Inheritance Patterns, Dominance and Cross-Resistance of Cry1Ab- and Cry1Ac-Selected Ostrinia furnacalis (Guenée)
Abstract
:1. Introduction
2. Results
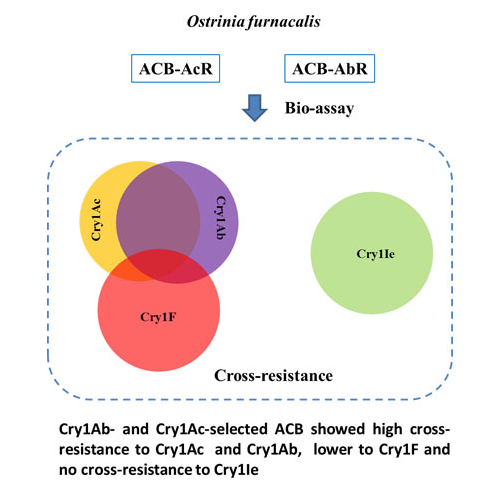
2.1. Levels of Cross-Resistance
| Bt toxins | Colony | LC50 (95%FL) μg/g | Resistance ratio RR50 | χ2 | Slope ± SE |
|---|---|---|---|---|---|
| Cry1Ab | ACB-BtS | 0.12 (0.05–0.21) | - | 8.19 | 1.05 ± 0.11 |
| ACB-AbR | 4.73 (2.67–8.07) | 39.42 | 19.91 | 1.0 ± 0.06 | |
| F1 (S×R) | 1.40 (0.86–2.36) | 11.67 | 6.78 | 0.85 ± 0.08 | |
| F1 (R×S) | 2.01 (1.25–3.34) | 16.75 | 2.19 | 0.77 ± 0.07 | |
| Cry1Ac | ACB-BtS | 0.10 (0.07–0.15) | - | 8.44 | 1.05 ± 0.06 |
| ACB-AbR | 11.34 (6.88–23.77) | 113.40 | 3.34 | 0.86 ± 0.24 | |
| F1 (S×R) | 1.29 (0.88–1.89) | 12.90 | 9.03 | 0.89 ± 0.08 | |
| F1 (R×S) | 1.82 (1.12–3.05) | 18.20 | 5.52 | 0.82 ± 0.07 | |
| Cry1F | ACB-BtS | 0.64 (0.46–1.05) | - | 3.60 | 0.90 ± 0.09 |
| ACB-AbR | 31.20 (17.98–114.40) | 48.75 | 12.10 | 0.88 ± 0.19 | |
| F1 (S×R) | 1.99 (1.24–3.31) | 3.11 | 5.78 | 0.81 ± 0.07 | |
| F1 (R×S) | 2.97 (1.87–4.95) | 4.64 | 4.08 | 0.70 ± 0.07 | |
| Cry1Ie | ACB-BtS | 1.36 (0.54–2.83) | - | 15.77 | 1.80 ± 0.13 |
| ACB-AbR | 1.95 (1.23–3.14) | 1.60 | 12.20 | 1.08 ± 0.10 | |
| F1 (S×R) | 1.00 (0.58–1.76) | 0.74 | 7.09 | 0.90 ± 0.08 | |
| F1 (R×S) | 1.10 (0.74–1.64) | 0.81 | 3.50 | 0.87 ± 0.08 |
| Bt Toxins | Colony | LC50 (95% FL) μg/g | Resistance Ratio RR50 | χ2 | Slope ± SE |
|---|---|---|---|---|---|
| Cry1Ab | ACB-BtS | 0.12 (0.05–0.21) | - | 8.19 | 1.05 ± 0.11 |
| ACB-AcR | 1.17 (0.65–1.96) | 9.75 | 18.85 | 0.95 ± 0.06 | |
| S×R | 1.20 (0.82–1.74) | 10.00 | 4.70 | 0.94 ± 0.08 | |
| R×S | 1.51 (1.03–2.23) | 12.58 | 3.16 | 0.91 ± 0.08 | |
| Cry1Ac | ACB-BtS | 0.10 (0.07–0.15) | - | 8.44 | 1.05 ± 0.06 |
| ACB-AcR | 7.88 (3.59–18.61) | 78.80 | 28.94 | 0.79 ± 0.06 | |
| S×R | 1.28 (0.89–1.84) | 12.80 | 4.89 | 1.99 ± 0.08 | |
| R×S | 2.63 (1.71–4.19) | 26.30 | 3.11 | 0.76 ± 0.07 | |
| Cry1F | ACB-BtS | 0.64 (0.46–1.05) | - | 3.60 | 0.90 ± 0.09 |
| ACB-AcR | 11.61 (6.70–23.28) | 18.14 | 8.54 | 0.61 ± 0.05 | |
| S×R | 2.70 (1.57–5.09) | 4.22 | 6.72 | 0.84 ± 0.08 | |
