The Key Role of Peltate Glandular Trichomes in Symbiota Comprising Clavicipitaceous Fungi of the Genus Periglandula and Their Host Plants
Abstract
:1. Introduction
2. Results
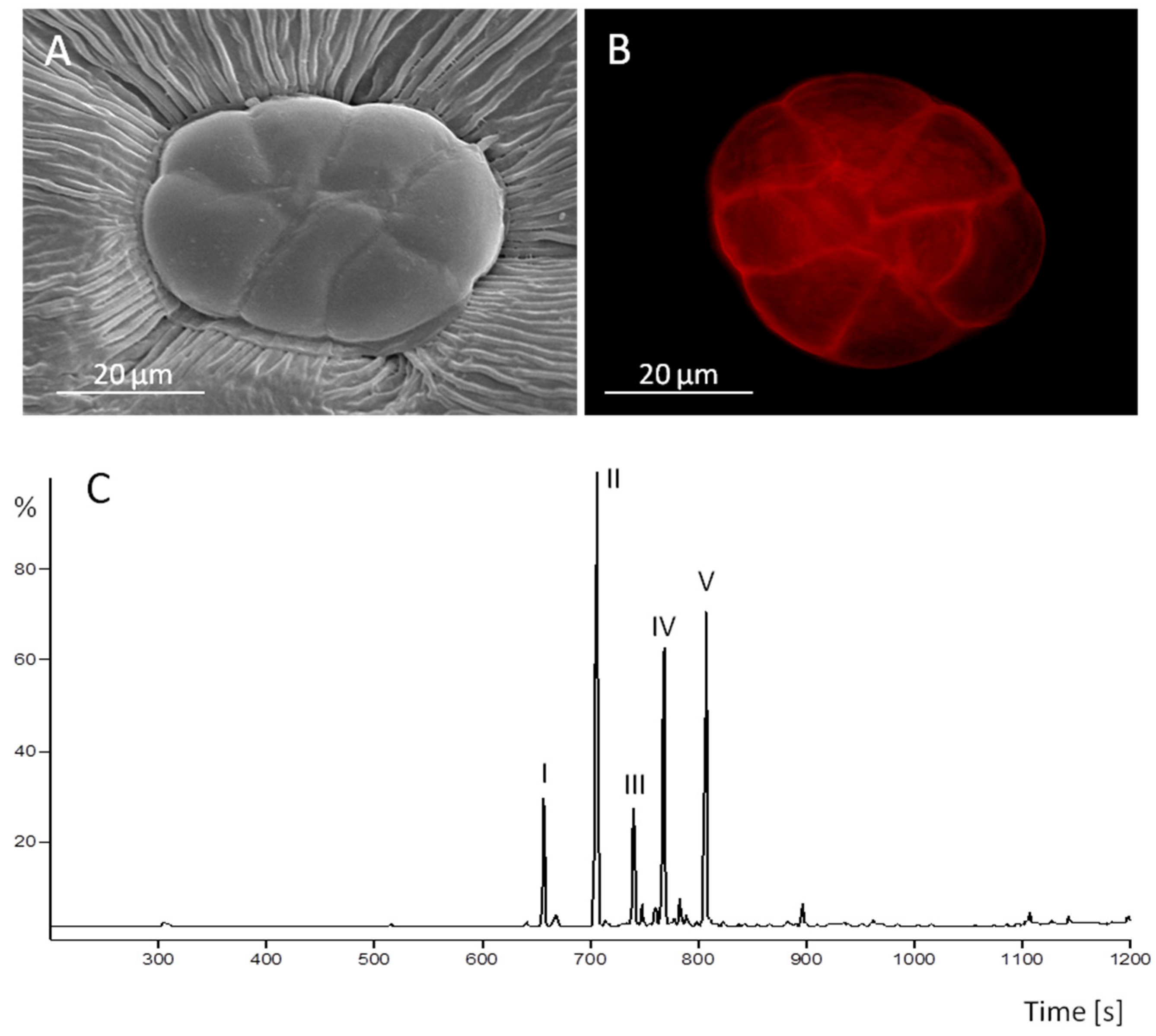
2.1. The Oil Secreting Function of Peltate Glandular Trichomes Present on the Adaxial Leaf Surface of Convolvulaceous Plants
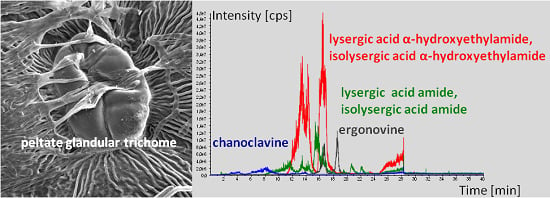
2.2. Ergot Alkaloid Biosynthesis in the Fungal Mycelium

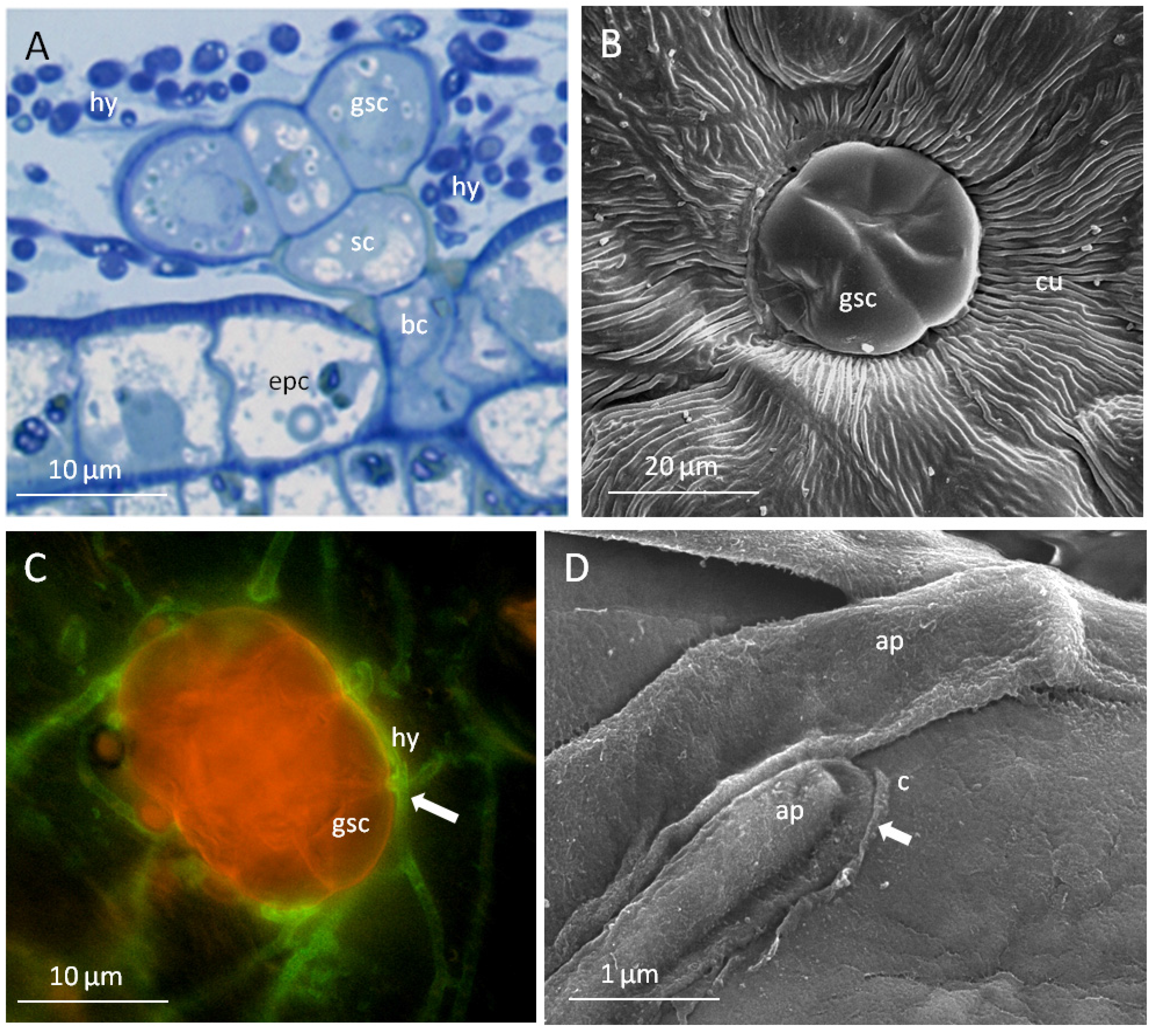
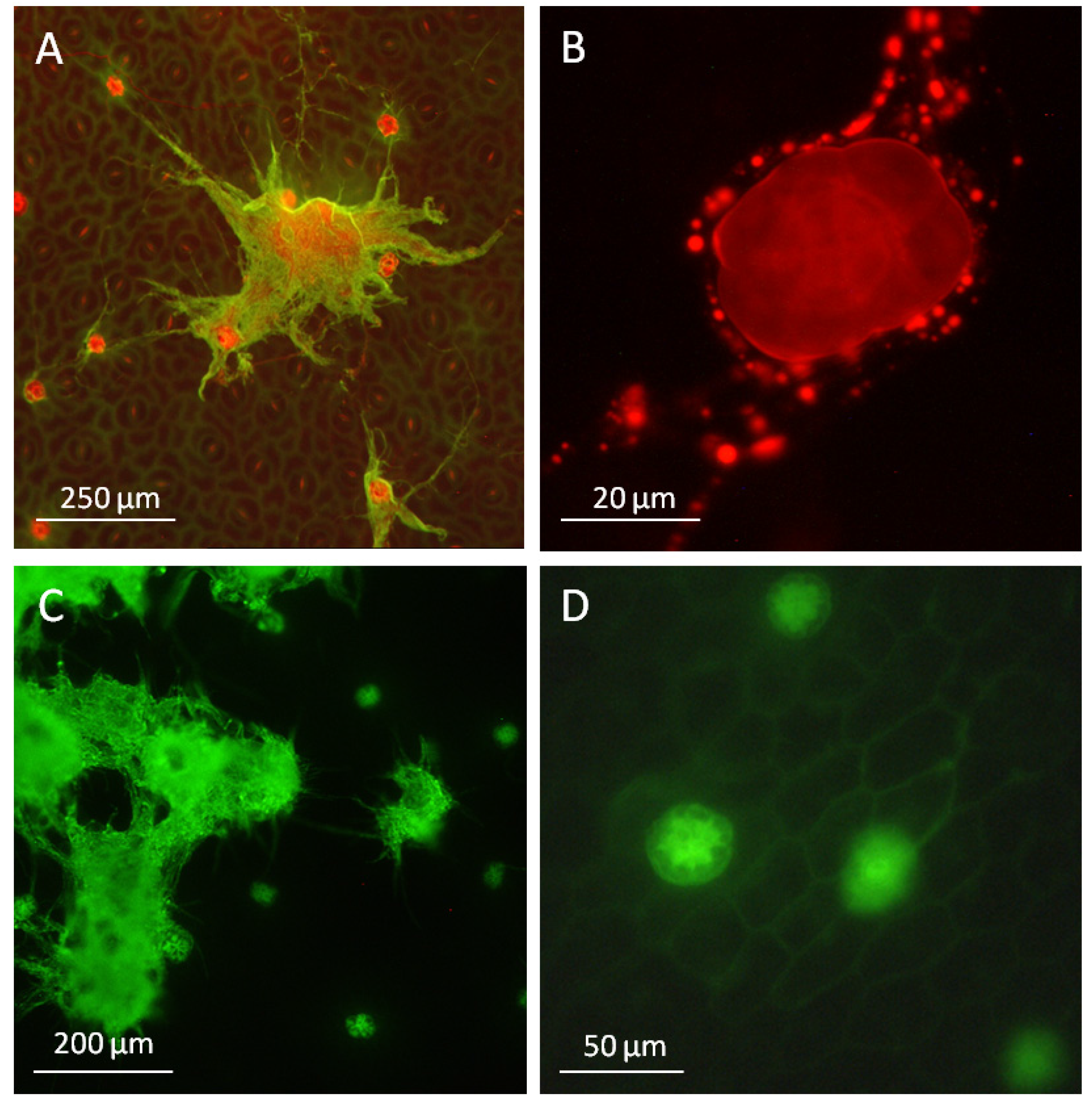
2.3. Peltate Glandular Trichomes of Convolvulaceous Plants and Periglandula Hyphae are Part of a Functional Entity
2.3.1. Kinetic Experiments

| Stage | Leaves | Ergosterol | Ergoline alkaloids | Glandular trichomes | |||
|---|---|---|---|---|---|---|---|
| Length of Rhachis (cm) | μg (gFW)−1 | % | μg (gFW)−1 | % | Number (mm2)−1 | % | |
| A | |||||||
| Bud | 1.0–2.0 | 13.9 | 100.0 | 48.3 | 100.0 | 77.0 | 100.0 |
| Open bud | 1.5–2.0 | 8.3 | 69.7 | 15.9 | 32.9 | 34.0 | 44.2 |
| Medium sized leaf | 2.5–4.0 | 0.9 | 6.5 | 11.9 | 24.6 | 16.5 | 21.4 |
| Fully expanded leaf | 4.5–6.5 | 1.7 | 12.2 | 6.4 | 13.3 | 8.3 | 10.8 |
| B | |||||||
| Bud | 1.5–3.0 | 13.5 | 100.0 | 110.8 | 100.0 | 37.8 | 100.0 |
| Medium sized leaf | 3.5–5.0 | trace | >0 | 53.3 | 48.1 | 10.6 | 28.0 |
| Fully expanded leaf | 5.5–7.0 | trace | >0 | 9.5 | 8.6 | 4.7 | 12.4 |
