; Kanchanawarin, C.; Katzenmeier, G.; Angsuthanasombat, C. Importance of polarity of the α4-α5 loop residue-Asn166 in the pore-forming domain of the Bacillus thuringiensis Cry4Ba toxin: Implications for ion permeation and pore opening. Biochim. Biophys. Acta Biomembr. 2014, 1838, 319–327. [Google Scholar] [CrossRef]
- Marchioretto, M.; Podobnik, M.; Dalla Serra, M.; Anderluh, G. What planar lipid membranes tell us about the pore-forming activity of cholesterol-dependent cytolysins. Biophys. Chem. 2013, 182, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Knapp, O.; Maier, E.; Waltenberger, E.; Mazuet, C.; Benz, R.; Popoff, M.R. Residues involved in the pore-forming activity of the Clostridium perfringens iota toxin. Cell. Microbiol. 2015, 17, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Menestrina, G.; Moser, C.; Pellet, S.; Welch, R. Pore-formation by Escherichia coli hemolysin (HlyA) and other members of the RTX toxins family. Toxicology 1994, 87, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Menestrina, G.; Ropele, M. Voltage-dependent gating properties of the channel formed by Escherichia coli hemolysin in planar lipid membranes. Biosci. Rep. 1989, 9, 465–473. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurehong, C.; Kanchanawarin, C.; Powthongchin, B.; Katzenmeier, G.; Angsuthanasombat, C. Membrane-Pore Forming Characteristics of the Bordetella pertussis CyaA-Hemolysin Domain. Toxins 2015, 7, 1486-1496. https://doi.org/10.3390/toxins7051486
Kurehong C, Kanchanawarin C, Powthongchin B, Katzenmeier G, Angsuthanasombat C. Membrane-Pore Forming Characteristics of the Bordetella pertussis CyaA-Hemolysin Domain. Toxins. 2015; 7(5):1486-1496. https://doi.org/10.3390/toxins7051486
Chicago/Turabian StyleKurehong, Chattip, Chalermpol Kanchanawarin, Busaba Powthongchin, Gerd Katzenmeier, and Chanan Angsuthanasombat. 2015. "Membrane-Pore Forming Characteristics of the Bordetella pertussis CyaA-Hemolysin Domain" Toxins 7, no. 5: 1486-1496. https://doi.org/10.3390/toxins7051486
APA StyleKurehong, C., Kanchanawarin, C., Powthongchin, B., Katzenmeier, G., & Angsuthanasombat, C. (2015). Membrane-Pore Forming Characteristics of the Bordetella pertussis CyaA-Hemolysin Domain. Toxins, 7(5), 1486-1496. https://doi.org/10.3390/toxins7051486