| R×S | 4.76 (2.45–10.70) | 7.44 | 8.39 | 0.75 ± 0.07 | |
| Cry1Ie | ACB-BtS | 1.36 (0.54–2.83) | - | 15.77 | 1.8 ± 0.13 |
| ACB-AcR | 1.88 (0.98–3.42) | 1.38 | 23.60 | 0.96 ± 0.06 | |
| S×R | 1.34 (0.73–2.44) | 0.99 | 9.80 | 0.98 ± 0.08 | |
| R×S | 1.73 (1.11–2.77) | 1.27 | 5.96 | 0.92 ± 0.08 |
2.2. Estimation of Dominance
| Toxin | Concentration (µg/g) | h value (Crosses) | |||
|---|---|---|---|---|---|
| ACB-AbRS | ACB-SAbR | ACB-AcRS | ACB-SAcR | ||
| Cry1Ab | 0.10 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1.00 | 0.90 | 0.78 | 1.00 | 1.00 | |
| 10.00 | 0.44 | 0.35 | 0.80 | 0.74 | |
| Cry1Ac | 0.10 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1.00 | 0.98 | 0.79 | 0.83 | 0.67 | |
| 10.00 | 0.52 | 0.20 | 0.43 | 0.20 | |
| Cry1F | 0.10 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1.00 | 0.90 | 0.80 | 0.70 | 1.00 | |
| 10.00 | 0.60 | 0.40 | 0.40 | 0.50 | |
| 100.00 | 0.30 | 0.10 | 0.15 | 0.06 | |
| Toxins | Colony | LC50 (95% FL) µg/g | Resistance ratio | χ2 | Slope ± SE |
|---|---|---|---|---|---|
| Cry1Ab | ACB-AbR | 4.73 (2.67–8.07) | 39.42 | 19.91 | 1.00 ± 0.06 |
| ACB-AcR | 1.17 (0.65–1.96) | 9.75 | 18.85 | 0.95 ± 0.06 | |
| ACB-AbcR | 2.85 (1.96–4.01) | 23.75 | 11.87 | 1.30 ± 0.08 | |
| ACB-AcbR | 1.55 (0.93–2.43) | 12.92 | 18.80 | 1.24 ± 0.07 | |
| Cry1Ac | ACB-AbR | 11.34 (6.88–23.77) | 113.40 | 3.34 | 0.86 ± 0.24 |
| ACB-AcR | 7.88 (3.59–18.61) | 78.80 | 28.94 | 0.79 ± 0.06 | |
| ACB-AbcR | 10.25 (7.94–13.35) | 102.50 | 5.14 | 0.88 ± 0.06 | |
| ACB-AcbR | 19.81 (15.41–25.87) | 198.10 | 3.90 | 0.99 ± 0.08 | |
| Cry1F | ACB-AbR | 31.20 (17.98–114.40) | 48.75 | 12.10 | 0.88 ± 0.19 |
| ACB-AcR | 11.61 (6.70–23.28) | 18.14 | 8.54 | 0.61 ± 0.05 | |
| ACB-AbcR | 35.80 (22.00–67.61) | 55.94 | 1.24 | 0.59 ± 0.06 | |
| ACB-AcbR | 47.78 (29.56–91.45) | 74.66 | 4.13 | 0.66 ± 0.07 | |
| Cry1Ie | ACB-AbR | 1.95 (1.23–3.14) | 1.60 | 12.20 | 1.08 ± 0.10 |
| ACB-AcR | 1.88 (0.98–3.42) | 1.38 | 23.60 | 0.96 ± 0.06 | |
| ACB-AbcR | 1.66 (1.00–2.57) | 1.22 | 16.69 | 1.16 ± 0.08 | |
| ACB-AcbR | 1.12 (0.61–1.94) | 0.82 | 21.65 | 0.99 ± 0.06 |