2.3.2. The Glandular Trichomes as Interface of the Plant/Fungus Symbiotum

3. Discussion
4. Experimental Section
4.1. Previously Published Materials and Methods
4.2. Identification of Terpenoids
4.3. Identification and Quantitative Estimation of Ergot Alkaloids by HPLC/MS/MS
4.4. Quantitative Estimation of Ergosterol as a Measure of Fungal Mycelium
4.5. Production and Characterisation of a Polyclonal Antibody against the 4-(γ,γ -Dimethylallyl) Tryptophan Synthase (DmaW) from P. ipomoeae

4.6. Microscopic Techniques
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buckingham, J. Dictionary of Natural Products; Chapman and Hall: London, UK, 1994. [Google Scholar]
- Zenk, M.H. Biochemie und physiologie sekundaerer pflanzenstoffe. Ber. Dtsch. Bot. Ges. 1967, 80, 573–591. [Google Scholar]
- Eisner, T. For Love of Insects; Harvard University Press: Cambridge, MA, USA, 2003. [Google Scholar]
- White, J.F., Jr.; Bacon, C.W.; Hywel-Jones, N.L.; Spatafora, J.W. Clavicipitalean Fungi, Evolutionary Biology, Chemistry, Biocontrol, and Cultural Impacts, Mycology Series; Dekker: New York, NY, USA, 2003. [Google Scholar]
- Harborne, J.B. Introduction to Ecological Biochemistry, 4th ed.; Academic: London, UK, 2004. [Google Scholar]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Steiner, U.; Ahimsa-Mueller, M.A.; Markert, A.; Kucht, S.; Groβ, J.; Kauf, N.; Kuzma, M.; Zych, M.; Lamshoeft, M.; Furmanowa, M.; et