Passive Immunotherapy Protects against Enteric Invasion and Lethal Sepsis in a Murine Model of Gastrointestinal Anthrax
Abstract
:1. Introduction
2. Results
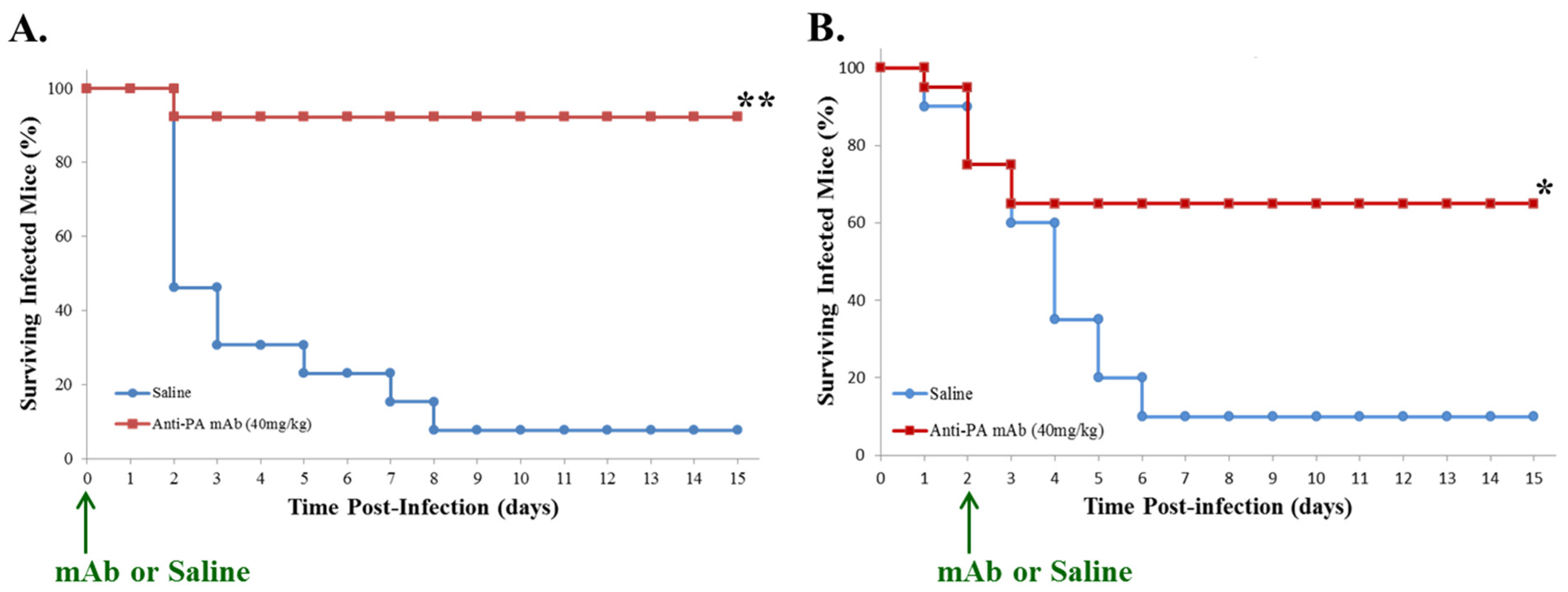
2.1. Post-Exposure Administration of Anti-PA mAb Prevents Lethality in Mice with GI Anthrax
2.2. Anti-PA mAb Treatment Blocks Intestinal Pathology Associated with GI Anthrax in Mice


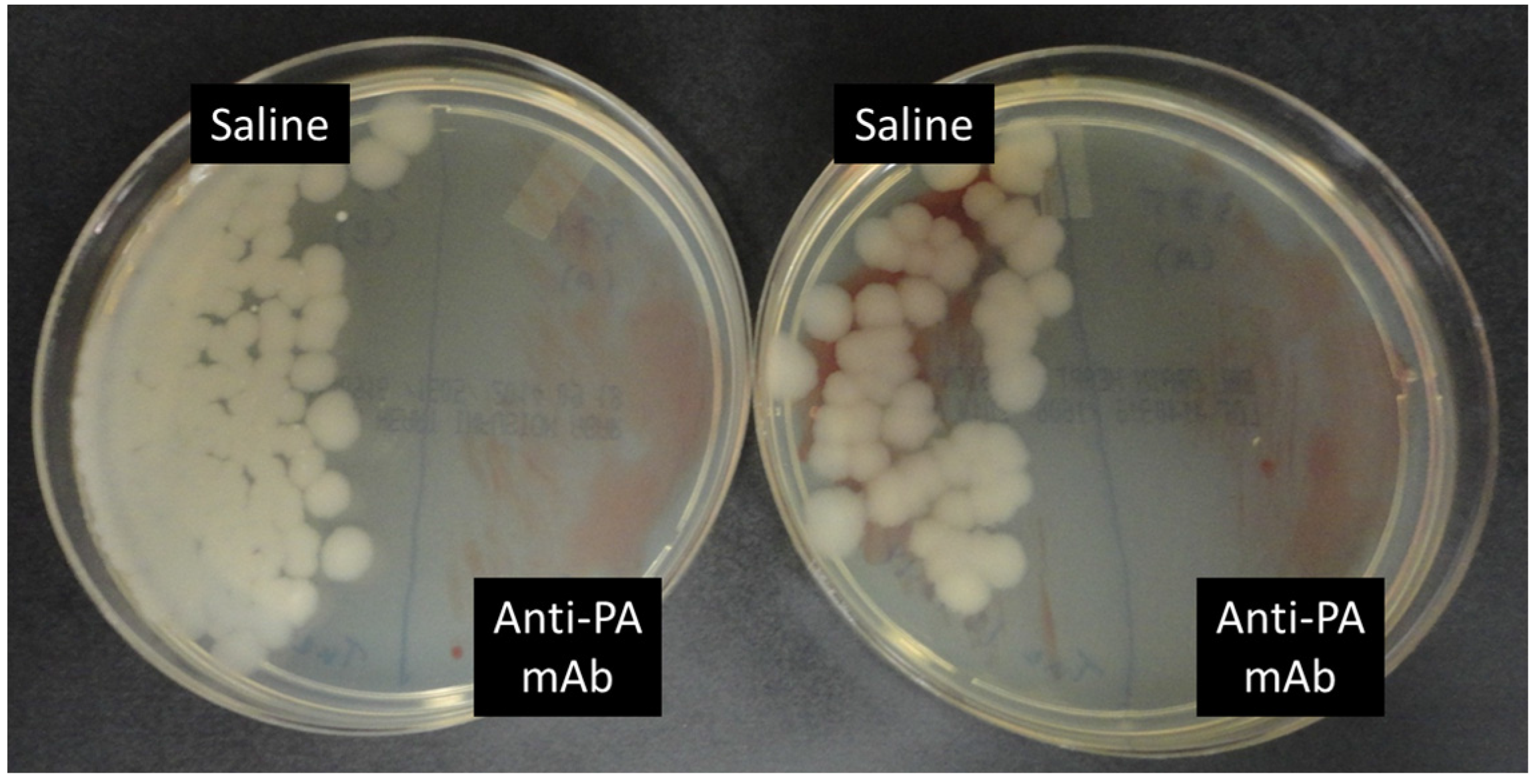
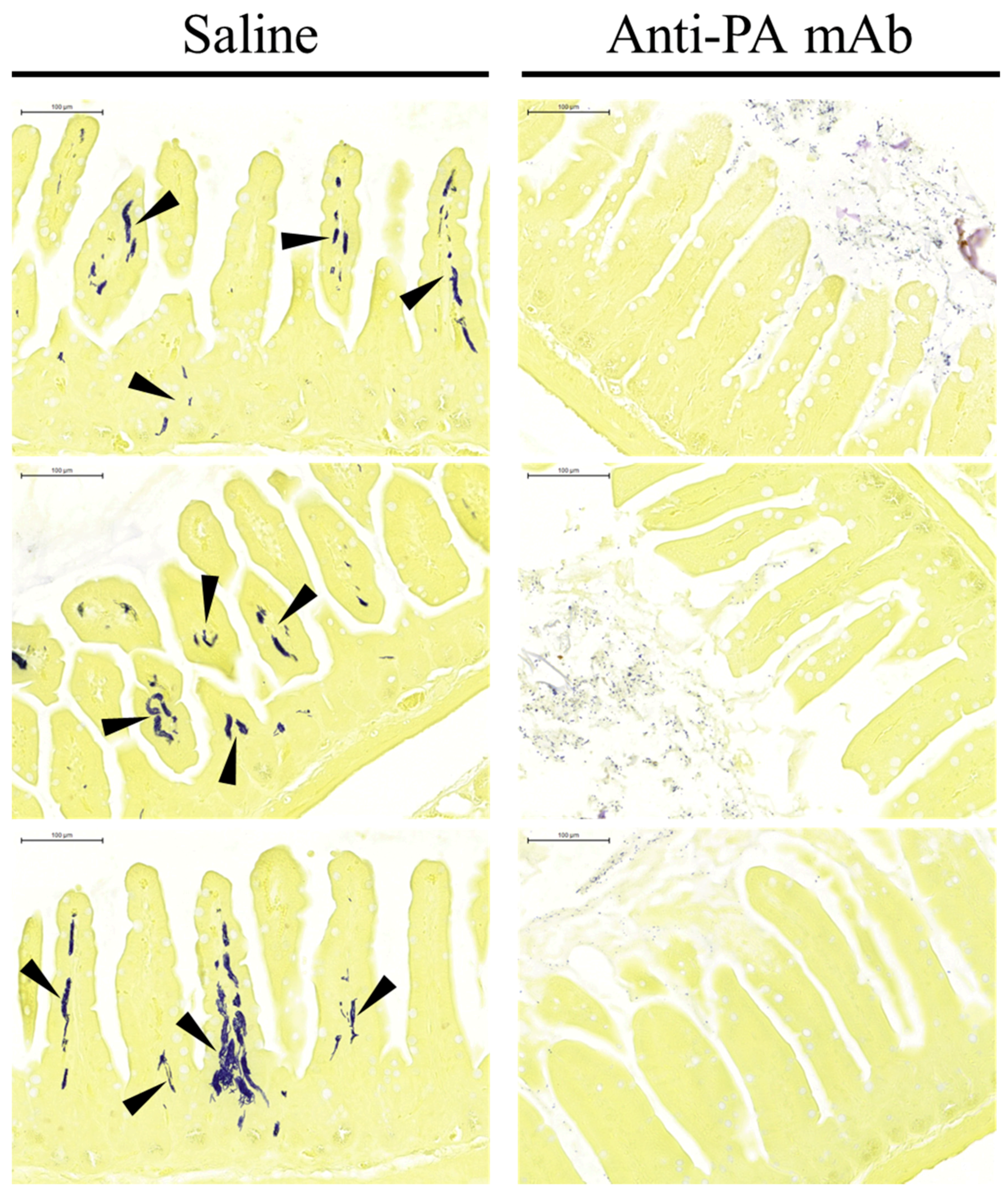
2.3. Anti-PA mAb Blocks the Penetration of B. anthracis into the Gut Epithelium
| Pathological findings | Saline | Anti-anthrax mAb |
|---|---|---|
| Intra-mucosal invasion | 5/5 | 0/5 |
| Congestion | 5/5 | 0/5 |
| Villous edema | 5/5 | 0/5 |
| Lymphocytolysis | 4/5 | 0/5 |
| Mucosal erosion/ulceration | 3/5 | 0/5 |
| Enteric mucosal atrophy | 2/5 | 0/5 |
3. Discussion
4. Experimental Section
4.1. Ethics Statement
4.2. Gastrointestinal Challenge with Bacillus Anthracis Sterne Strain
4.3. Therapeutic Agent
4.4. Murine Survival Study
4.5. Paired Treatment Analysis
4.6. Pathology Assessment
4.7. Statistical Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- Brachman, P.S. Bioterrorism: An update with a focus on anthrax. Am. J. Epidemiol. 2002, 155, 981–987. [Google Scholar] [CrossRef]
- Jernigan, D.B.; Raghunathan, P.L.; Bell, B.P.; Brechner, R.; Bresnitz, E.A.; Butler, J.C.; Cetron, M.; Cohen, M.; Doyle, T.; Fischer, M.; et al. Investigation of bioterrorism-related anthrax, United States, 2001: Epidemiologic findings. Emerg. Infect. Dis. 2002, 8, 1019–1028. [Google Scholar] [CrossRef]
- Bush, L.M.; Abrams, B.H.; Beall, A.; Johnson, C.C. Index case of fatal inhalational anthrax due to bioterrorism in the United States. N. Engl. J. Med. 2001, 345, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Loving, C.L.; Kennett, M.; Lee, G.M.; Grippe, V.K.; Merkel, T.J. Murine aerosol challenge model of anthrax. Infect. Immun. 2007, 75, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.; Barnewall, R.; Babin, M.; Hunt, R.; Estep, J.; Nielsen, C.; Carnes, R.; Carney, J. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab. Investig. 