Using S. cerevisiae as a Model System to Investigate V. cholerae VopX-Host Cell Protein Interactions and Phenotypes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Full Length VopX Sequences are Required for Growth Inhibition in Yeast
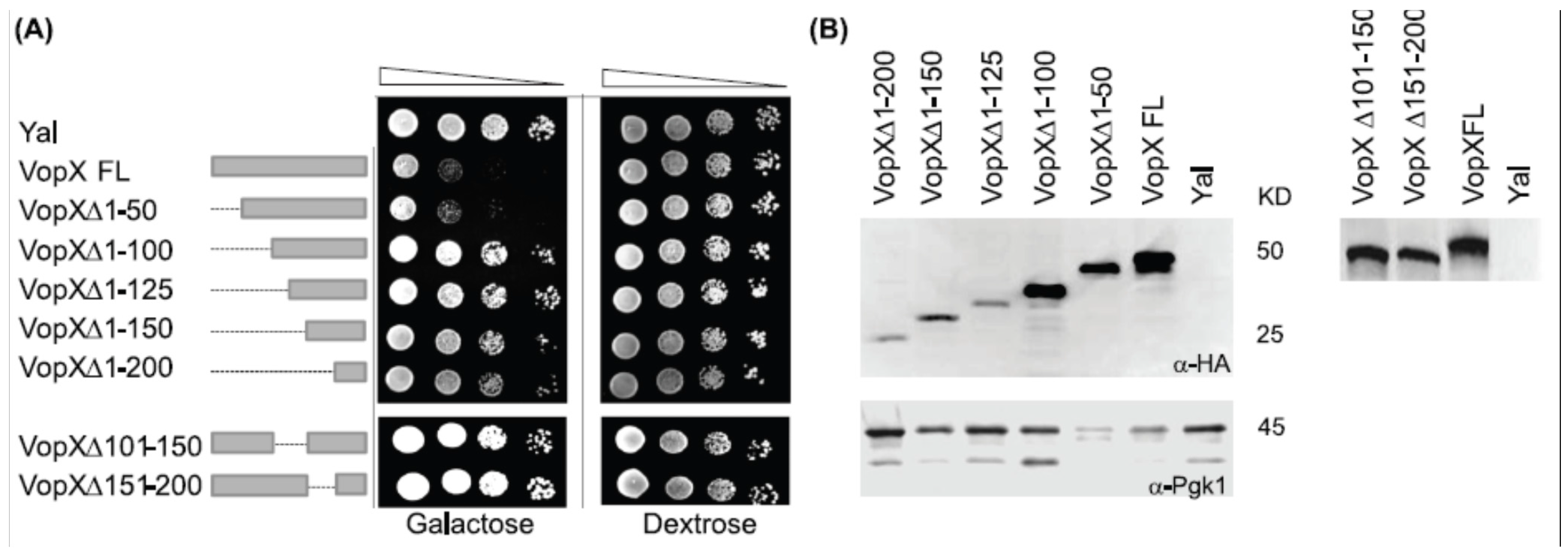
2.2. Temperature and Sorbitol Alter the VopX Induced Growth Defect

2.3. VopX Induces Rlm1 Responsive Element-Promoter Activity
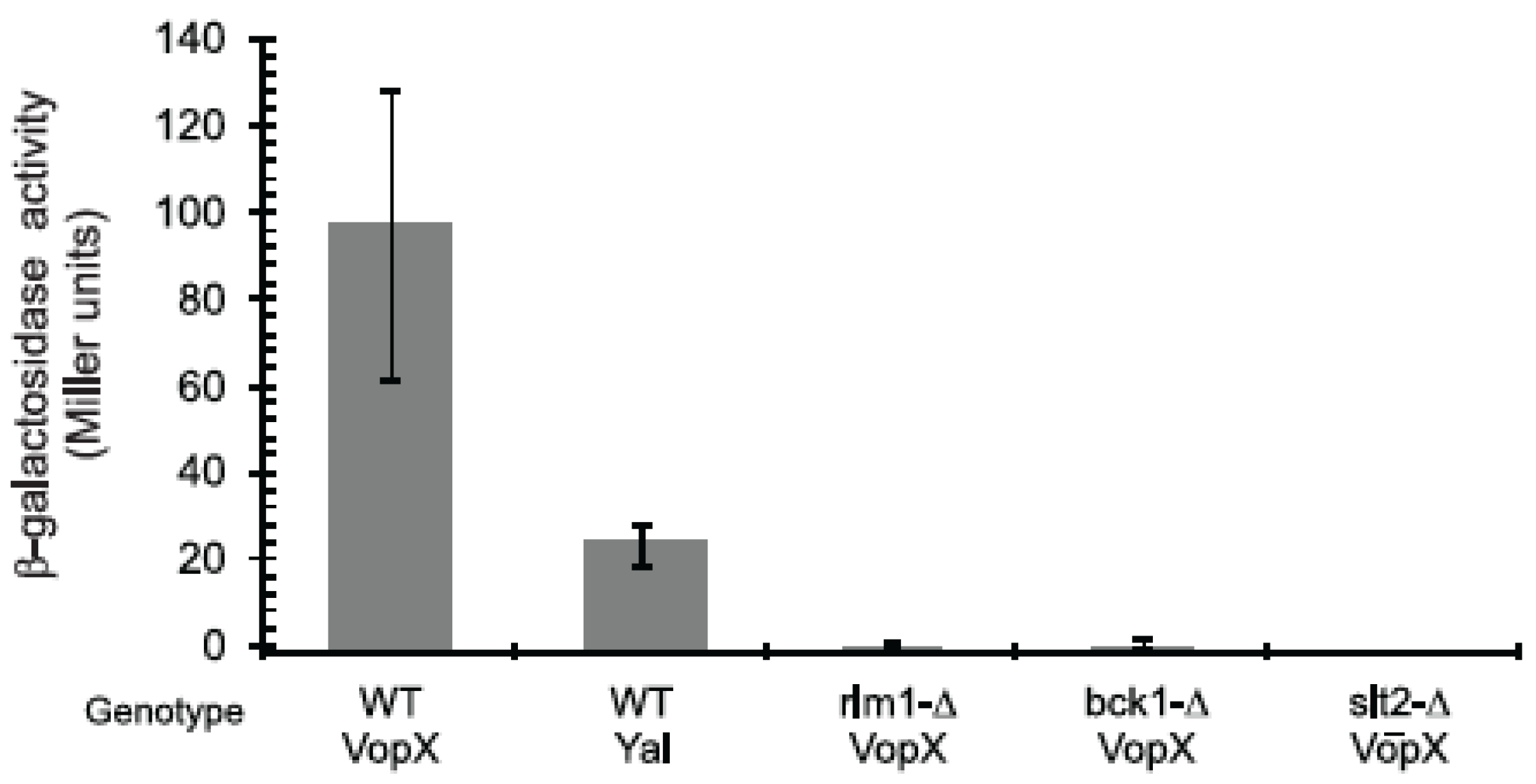
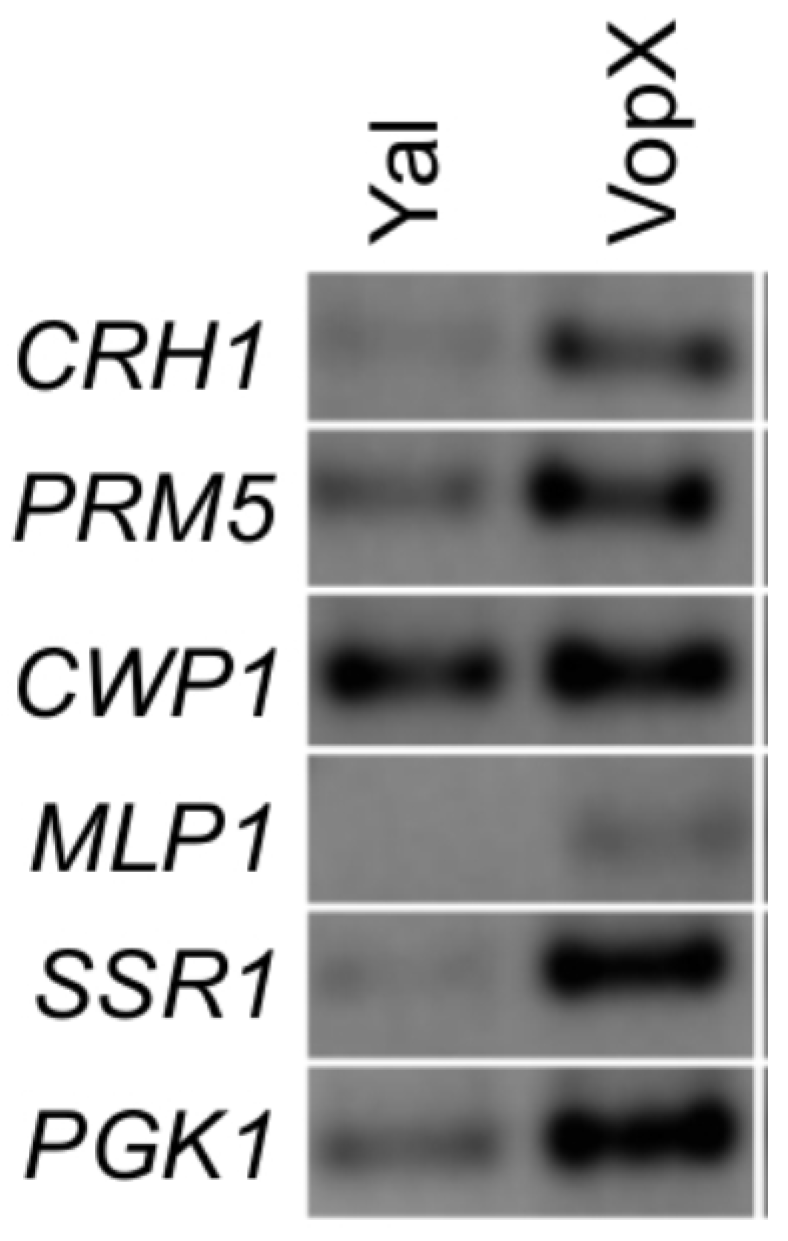
2.4. VopX Expression Activates Expression of Genes Downstream of RLM1
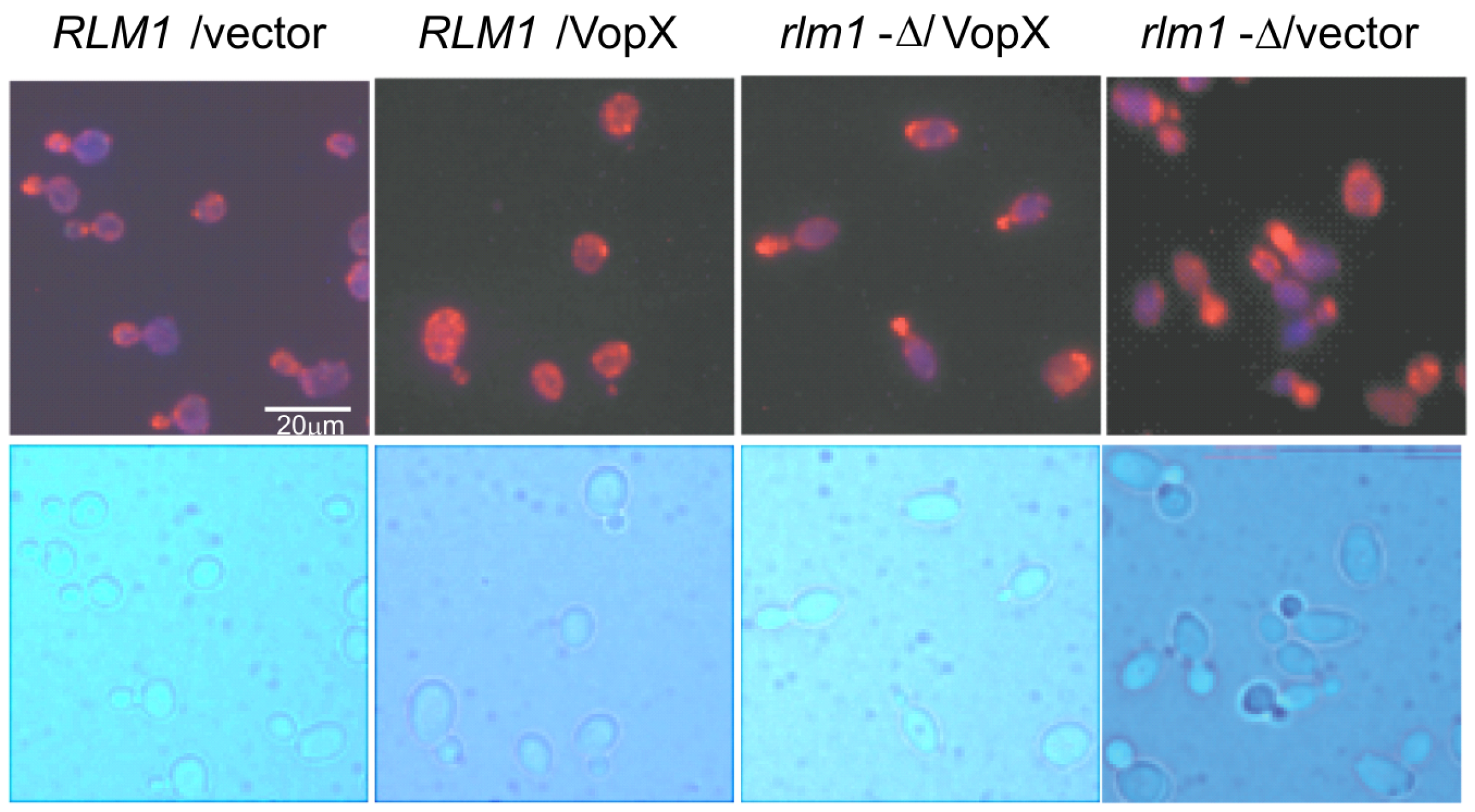
2.5. VopX Expression Alters Cell Cycle Progression

2.6. VopX Disrupts Actin Localization during the Budding Stage of the Cell Cycle
3. Experimental Section
3.1. Strains, Media, and Standard Techniques
3.2. Yeast Growth Inhibition Assay
3.3. pCHS02 Plasmid Construction
3.4. β-Galactosidase Assay
3.5. RT-PCR
3.6. Actin Staining
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sack, D.A.; Sack, R.B.; Nair, G.B.; Siddique, A.K. Cholera. Lancet 2004, 363, 223–233. [Google Scholar] [CrossRef]
- Chatterjee, S.N.; Chaudhuri, K. Lipopolysaccharides of Vibrio cholerae: I. Physical and chemical characterization. Biochim. Biophys. Acta 2003, 1639, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Faruque, S.M.; Chowdhury, N.; Kamruzzaman, M.; Dziejman, M.; Rahman, M.H.; Sack, D.A.; Nair, G.B.; Mekalanos, J.J. Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc. Natl. Acad. Sci. USA 2004, 101, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Faruque, S.M.; Albert, M.J.; Mekalanos, J.J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 1998, 62, 1301–1314. [Google Scholar] [PubMed]