al. Molecular characterization of a seed transmitted clavicipitaceous fungus occurring on dicotyledonous plants (Convolvulaceae). Planta 2006, 224, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Ahimsa-Mueller, M.A.; Markert, A.; Hellwig, S.; Knoop, V.; Steiner, U.; Drewke, C.; Leistner, E. Clavicipitaceous fungi associated with ergoline alkaloid-containing Convolvulaceae. J. Nat. Prod. 2007, 70, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Leistner, E.; Steiner, U. The Mycota XV; Anke, T., Weber, D., Eds.; Springer: Berlin, Germany, 2009; pp. 197–208. [Google Scholar]
- Steiner, U.; Leistner, E. Ergoline Alkaloids in convolvulaceous host plants originate from epibiotic clavicipitaceous fungi of the genus Periglandula. Fungal Ecol. 2012, 5, 316–321. [Google Scholar] [CrossRef]
- Steiner, U.; Leibner, S.; Schardl, C.L.; Leuchtmann, A.; Leistner, E. Periglandula, a new fungal genus within the Clavicipitaceae and its association with Convolvulaceae. Mycologia 2011, 103, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Kucht, S.; Groß, J.; Hussein, Y.; Grothe, T.; Keller, U.; Basar, S.; Koenig, W.A.; Steiner, U.; Leistner, E. Elimination of ergoline alkaloids following treatment of Ipomoea asarifolia (Convolvulaceae) with fungicides. Planta 2004, 219, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Eserman, L.A.; Tiley, G.P.; Jarret, R.L.; Leebens-Mack, J.H.; Miller, R.E. Phylogenetics and diversification of Morning Glories (tribe Ipomoeeae, Convolvulaceae) based on whole plastome sequences. Am. J. Bot. 2014, 101, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eich, E. Solanaceae and Convolvulaceae Secondary Metabolites: Biosynthesis, Chemotaxonomy, Biological and Economic Significance (A Handbook); Springer: Berlin, Germany, 2008. [Google Scholar]
- Steiner, U.; Hellwig, S.; Leistner, E. Specificity in the interaction between an epibiotic clavicipitalean fungus and its convolvulaceous host in a fungus/plant symbiotum. Plant Signal. Behav. 2008, 3, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Markert, A.; Steffan, N.; Ploss, K.; Hellwig, K.; Steiner, U.; Drewke, C.; Li, S.M.; Boland, W.; Leistner, E. Biosynthesis and accumulation of ergoline alkaloids in a mutualistic association between Ipomoea asarifolia (Convolvulaceae) and a clavicipitalean fungus. Plant Physiol. 2008, 147, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Young, A.C.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B.; et al. Plant symbiotic fungi as chemical engineers: Multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 2013, 9, e1003323. [Google Scholar] [CrossRef] [PubMed]