2.3. Inheritance of Resistance
| Strains | Concentration (µg/g) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.05 | 0.25 | 0.50 | 2.50 | 5.00 | 25.00 | 50.00 | ||
| ACB-AbRS×ACBAbR | Actual mortality (%) | 18.06 | 27.08 | 41.67 | 70.14 | 78.47 | 93.75 | 97.22 |
| Expected mortality (%) | 11.63 | 16.03 | 23.09 | 42.71 | 53.47 | 73.44 | 85.59 | |
| χ2 | 5.78 | 13.06 | 27.98 | 44.28 | 36.17 | 30.46 | 15.80 | |
| P | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Mean difference (%) | 17.20 | |||||||
| ACB-AcRS×ACB-BtS | Actual mortality (%) | 21.53 | 40.97 | 48.61 | 79.17 | 86.11 | 95.14 | 97.92 |
| Expected mortality (%) | 14.49 | 21.70 | 32.46 | 49.13 | 64.97 | 87.67 | 93.23 | |
| χ2 | 1.21 | 2.92 | 3.26 | 2.70 | 1.82 | 0.83 | 1.00 | |
| P | 0.27 | 0.09 | 0.07 | 0.10 | 0.18 | 0.36 | 0.32 | |
| Mean difference (%) | 4.64 | |||||||
| Strains | Concentration (µg/g) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.05 | 0.25 | 0.50 | 2.50 | 5.00 | 25.00 | 50.00 | ||
| ACB-AbRS×ACB-AbR | Actual mortality (%) | 17.36 | 21.53 | 28.47 | 43.06 | 59.72 | 77.78 | 94.44 |
| Expected mortality (%) | 19.30 | 24.10 | 28.82 | 41.15 | 51.22 | 69.79 | 83.16 | |
| χ2 | 0.35 | 0.52 | 0.01 | 0.22 | 4.17 | 4.36 | 13.09 | |
| P | 0.56 | 0.47 | 0.93 | 0.64 | 0.04 | 0.04 | 0.00 | |
| Mean difference (%) | 4.93 | |||||||
| ACB-AcRS×ACB-BtS | Actual mortality (%) | 20.83 | 40.28 | 49.31 | 72.22 | 83.33 | 90.97 | 95.14 |
| Expected mortality (%) | 10.42 | 16.27 | 21.98 | 38.54 | 48.09 | 68.89 | 80.21 | |
| χ2 | 1.52 | 0.93 | 1.09 | 7.72 | 4.78 | 6.67 | 5.78 | |
| P | 0.22 | 0.34 | 0.30 | 0.01 | 0.03 | 0.01 | 0.02 | |
| Mean difference (%) | 3.89 | |||||||
3. Discussion
4. Experimental Section
4.1. Insect Colonies
4.2. Bt Toxins
4.3. Diet Bioassays
4.4. Genetic Crosses
- (1)
- ACB-AbR×ACB-BtS (ACB-AbRS);
- (2)
- ACB-BtS×ACB-AbR (ACB-SAbR);
- (3)
- ACB-AcR×ACB-BtS (ACB-AcRS);
- (4)
- ACB-BtS×ACB-AcR (ACB-SAcR);
- (5)
- (ACB-AbR×ACB-BtS)×ACB-AbR (ACB-AbRS×ACB-AbR);
- (6)
- (ACB-AcR×ACB-BtS)×ACB-BtS (ACB-AcRS×ACB-BtS);
- (7)
- ACB-AbR×ACB-AcR (ACB-AbcR);
- (8)
- ACB-AcR×ACB-AbR (ACB-AcbR).