2003, 83, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.R.; Lovchik, J.; Hutt, J.; Lipscomb, M.F.; Wang, E.; Heninger, S.; Berliba, L.; Garrison, K. Murine model of pulmonary anthrax: Kinetics of dissemination, histopathology, and mouse strain susceptibility. Infect. Immun. 2004, 72, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.; Turnbull, P.C. Anthrax in animals. Mol. Aspects Med. 2009, 30, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Fasanella, A.; Galante, D.; Garofolo, G.; Jones, M.H. Anthrax undervalued zoonosis. Vet. Microbiol. 2010, 140, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Shields, M.J.; Hahn, K.R.; Janzen, T.W.; Goji, N.; Thomas, M.C.; Kingombe, C.B.; Paquet, C.; Kell, A.J.; Amoako, K.K. Immunomagnetic capture of Bacillus anthracis spores from food. J. Food Prot. 2012, 75, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.C.; Kornacki, J.L. Bacillus anthracis: Current knowledge in relation to contamination of food. J. Food Prot. 2003, 66, 691–699. [Google Scholar] [PubMed]
- Perdue, M.L.; Karns, J.; Higgins, J.; van Kessel, J.A. Detection and fate of Bacillus anthracis (Sterne) vegetative cells and spores added to bulk tank milk. J. Food Prot. 2003, 66, 2349–2354. [Google Scholar] [PubMed]
- Novak, J.S.; Call, J.; Tomasula, P.; Luchansky, J.B. An assessment of pasteurization treatment of water, media, and milk with respect to Bacillus spores. J. Food Prot. 2005, 68, 751–757. [Google Scholar] [PubMed]
- Xu, S.; Labuza, T.P.; Diez-Gonzalez, F. Thermal inactivation of Bacillus anthracis spores in cow’s milk. Appl. Environ. Microbiol. 2006, 72, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Sun, C.; Uslu, K.; Auth, R.D.; Fang, H.; Ouyang, W.; Frucht, D.M. A New Murine Model for Gastrointestinal Anthrax Infection. PLoS ONE 2013, 8, e66943. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; Manchester, M. Anti-toxin antibodies in prophylaxis and treatment of inhalation anthrax. Future Microbiol. 2009, 4, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.M.; Welkos, S.L.; Pitt, M.L.; Ezzell, J.W.; Worsham, P.L.; Rose, K.J.; Ivins, B.E.; Lowe, J.R.; Howe, G.B.; Mikesell, P.; et al. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 1993, 167, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Fang, H.; Xie, T.; Auth, R.D.; Patel, N.; Murray, P.R.; Snoy, P.J.; Frucht, D.M. Anthrax lethal toxin disrupts intestinal barrier function and causes systemic infections with enteric bacteria. PLoS ONE 2012, 7, e33583. [Google Scholar] [CrossRef] [PubMed]
