Alteration in the Expression of Cytochrome P450s (CYP1A1, CYP2E1, and CYP3A11) in the Liver of Mouse Induced by Microcystin-LR
Abstract
:1. Introduction
2. Result
2.1. Effects of MCLR on mRNA Level, Protein Content, and Enzymic Activity of CYP1A1 in Mouse Liver


2.2. CYP2E1 Transcription, Protein Content, and ANH Activity


2.3. CYP3A11 mRNA Level, Protein Content, and ERND Activity
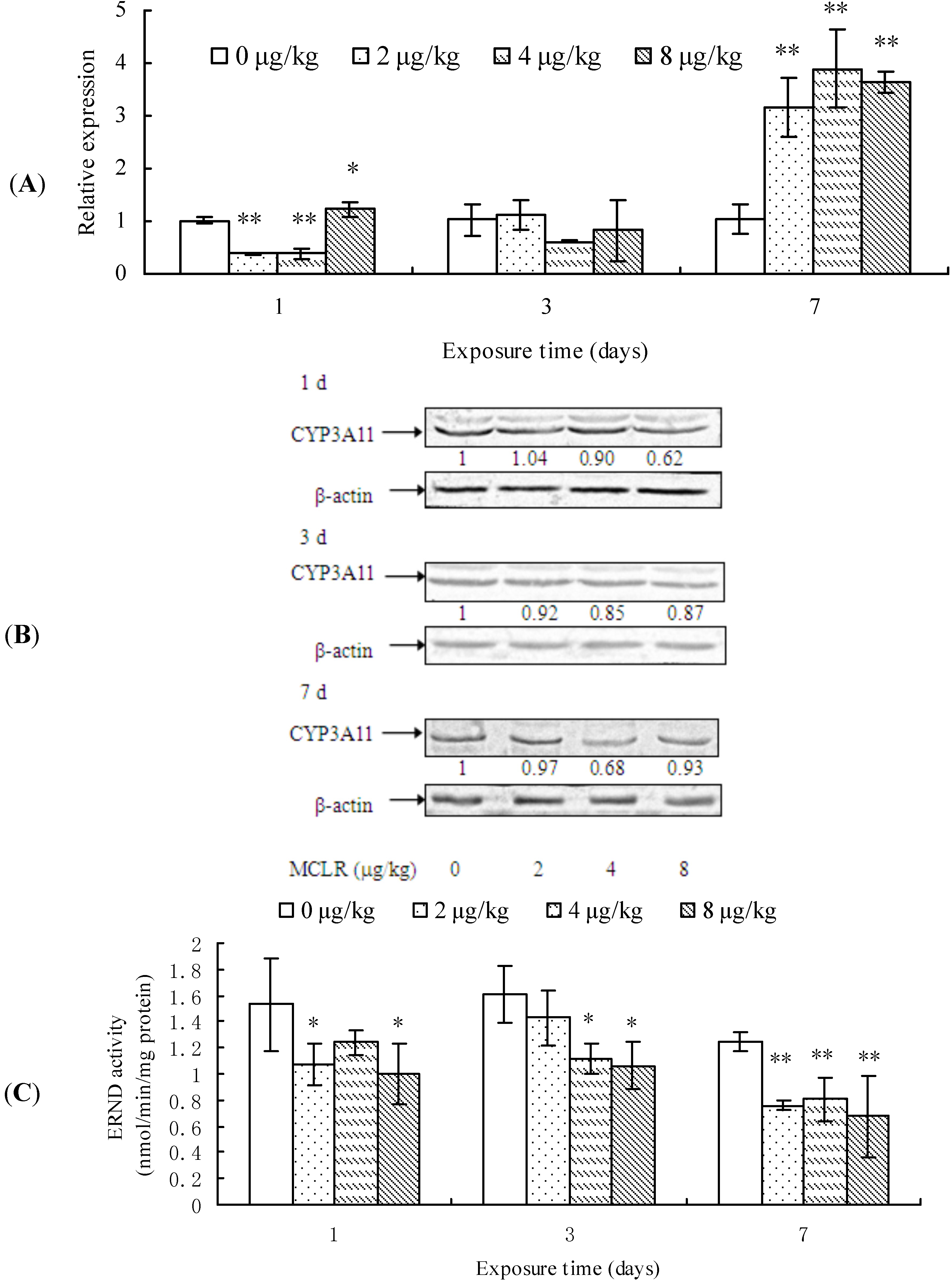
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals and MCLR-Exposure
4.3. RNA Extraction and Real Time PCR
| Gene | Forward (5' to 3') | Reverse (5' to 3') |
|---|---|---|
| CYP1A1 | GGTTAACCATGACCGGGAACT | TGCCCAAACCAAAGAGAGTGA |
| CYP2E1 | AAGCGCTTCGGGCCAG | TAGCCATGCAGGACCACGA |
| CYP3A11 | ATTCCTGGGCCCAAACCTCTGCCA | TGGGTCTGTGACAGCAAGGAGAGGC |
| β-actin | CACCCGCGAGCACAGCTTCTTT | TTGTCGACGACCAGCGCAGCGATA |
4.4. Western Blot Analysis
4.5. Preparation of Microsomes and Enzyme Activity Assay
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharm. 2005, 203, 264–272. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Qin, W.; Xu, L.; Wang, T.; Cheng, S.; Yang, L. Effects of microcystin-LR exposure on matrix metalloproteinase-2/-9 expression and cancer cell migration. Ecotoxicol. Environ. Saf. 2012, 77, 88–93. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W. Cyanobacteria secondary metabolites-the cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.M. The toxicology of microcystins. Toxicon 1998, 36, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Nam Ong, C. Role of oxidative stress and mitochondrial changes in cyanobacteria-induced apoptosis and hepatotoxicity. FEMS Microbiol. Lett. 2003, 220, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nong, Q.; Komatsu, M.; Izumo, K.; Indo, H.P.; Xu, B.; Aoyama, K.; Majima, H.J.; Horiuchi, M.; Morimoto, K.; Takeuchi, T.; et al. Involvement of reactive oxygen species in Microcystin-LR-induced cytogenotoxicity. Free Radic. Res. 2007, 41, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Štraser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. Rev. Mutat. 2011, 727, 16–41. [Google Scholar] [CrossRef]
- Jayaraj, R.; Anand, T.; Rao, P.V.L. Activity and gene expression profile of certain antioxidant enzymes to microcystin-LR induced oxidative stress in mice. Toxicology 2006, 220, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Xie, P.; Guo, X.; Fan, H.; Yu, D.; Zeng, C.; Chen, L. The role of glutathione detoxification pathway in MCLR-induced hepatotoxicity in SD rats. Environ. Toxicol. 2014. [Google Scholar] [CrossRef]
- Moreno, I.; Pichardo, S.; Jos, A.; Gómez-Amores, L.; Mate, A.; Vazquez, C.M.; Cameán, A.M. Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin-LR administered intraperitoneally. Toxicon 2005, 45, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Cazenave, J.; Bistoni, M.D.L.A.; Pesce, S.F.; Wunderlin, D.A. Differential detoxification and antioxidant response in diverse organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquat. Toxicol. 2006, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cai, C.; Fang, W.; Wang, J.; Zhang, Y.; Liu, J.; Jia, X. Oxidative damage and apoptosis induced by microcystin-LR in the liver of Rana nigromaculata in vivo. Aquat. Toxicol. 2013, 140–141, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Song, L.; Liu, J. Responses of antioxidant systems in the hepatocytes of common carp (Cyprinus carpio L.) to the toxicity of microcystin-LR. Toxicon 2003, 42, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. Oxidative stress induced by microcystin-LR on PLHC-1 fish cell line. Toxicol. In Vitro 2009, 23, 1445–1449. [Google Scholar] [CrossRef]