- Singh, D.V.; Matte, M.H.; Matte, G.R.; Jiang, S.; Sabeena, F.; Shukla, B.N.; Sanyal, S.C.; Huq, A.; Colwell, R.R. Molecular analysis of Vibrio cholerae O1, O139, non-O1, and non-O139 strains: Clonal relationships between clinical and environmental isolates. Appl. Environ. Microbiol. 2001, 67, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Rivera, I.N.; Chun, J.; Huq, A.; Sack, R.B.; Colwell, R.R. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Appl. Environ. Microbiol. 2001, 67, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.J.; Choi, S.Y.; Grim, C.J.; Onifade, T.J.; Cinar, H.N.; Tall, B.D.; Taviani, E.; Hasan, N.A.; Abdullah, A.H.; Carter, L.; et al. Genomic and phenotypic characterization of Vibrio cholerae non-O1 isolates from a US Gulf Coast cholera outbreak. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Ghosh, K.; Raychoudhuri, A.; Chowdhury, G.; Bhattacharya, M.K.; Mukhopadhyay, A.K.; Ramamurthy, T.; Bhattacharya, S.K.; Klose, K.E.; Nandy, R.K.; et al. Incidence, virulence factors, and clonality among clinical strains of non-O1, non-O139 Vibrio cholerae isolates from hospitalized diarrheal patients in Kolkata, India. J. Clin. Microbiol. 2009, 47, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.C.; Serruto, D.; Dziejman, M.; Brieher, W.; Mekalanos, J.J. A type III secretion system in Vibrio cholerae translocates a formin/spire hybrid-like actin nucleator to promote intestinal colonization. Cell Host Microbe 2007, 1, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Shin, O.S.; Tam, V.C.; Suzuki, M.; Ritchie, J.M.; Bronson, R.T.; Waldor, M.K.; Mekalanos, J.J. Type