- Comer, J.E.; Chopra, A.K.; Peterson, J.W.; Konig, R. Direct inhibition of T-lymphocyte activation by anthrax toxins in vivo. Infect. Immun. 2005, 73, 8275–8281. [Google Scholar] [CrossRef] [PubMed]
- Dang, O.; Navarro, L.; Anderson, K.; David, M. Cutting edge: Anthrax lethal toxin inhibits activation of IFN-regulatory factor 3 by lipopolysaccharide. J. Immunol. 2004, 172, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Lingappa, J.; Leppla, S.H.; Agrawal, S.; Jabbar, A.; Quinn, C.; Pulendran, B. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 2003, 424, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Duesbery, N.S.; Webb, C.P.; Leppla, S.H.; Gordon, V.M.; Klimpel, K.R.; Copeland, T.D.; Ahn, N.G.; Oskarsson, M.K.; Fukasawa, K.; Paull, K.D.; et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 1998, 280, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Leppla, S.H. Anthrax toxin edema factor: A bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 1982, 79, 3162–3166. [Google Scholar] [CrossRef] [PubMed]
- Paccani, S.R.; Tonello, F.; Ghittoni, R.; Natale, M.; Muraro, L.; D’Elios, M.M.; Tang, W.J.; Montecucco, C.; Baldari, C.T. Anthrax toxins suppress T lymphocyte activation by disrupting antigen receptor signaling. J. Exp. Med. 2005, 201, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, M.; Haines, D.; Young, H.A.; Leppla, S.H. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J. Clin. Investig. 2003, 112, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Xu, L.; Chen, T.Y.; Cyr, J.M.; Frucht, D.M. Anthrax lethal toxin has direct and potent inhibitory effects on B cell proliferation and immunoglobulin production. J. Immunol. 2006, 176, 6155–6161. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Cordoba-Rodriguez, R.; Lankford, C.S.; Frucht, D.M. Anthrax lethal toxin blocks MAPK kinase-dependent IL-2 production in CD4+ T cells. J. Immunol. 2005, 174, 4966–4971. [Google Scholar] [CrossRef] [PubMed]