- Weng, D.; Lu, Y.; Wei, Y.; Liu, Y.; Shen, P. The role of ROS in microcystin-LR-induced hepatocyte apoptosis and liver injury in mice. Toxicology 2007, 232, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Lah, T.T.; Filipic, M. The role of reactive oxygen species in microcystin-LR-induced DNA damage. Toxicology 2004, 200, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shan, Z.; Xu, W.; Wang, X.; Zhou, J.; Kong, D.; Xu, J. Microcystin-LR induced reactive oxygen species mediate cytoskeletal disruption and apoptosis of hepatocytes in Cyprinus carpio L. PLoS One 2013, 8, e84768. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kashyap, A.; Pandey, R.V.; Saini, K.S. Novel advances in cytochrome P450 research. Drug Discov. Today 2011, 16, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R. Comparison of P450s from human and fugu: 420 Million years of vertebrate P450 evolution. Arch. Biochem. Biophys. 2003, 409, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W.; Dalton, T.P.; Okey, A.B.; Gonzalez, F.J. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 2004, 279, 23847–23850. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Ozgun, O.; Arinç, E.; Arslan, S. Diverse action of acrylamide on cytochrome P450 and glutathione S-transferase isozyme activities, mRNA levels and protein levels in human hepatocarcinoma cells. Cell Biol. Toxicol. 2012, 28, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef]
- Thummel, K.E.; Wilkinson, G.R. In vitro and in vivo drug interactions involving human CYP3A. Annu. Rev. Pharmacol. 1998, 38, 389–430. [Google Scholar] [CrossRef]
- Hudder, A.; Song, W.; O’Shea, K.E.; Walsh, P.J. Toxicogenomic evaluation of microcystin-LR treated with ultrasonic irradiation. Toxicol. Appl. Pharm. 2007, 220, 357–364. [Google Scholar] [CrossRef]
- Guo, F.; An, T.; Rein, K.S. The algal hepatoxoxin okadaic acid is a substrate for human cytochromes CYP3A4 and CYP3A5. Toxicon 2010, 55, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, J.; Fang, Q.; Li, Y. Transcription alterations of microRNAs, cytochrome P4501A1 and 3A65, and AhR and PXR in the liver of zebrafish exposed to crude microcystins. Toxicon 2013, 73, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Isin, E.M.; Guengerich, F.P. Complex reactions catalyzed by cytochrome P450 enzymes. BBA Gen. Subj. 2007, 1770, 314–329. [Google Scholar] [CrossRef]
- Moore, M.J.; Mitrofanov, I.V.; Valentini, S.S.; Volkov, V.V.; Kurbskiy, A.V.; Zhimbey, E.N.; Eglinton, L.B.; Stegeman, J.J. Cytochrome P4501A expression, chemical contaminants and histopathology in roach, goby and sturgeon and chemical contaminants in sediments from the Caspian Sea, Lake Balkhash and the Ily River Delta, Kazakhstan. Mar. Pollut. Bull. 2003, 46, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Song, Y.; Kai, J.; Yang, X.; Ji, P. Evaluation of EROD and CYP3A4 activities in earthworm Eisenia fetida as biomarkers for soil heavy metal contamination. J. Hazard. Mater. 