- Groeger, D.; Floss, H.G. The Alkaloids- Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: New York, NY, USA, 1998; Volume 50, pp. 171–218. [Google Scholar]
- Clay, K. Fungi and the food of the gods. Nature 2004, 427, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Tudzynski, P.; Scheffer, J. Claviceps purpurea: Molecular aspects of a unique pathogenic lifestyle. Mol. Plant Pathol. 2004, 5, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.F.; White, J.F., Jr. Evolutionary development of the Clavicipitaceae. In The Fungal Community, Its Organisation and Role in the Ecosystem; Dighton, J., White, J.F., Oudemans, P., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 505–518. [Google Scholar]
- Schardl, C.; Leuchtmann, A. The Epichloe endophytes of grasses and the symbiotic continuum. In The Fungal Community, Its Organisation and Role in the Ecosystems; Dighton, J., White, J.F., Oudemans, P., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 475–503. [Google Scholar]
- Schardl, C.L.; Panaccione, D.G.; Tudzynski, P. The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic: New York, NY, USA, 2006; Volume 63, pp. 45–86. [Google Scholar]
- Hochmuth, D.H.; Joulain, D.; Koenig, W.A. MassFinder software and Data Bank, University of Hamburg. 2002. Available online: http://www.chemie.uni-hamburg.de/oc/koenig/massfinder.html (accessed on 2 October 2003).