III secretion is essential for the rapidly fatal diarrheal disease caused by non-O1, non-O139 Vibrio cholerae. MBIO 2011, 2, e00106-11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Johnson, J.A.; Pusch, G.D.; Morris, J.G., Jr.; Stine, O.C. The genome of non-O1 Vibrio cholerae NRT36S demonstrates the presence of pathogenic mechanisms that are distinct from those of O1 Vibrio cholerae. Infect. Immun. 2007, 75, 2645–2647. [Google Scholar] [CrossRef] [PubMed]
- Dziejman, M.; Serruto, D.; Tam, V.C.; Sturtevant, D.; Diraphat, P.; Faruque, S.M.; Rahman, M.H.; Heidelberg, J.F.; Decker, J.; Li, L.; et al. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc. Natl. Acad. Sci. USA 2005, 102, 3465–3470. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Miller, K.A.; Chaand, M.; Butler, J.S.; Dziejman, M. Identification of Vibrio cholerae type III secretion system effector proteins. Infect. Immun. 2011, 79, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, X.; Oswald, E. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J. Bacteriol. 2004, 186, 5486–5495. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.W.; Slagowski, N.L.; Eze, N.A.; Giddings, K.S.; Morrison, M.F.; Siggers, K.A.; Starnbach, M.N.; Lesser, C.F. Yeast functional genomic screens lead to identification of a role for a bacterial effector in innate immunity regulation. PLoS Pathog. 2007, 3. [Google Scholar] [CrossRef] [PubMed]