2012, 243, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Elbekai, R.H.; El-Kadi, A.O.S. Modulation of aryl hydrocarbon receptor-regulated gene expression by arsenite, cadmium, and chromium. Toxicology 2004, 202, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chang, Z.; Bae, M.; Oh, S.M.; Chung, K.; Park, J. Molecular characterization of the aryl hydrocarbon receptor (AhR) pathway in goldfish (Carassius auratus) exposure to TCDD: The mRNA and protein levels. Fish Shellfish Immunol. 2013, 35, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Farmahin, R.; Crump, D.; Jones, S.; Mundy, L.; Kennedy, S. Cytochrome P4501A induction in primary cultures of embryonic European starling hepatocytes exposed to TCDD, PeCDF and TCDF. Ecotoxicology 2013, 22, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Sérée, E.; Villard, P.H.; Hevér, A.; Guigal, N.; Puyoou, F.; Charvet, B.; Point-Scomma, H.; Lechevalier, E.; Lacarelle, B.; Barra, Y.; et al. Modulation of MDR1 and CYP3A expression by dexamethasone: Evidence for an inverse regulation in adrenals. Biochem. Biophys. Res. Commun. 1998, 252, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.P.; Codd, G.A. Immunological and toxicological studies on Microcystis aeruginosa peptide toxin. Br. Phycol. J. 1987, 22, 301. [Google Scholar]
- Tabrez, S.; Ahmad, M. Cytochrome P450 system as potential biomarkers of certain toxicants: Comparison between plant and animal models. Environ. Monit. Assess. 2013, 185, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Cai, H.; Wang, J.; Liu, G. Detrimental effects of metronidazole on the liver of freshwater common carp (Cyprinus carpio L.). Bull. Environ. Contam. Toxicol. 2013, 91, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Dévier, M.; le Dû-Lacoste, M.; Akcha, F.; Morin, B.; Peluhet, L.; le Menach, K.; Burgeot, T.; Budzinski, H. Biliary PAH metabolites, EROD activity and DNA damage in dab (Limanda limanda) from Seine Estuary (France). Environ. Sci. Pollut. Res. Int. 2013, 20, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Farmahin, R.; Kennedy, S. Ethoxyresorufin-O-deethylase (EROD) induction by TCDD, PeCDF and PCB 126 in bobwhite quail hepatocytes. Ecotoxicology 2014, 23, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.W.; Lorenzen, A.; James, C.A.; Norstrom, R.J. Ethoxyresorufin-O-deethylase (EROD) and porphyriainduction in chicken embryo hepatocyte cultures—A new bioassay of PCB, PCDD, and related chemical contamination in wildlife. Chemosphere 1992, 25, 193–196. [Google Scholar] [CrossRef]
- Amara, I.E.A.; Anwar-Mohamed, A.; El-Kadi, A.O.S. Posttranslational mechanisms modulating the expression of the cytochrome P450 1A1 gene by methylmercury in HepG2 cells: A role of heme oxygenase-1. Toxicol. Lett. 2013, 219, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Brüschweiler, B.; Würgler, F.; Fent, K. Inhibitory effects of heavy metals on cytochrome P4501A induction in permanent fish hepatoma cells. Arch. Environ. Contam. Toxicol. 1996, 31, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.; Kim, D.; Iwasaki, M. Role of human cytochrome P450IIE1 in the oxidation of many low molecular weight cancer suspects. Chem. Res. Toxicol. 1991, 4, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Caradonna, F. Cytochrome P450 2E1 variable number tandem repeat polymorphisms and health risks: A genotype-phenotype study in cancers associated with drinking and/or smoking. Mol. Med. Rep. 2012, 6, 416–420. [Google Scholar] [PubMed]