- Ahimsa-Müller, M.A. The Colonization of Ergoline Alkaloid Containing Convolvulaceae Plants by Clavicipitaceous Fungi. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2007. [Google Scholar]
- Jenett-Siems, K.; Kaloga, M.; Eich, E. Ergobalansine/Ergobalansinine, a proline-free peptide-type alkaloid of the fungal genus Balansia., is a constituent of Ipomoea piurensis. J. Nat. Prod. 1994, 57, 1304–1306. [Google Scholar] [CrossRef]
- Jenett-Siems, K.; Kaloga, M.; Eich, E. Additions and corrections. J. Nat. Prod. 2004, 67, 2160. [Google Scholar] [CrossRef]
- Lee, S.L.; Floss, H.G.; Heinstein, P. Purification and properties of dimethylallylpyrophosphate: Tryptophan dimethylallyl transferase, the first enzyme of ergot alkaloid biosynthesis in Claviceps. sp.SD 58. Arch. Biochem. Biophys. 1976, 77, 84–94. [Google Scholar] [CrossRef]
- Cress, W.A.; Chayet, L.T.; Rilling, H.C. Crystallisation and partial characterization of dimethylallyl pyrophosphate: L-tryptophan dimethylallyl transferase from Claviceps. sp. SD 58. J. Biol. Chem. 1981, 256, 10917–10923. [Google Scholar] [PubMed]
- Wallwey, C.; Li, S.M. Ergot alkaloids: Structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes. Nat. Prod. Rep. 2011, 28, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Frohmann, M.A.; Dush, M.K.; Martin, G.R. Rapid production of full-length cDNAs from rare transcripts: Amplification using a single gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. USA 1988, 85, 8998–9002. [Google Scholar] [CrossRef] [PubMed]
- Mulac, D.; Lepski, S.; Ebert, F.; Schwerdtle, T.; Humpf, H.-U. Cytotoxicity and fluorescence visualisation of ergot alkaloids in human cell lines. J. Agric. Food Chem. 2012, 61, 462–471. [Google Scholar] [CrossRef]
- Schwadorf, K.; Müller, H.-M. Determination of ergosterol in cereals, mixed feed components, and mixed feeds by liquid chromatography. J. Assoc. Anal. Chem. 1989, 72, 457–462. [Google Scholar]
- White, J.F., Jr.; Camp, C.R. A study of water relations of Epichloe amarillans, an endophyte of the grass Agrostis hiemalis. Symbiosis 1995, 18, 15–25. [Google Scholar]
- White, J.F., Jr.; Breen, J.P.; Morgan-Jones, G. Substrate utilization in selected Acremonium, Atkinsonella. and Balansia. species. Mycologia 1991, 83, 601–610. [Google Scholar] [CrossRef]
- Loesecke, W.; Neumann, D.; Schmauder, H.-P.; Groeger, D. Changes in cytoplasmic ultrastructure during submerged cultivation of a peptide alkaloids-producing strain of Claviceps purpurea (Fr.) Tul. Z. Allg. Mikrobiol. 1982, 22, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Beaulieu, W.T.; Mott, I.W.; Riet-Correa, F.; Gardner, D.R.; Grum, D.; Pfister, J.A.; Clay, K.; Marcolongo-Pereira, C. Production of the alkaloid swainsonine by a fungal endosymbiont of the Ascomycete order Chaetothyriales in the host Ipomoea carnea. J. Agric. Food Chem. 2013, 61, 3797–3803. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.T.; Bacon, C.W.; Luttrell, E.S. Reciprocal translocation of carbohydrates between host and fungus in Bahiagrass infected with Myriogenospora atramentosa. Physiol. Biochem. 1985, 75, 407–411. [Google Scholar]
- Lehtonen, P.; Helander, M.; Wink, M.; Sporer, F.; Saikkonen, K. Transfer of endophyte-origin defensive alkaloids from a grass to a hemiparasitic plant. Ecol. Lett. 2005, 8, 1256–1263. [Google Scholar] [CrossRef]
- Shitan, N.; Yazaki, K. Accumulation and membrane transport of plant alkaloids. Curr. Pharm. Biotechnol. 2007, 8, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Sugiyama, A.; Morita, M.; Shitan, N. Secondary transport as an efficient membrane transport mechanism for plant secondary metabolites. Phytochem. Rev. 2008, 7, 513–524. [Google Scholar] [CrossRef]
- Broun, P.; Liu, Y.; Queen, E.; Schwarz, Y.; Abenes, M.L.; Leibman, M. Importance of transcription factors in the regulation of secondary metabolism and their relevance to the control of terpenoid accumulation. Phytochem. Rev. 2006, 5, 27–38. [Google Scholar] [CrossRef]