- Froude, J.W., 2nd; Thullier, P.; Pelat, T. Antibodies against anthrax: mechanisms of action and clinical applications. Toxins 2011, 3, 1433–1452. [Google Scholar] [CrossRef] [PubMed]
- Young, J.A.; Collier, R.J. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 2007, 76, 243–265. [Google Scholar] [PubMed]
- Bradley, K.A.; Mogridge, J.; Mourez, M.; Collier, R.J.; Young, J.A. Identification of the cellular receptor for anthrax toxin. Nature 2001, 414, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Scobie, H.M.; Rainey, G.J.; Bradley, K.A.; Young, J.A. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 5170–5174. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S. Raxibacumab. MAbs 2009, 1, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Kummerfeldt, C.E. Raxibacumab: potential role in the treatment of inhalational anthrax. Infect. Drug Resist. 2014, 7, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Moayeri, M.; Purcell, R. Monoclonal antibody therapies against anthrax. Toxins 2011, 3, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Migone, T.S.; Subramanian, G.M.; Zhong, J.; Healey, L.M.; Corey, A.; Devalaraja, M.; Lo, L.; Ullrich, S.; Zimmerman, J.; Chen, A.; et al. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 2009, 361, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Petosa, C.; Collier, R.J.; Klimpel, K.R.; Leppla, S.H.; Liddington, R.C. Crystal structure of the anthrax toxin protective antigen. Nature 1997, 385, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Corey, A.; Migone, T.S.; Bolmer, S.; Fiscella, M.; Ward, C.; Chen, C.; Meister, G. Bacillus anthracis protective antigen kinetics in inhalation spore-challenged untreated or Levofloxacin/Raxibacumab-treated New Zealand white rabbits. Toxins 2013, 5, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Migone, T.S.; Bolmer, S.; Zhong, J.; Corey, A.; Vasconcelos, D.; Buccellato, M.; Meister, G. Added benefit of raxibacumab to antibiotic treatment of inhalational anthrax. Antimicrob. Agents Chemother. 2015, 59, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Sun, C.; Xu, L.; Owen, R.J.; Auth, R.D.; Snoy, P.J.; Frucht, D.M. Neutrophil elastase mediates pathogenic effects of anthrax lethal toxin in the murine intestinal tract. J. Immunol. 2010, 185, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Sternbach, G. The history of anthrax. J. Emerg. Med. 2003, 24, 463–467. [Google Scholar] [CrossRef]