- Lesser, C.F.; Miller, S.I. Expression of microbial virulence proteins in Saccharomyces cerevisiae models mammalian infection. EMBO J. 2001, 20, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Siggers, K.A.; Lesser, C.F. The Yeast Saccharomyces cerevisiae: A versatile model system for the identification and characterization of bacterial virulence proteins. Cell Host Microbe 2008, 4, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Slagowski, N.L.; Kramer, R.W.; Morrison, M.F.; LaBaer, J.; Lesser, C.F. A functional genomic yeast screen to identify pathogenic bacterial proteins. PLoS Pathog. 2008, 4. [Google Scholar] [CrossRef] [PubMed]
- Curak, J.; Rohde, J.; Stagljar, I. Yeast as a tool to study bacterial effectors. Curr. Opin. Microbiol. 2009, 12, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Stirling, F.R.; Evans, T.J. Effects of the type III secreted pseudomonal toxin ExoS in the yeast Saccharomyces cerevisiae. Microbiology 2006, 152, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Sisko, J.L.; Spaeth, K.; Kumar, Y.; Valdivia, R.H. Multifunctional analysis of Chlamydia-specific genes in a yeast expression system. Mol. Microbiol. 2006, 60, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.E.; Thorner, J. Function and regulation in MAPK signaling pathways: Lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 2007, 1773, 1311–1340. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Elion, E.A. MAP kinase pathways. J. Cell Sci. 2005, 118, 3569–3572. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.L.; Sharrow, S.O.; Gart, J.J. Cell cycle of Saccharomycescerevisiae in populations growing at different rates. Proc. Natl. Acad. Sci. USA 1977, 74, 3850–3854. [Google Scholar] [CrossRef] [PubMed]