- Koop, D. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992, 6, 724–730. [Google Scholar] [PubMed]
- Arinç, E.; Adali, O.; Gençler-Özkan, A.M. Induction of N-nitrosodimethylamine metabolism in liver and lung by in vivo pyridine treatments of rabbits. Arch. Toxicol. 2000, 74, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cederbaum, A. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol. Sci. 1996, 89, 515–523. [Google Scholar] [CrossRef]
- Westerink, W.M.A.; Schoonen, W.G.E.J. Cytochrome P450 enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol. In Vitro 2007, 21, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P-450 3A4: Regulation and role in drug metabolism. Annu. Rev. Pharmacol. 1999, 39, 1–17. [Google Scholar] [CrossRef]
- Lan, L.B.; Dalton, T.J.; Schuetz, E.G. Mdr1 limits CYP3A metabolism in vivo. Mol. Pharmacol. 2000, 58, 863–869. [Google Scholar] [PubMed]
- Elbekai, R.H.; El-kadi, A.O.S. The role of oxidative stress in the modulation of aryl hydrocarbon receptor-regulated genes by As3+, Cd3+, and Cr6+. Free Radic. Biol. Med. 2005, 39, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Galal, A.; Patrick, D.S. 21-Aminosteroids prevent the down-regulation of hepatic cytochrome P450 induced by hypoxia and inflammation in conscious rabbits. Br. J. Pharmacol. 1999, 128, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, M.E.; El-Kadi, A.O.S.; Brocks, D.R. The effect of β-naphthoflavone on the metabolism of amiodarone by hepatic and extra-hepatic microsomes. Toxicol. Lett. 2010, 195, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sinal, C.J.; Webb, C.D.; Bend, J.R. Differential in vivo effects of α-naphthoflavone and β-naphthoflavone on CYP1A1 and CYP2E1 in rat liver, lung, heart, and kidney. J. Biochem. Mol. Toxicol. 1999, 13, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Ito, A.; Sato, R. Evidence for biochemically different types of vesicles in the hepatic microsomal fraction. J. Biochem. 1966, 60, 417–428. [Google Scholar] [PubMed]
- Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 1953, 55, 416–421. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Liu, Y.; Li, X. Alteration in the Expression of Cytochrome P450s (CYP1A1, CYP2E1, and CYP3A11) in the Liver of Mouse Induced by Microcystin-LR. Toxins 2015, 7, 1102-1115. https://doi.org/10.3390/toxins7041102
Zhang B, Liu Y, Li X. Alteration in the Expression of Cytochrome P450s (CYP1A1, CYP2E1, and CYP3A11) in the Liver of Mouse Induced by Microcystin-LR. Toxins. 2015; 7(4):1102-1115. https://doi.org/10.3390/toxins7041102
Chicago/Turabian StyleZhang, Bangjun, Yang Liu, and Xiaoyu Li. 2015. "Alteration in the Expression of Cytochrome P450s (CYP1A1, CYP2E1, and CYP3A11) in the Liver of Mouse Induced by Microcystin-LR" Toxins 7, no. 4: 1102-1115. https://doi.org/10.3390/toxins7041102
APA StyleZhang, B., Liu, Y., & Li, X. (2015). Alteration in the Expression of Cytochrome P450s (CYP1A1, CYP2E1, and CYP3A11) in the Liver of Mouse Induced by Microcystin-LR. Toxins, 7(4), 1102-1115. https://doi.org/10.3390/toxins7041102