- Schwartz, M. Dr. Jekyll and Mr. Hyde: A short history of anthrax. Mol. Aspects Med. 2009, 30, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Barras, V.; Greub, G. History of biological warfare and bioterrorism. Clin. Microbiol. Infect. 2014, 20, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Mwenye, K.S.; Siziya, S.; Peterson, D. Factors associated with human anthrax outbreak in the Chikupo and Ngandu villages of Murewa district in Mashonaland East Province, Zimbabwe. Cent. Afr. J. Med. 1996, 42, 312–315. [Google Scholar] [PubMed]
- Siamudaala, V.M.; Bwalya, J.M.; Munang’andu, H.M.; Sinyangwe, P.G.; Banda, F.; Mweene, A.S.; Takada, A.; Kida, H. Ecology and epidemiology of anthrax in cattle and humans in Zambia. Jpn. J. Vet. Res. 2006, 54, 15–23. [Google Scholar] [PubMed]
- Owen, J.L.; Yang, T.; Mohamadzadeh, M. New insights into gastrointestinal anthrax infection. Trends Mol. Med. 2015, 21, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Sirisanthana, T.; Nelson, K.E.; Ezzell, J.W.; Abshire, T.G. Serological studies of patients with cutaneous and oral-oropharyngeal anthrax from northern Thailand. Am. J. Trop. Med. Hyg. 1988, 39, 575–581. [Google Scholar] [PubMed]
- Beatty, M.E.; Ashford, D.A.; Griffin, P.M.; Tauxe, R.V.; Sobel, J. Gastrointestinal anthrax: Review of the literature. Arch. Intern. Med. 2003, 163, 2527–2531. [Google Scholar] [CrossRef] [PubMed]
- Ndiva Mongoh, M.; Dyer, N.W.; Stoltenow, C.L.; Hearne, R.; Khaitsa, M.L. A review of management practices for the control of anthrax in animals: The 2005 anthrax epizootic in North Dakota—Case study. Zoonoses Public Health 2008, 55, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kassenborg, H.; Danila, R.; Snippes, P.; Wiisanen, M.; Sullivan, M.; Smith, K.E.; Crouch, N.; Medus, C.; Weber, R.; Korlath, J.; et al. From the Centers for Disease Control and Prevention. Human ingestion of Bacillus anthracis-contaminated meat—Minnesota, August 2000. JAMA 2000, 284, 1644–1646. [Google Scholar]