- De Lucena, R.M.; Elsztein, C.; Simões, D.A.; de Morais, M.A., Jr. Participation of CWI, HOG and Calcineurin pathways in the tolerance of Saccharomyces cerevisiae to low pH by inorganic acid. J. Appl. Microbiol. 2012, 113, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, M.; Peterfreund, G.L.; Tsou, A.M.; Selamoglu, N.; Daldal, F.; Zhong, Z.; Kan, B.; Zhu, J. Vibrio cholerae anaerobic induction of virulence gene expression is controlled by thiol-based switches of virulence regulator AphB. Proc. Natl. Acad. Sci. USA 2011, 108, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.B.; Mylonakis, E. Our paths might cross: The role of the fungal cell wall integrity pathway in stress response and cross talk with other stress response pathways. Eukaryot. Cell 2009, 8, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Gelperin, D.M.; White, M.A.; Wilkinson, M.L.; Kon, Y.; Kung, L.A.; Wise, K.J.; Lopez-Hoyo, N.; Jiang, L.; Piccirillo, S.; Yu, H.; et al. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev. 2005, 19, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Shen, X.; Yan, G.; Ma, D.; Bai, X.; Li, S.; Jiang, Y. A MAP kinase dependent feedback mechanism controls Rho1 GTPase and actin distribution in yeast. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.S.; Sobering, A.K.; Romeo, M.J.; Levin, D.E. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 2002, 46, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.S.; Levin, D.E. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 1999, 34, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Ohya, Y. The cell wall integrity checkpoint: Coordination between cell wall synthesis and the cell cycle. Yeast 2010, 27, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed]