- Hückelhoven, R. Transport and secretion in plant-microbe interactions. Curr. Opin. Plant Biol. 2007, 10, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, N.; Haarmann, T.; Pazoutova, S.; Jung, M.; Tudzynski, P. The ergot alkaloid gene cluster: Functional analyses and evolutionary aspects. Phytochemistry 2009, 70, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The challenges of moving chemicals within and out of cells: Insights into the transport of plant natural products. Planta 2004, 219, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.M.; Stoopen, G.; Menzel, T.R.; Gols, R.; Bouwmeester, H.J.; Dicke, M.; Jongsma, M.A. Bidirectional secretions from glandular trichomes of Pyrethrum enable immunization of seedlings. Plant Cell 2012, 24, 4252–4265. [Google Scholar] [CrossRef] [PubMed]
- Owen, T.P.; Lennon, K.A.; Santo, M.J.; Anderson, A.N. Pathways for nutrient transport in the pitchers of the carnivorous plant Nepenthes alata. Ann. Bot. 1999, 84, 459–466. [Google Scholar] [CrossRef]
- Darwin, C. Insectivorous Plants; John Murray: London, UK, 1875; Chapter I; pp. 1–18. [Google Scholar]
- Beaulieu, W.T.; Panaccione, D.G.; Hazekamp, C.S.; Mckee, M.C.; Ryan, K.L.; Clay, K. Differential allocation of seed-borne ergot alkaloids during early ontogeny of morning glories (Convolvulaceae). J. Chem. Ecol. 2013, 39, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Unsöld, I.A.; Li, S.-M. Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus fumigatus. Microbiology 2005, 151, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Steffan, N.; Unsöld, I.A.; Li, S.-M. Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus. Chem. Chem. 2007, 8, 1298–1307. [Google Scholar] [CrossRef]
- Kremer, A.; Westrich, L.; Li, S.-M. A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: Overproduction, purification and biochemical characterization. Microbiology 2007, 153, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, M.J. A formaldehyde glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Schumacher, C.F.A.; Steiner, U.; Dehne, H.-W.; Oerke, E.-C. Localized adhesion of nongerminated Venturia inaequalis conidia to leaves and artificial surfaces. Phytopathology 2008, 98, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Svitkina, T.M.; Shevelev, A.A.; Bershadsky, A.D.; Gelfand, V.I. Cytoskeleton of mouse embryo fibroblasts. Electron microscopy of platinum replicas. Eur. J. Cell Biol. 1984, 34, 64–74. [Google Scholar] [PubMed]
- Ouadt-Hallmann, A.; Löw, A.; Hamacher, J. Distribution of cherry leaf roll nepovirus (CLRV) in leaves of deciduous forest trees and herbaceous plants detected by tissue print immunopressblotting (TPI) of whole leaf blades. J. Plant Dis. Prot. 1996, 103, 449–454. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steiner, U.; Hellwig, S., née Kucht; Ahimsa-Müller, M.A.; Grundmann, N.; Li, S.-M.; Drewke, C.; Leistner, E. The Key Role of Peltate Glandular Trichomes in Symbiota Comprising Clavicipitaceous Fungi of the Genus Periglandula and Their Host Plants. Toxins 2015, 7, 1355-1373. https://doi.org/10.3390/toxins7041355
Steiner U, Hellwig S née Kucht, Ahimsa-Müller MA, Grundmann N, Li S-M, Drewke C, Leistner E. The Key Role of Peltate Glandular Trichomes in Symbiota Comprising Clavicipitaceous Fungi of the Genus Periglandula and Their Host Plants. Toxins. 2015; 7(4):1355-1373. https://doi.org/10.3390/toxins7041355
Chicago/Turabian StyleSteiner, Ulrike, Sabine Hellwig, née Kucht, Mahalia A. Ahimsa-Müller, Nicola Grundmann, Shu-Ming Li, Christel Drewke, and Eckhard Leistner. 2015. "The Key Role of Peltate Glandular Trichomes in Symbiota Comprising Clavicipitaceous Fungi of the Genus Periglandula and Their Host Plants" Toxins 7, no. 4: 1355-1373. https://doi.org/10.3390/toxins7041355
APA StyleSteiner, U., Hellwig, S., née Kucht, Ahimsa-Müller, M. A., Grundmann, N., Li, S.-M., Drewke, C., & Leistner, E. (2015). The Key Role of Peltate Glandular Trichomes in Symbiota Comprising Clavicipitaceous Fungi of the Genus Periglandula and Their Host Plants. Toxins, 7(4), 1355-1373. https://doi.org/10.3390/toxins7041355