- Shireley, L.; Dwelle, T.; Streitz, D.; Schuler, L. From the Centers for Disease Control and Prevention. Human anthrax associated with an epizootic among livestock—North Dakota, 2000. JAMA 2001, 286, 1307–1308. [Google Scholar]
- Torok, T.J.; Tauxe, R.V.; Wise, R.P.; Livengood, J.R.; Sokolow, R.; Mauvais, S.; Birkness, K.A.; Skeels, M.R.; Horan, J.M.; Foster, L.R. A large community outbreak of salmonellosis caused by intentional contamination of restaurant salad bars. JAMA 1997, 278, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.B. Historical trends related to bioterrorism: An empirical analysis. Emerg. Infect. Dis. 1999, 5, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Inglesby, T.V.; O’Toole, T.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Friedlander, A.M.; Gerberding, J.; Hauer, J.; Hughes, J.; et al. Anthrax as a biological weapon, 2002: Updated recommendations for management. JAMA 2002, 287, 2236–2252. [Google Scholar] [CrossRef] [PubMed]
- Dixon, T.C.; Meselson, M.; Guillemin, J.; Hanna, P.C. Anthrax. N. Engl. J. Med. 1999, 341, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, K.A.; Wright, M.E.; Shadomy, S.V.; Bradley, J.S.; Morrow, M.G.; Pavia, A.T.; Rubinstein, E.; Holty, J.E.; Messonnier, N.E.; Smith, T.L.; et al. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg. Infect. Dis. 2014, 20, e130687. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, Y.L.; Yang, T.; Sahay, B.; Zadeh, M.; Cheng, S.X.; Wang, G.P.; Owen, J.L.; Mohamadzadeh, M. Colonic immune suppression, barrier dysfunction, and dysbiosis by gastrointestinal Bacillus anthracis infection. PLoS ONE 2014, 9, e100532. [Google Scholar] [CrossRef] [PubMed]
- Rossi Paccani, S.; Benagiano, M.; Capitani, N.; Zornetta, I.; Ladant, D.; Montecucco, C.; D’Elios, M.M.; Baldari, C.T. The adenylate cyclase toxins of Bacillus anthracis and Bordetella pertussis promote Th2 cell development by shaping T cell antigen receptor signaling. PLoS Pathog. 2009, 5, e1000325. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Frucht, D.M. Bacillus anthracis: A multi-faceted role for anthrax lethal toxin in thwarting host immune defenses. Int. J. Biochem. Cell Biol. 2007, 39, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Ginzberg, H.H.; Cherapanov, V.; Dong, Q.; Cantin, A.; McCulloch, C.A.; Shannon, P.T.; Downey, G.P. Neutrophil-mediated epithelial injury during transmigration: Role of elastase. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G705–G717. [Google Scholar] [PubMed]
- Xie, T.; Auth, R.D.; Frucht, D.M. The effects of anthrax lethal toxin on host barrier function. Toxins 2011, 3, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Noack, D.; Wood, M.; Perego, M.; Knaus, U.G. Lung epithelial injury by B. anthracis lethal toxin is caused by MKK-dependent loss of cytoskeletal integrity. PLoS ONE 2009, 4, e4755. [Google Scholar] [CrossRef] [PubMed]
- D’Agnillo, F.; Williams, M.C.; Moayeri, M.; Warfel, J.M. Anthrax lethal toxin downregulates claudin-5 expression in human endothelial tight junctions. PLoS ONE 2013, 8, e62576. [Google Scholar] [CrossRef] [PubMed]
- Warfel, J.M.; D’Agnillo, F. Anthrax lethal toxin-mediated disruption of endothelial VE-cadherin is attenuated by inhibition of the Rho-associated kinase pathway. Toxins 2011, 3, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Xie, T.; Rotstein, D.; Fang, H.; Frucht, D.M. Passive Immunotherapy Protects against Enteric Invasion and Lethal Sepsis in a Murine Model of Gastrointestinal Anthrax. Toxins 2015, 7, 3960-3976. https://doi.org/10.3390/toxins7103960
Huang B, Xie T, Rotstein D, Fang H, Frucht DM. Passive Immunotherapy Protects against Enteric Invasion and Lethal Sepsis in a Murine Model of Gastrointestinal Anthrax. Toxins. 2015; 7(10):3960-3976. https://doi.org/10.3390/toxins7103960
Chicago/Turabian StyleHuang, Bruce, Tao Xie, David Rotstein, Hui Fang, and David M. Frucht. 2015. "Passive Immunotherapy Protects against Enteric Invasion and Lethal Sepsis in a Murine Model of Gastrointestinal Anthrax" Toxins 7, no. 10: 3960-3976. https://doi.org/10.3390/toxins7103960
APA StyleHuang, B., Xie, T., Rotstein, D., Fang, H., & Frucht, D. M. (2015). Passive Immunotherapy Protects against Enteric Invasion and Lethal Sepsis in a Murine Model of Gastrointestinal Anthrax. Toxins, 7(10), 3960-3976. https://doi.org/10.3390/toxins7103960