- Smethurst, D.G.; Dawes, I.W.; Gourlay, C.W. Actin—A biosensor that determines cell fate in yeasts. FEMS Yeast Res. 2014, 14, 89–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Alam, A.; Tam, V.; Hamilton, E.; Dziejman, M. vttRA and vttRB Encode ToxR family proteins that mediate bile-induced expression of type three secretion system genes in a non-O1/non-O139 Vibrio cholerae strain. Infect. Immun. 2010, 78, 2554–2570. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seward, C.H.; Manzella, A.; Alam, A.; Butler, J.S.; Dziejman, M. Using S. cerevisiae as a Model System to Investigate V. cholerae VopX-Host Cell Protein Interactions and Phenotypes. Toxins 2015, 7, 4099-4110. https://doi.org/10.3390/toxins7104099
Seward CH, Manzella A, Alam A, Butler JS, Dziejman M. Using S. cerevisiae as a Model System to Investigate V. cholerae VopX-Host Cell Protein Interactions and Phenotypes. Toxins. 2015; 7(10):4099-4110. https://doi.org/10.3390/toxins7104099
Chicago/Turabian StyleSeward, Christopher H., Alexander Manzella, Ashfaqul Alam, J. Scott Butler, and Michelle Dziejman. 2015. "Using S. cerevisiae as a Model System to Investigate V. cholerae VopX-Host Cell Protein Interactions and Phenotypes" Toxins 7, no. 10: 4099-4110. https://doi.org/10.3390/toxins7104099
APA StyleSeward, C. H., Manzella, A., Alam, A., Butler, J. S., & Dziejman, M. (2015). Using S. cerevisiae as a Model System to Investigate V. cholerae VopX-Host Cell Protein Interactions and Phenotypes. Toxins, 7(10), 4099-4110. https://doi.org/10.3390/toxins7104099