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferré, J.; van Rie, J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2002, 47, 501–533. [Google Scholar]
- James, C. Global Status of Commercialized Biotech/GM Crops: 2013; ISAAA Brief 46-2013: Executive Summary; International Service for the Acquisition of Agri-Biotech Applications (ISAAA): Ithaca, NY, USA, 2013. [Google Scholar]
- Mcgaughey, W.H. Insect resistance to the biological insecticide Bacillus thuringiensis. Science 1985, 229, 193–195. [Google Scholar] [PubMed]
- Tabashnik, B.E.; Cushing, N.L.; Finson, N.; Johnson, M.W. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 1990, 83, 1671–1676. [Google Scholar]
- Sayyed, A.H.; Schuler, T.H.; Wright, D.J. Inheritance of resistance to Bt canola in a field-derived population of Plutella xylostella. Pest Manag. Sci. 2003, 59, 1197–1202. [Google Scholar] [CrossRef]
- Crespo, A.L.B.; Spencer, T.A.; Alves, A.P.; Hellmich, R.L.; Blankenship, E.E.; Magalhães, L.C.; Siegfried, B.D. On-plant survival and inheritance of resistance to Cry1Ab toxin from Bacillus thuringiensis in a field-derived strain of European corn borer, Ostrinia nubilalis. Pest Manag. Sci. 2009, 65, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Grbic, M.; van Leeuwen, T.; Clark, R.M.; Rombauts, S.; Rouze, P.; Grbic, V.; Osborne, E.J.; Dermauw, W.; Ngoc, P.C.; Ortego, F.; et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 2011, 479, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Patin, A.L.; Dennehy, T.J.; Liu, Y.-B.; Carrière, Y.; Sims, M.A.; Antilla, L. Frequency of resistance to Bacillus thuringiensis in field populations of pink bollworm. Proc. Natl. Acad. Sci. USA 2000, 97, 12980–12984. [Google Scholar] [CrossRef] [PubMed]
- Dhurua, S.; Gujar, G.T. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag. Sci. 2011, 67, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Huang, Y.; Wu, H.; Huang, M.; Cong, S.; Tabashnik, B.E.; Wu, K. Increased frequency of pink bollworm resistance to Bt toxin Cry1Ac in China. PLoS One 2012, 7, e29975. [Google Scholar] [CrossRef] [PubMed]
- Janmaat, A.F.; Myers, J. Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia Ni. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 2003, 270, 2263–2270. [Google Scholar]
- McGaughey, W.H.; Beeman, R.W. Resistance to Bacillus thuringiensis in colonies of Indian meal moth and almond moth (Lepidoptera: Pyralidae). J. Econ. Entomol. 1988, 81, 28–33. [Google Scholar]
- Gould, F.; Anderson, A.; Reynolds, A.; Bumgarner, L.; Moar, W. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J. Econ. Entomol. 1995, 88, 1545–1559. [Google Scholar]
- Moar, W.J.; Pusztai-Carey, M.; van Faassen, H.; Bosch, D.; Frutos, R.; Rang, C.; Luo, K.; Adang, M.J. Development of Bacillus thuringiensis CryIC resistance by Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae). Appl. Environ. Microbiol. 1995, 61, 2086–2092. [Google Scholar] [PubMed]
- Zhou, D.R.; He, K.L.; Wang, Z.Y.; Ye, Z.H.; Wen, L.P.; Gao, Y.X.; Song, Y.Y. Asian Corn Borer and Its Integrated Management; Golden Shield Press: Beijing, China, 1995. [Google Scholar]
- Ying, W.Z.; He, K.L.; Yan, S. arge-scale augmentative biological control of AsianCorn borer using Trichogramma in China: A Success Story. In Proceedings of the Second International Symposium on Biological Control of Arthropods, Davos, Switzerland, 12–16 September 2005; pp. 487–494.
- He, K.; Wang, Z.; Zhou, D.; Wen, L.; Song, Y.; Yao, Z. Evaluation of transgenic Bt corn for resistance to the Asian corn borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 2003, 96, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Wang, Z.Y.; He, K.L.; Cong, B.; Bai, S.X.; Wen, L.P. Temporal and spatial expression of CrylAb toxin in transgenic Bt corn and its effects on Asian corn borer, Ostrinia furnacalis (Guenée). Sci. Agric. Sin. 2004, 37, 1155–1159. [Google Scholar]
- Chang, X.; Chang, X.Y.; He, K.L.; Wang, Z.Y.; Bai, S.X. Resistance evaluation of transgenic Bt maize to oriental armyworm. Acta Phytophylacica Sin. 2007, 34, 225–228. [Google Scholar]
- Chang, X.Y.; He, K.L.; Wang, Z.Y.; Bai, S.X. Evaluation of transgenic Bt maize for resistance to cotton bollworm. Acta Phytophylacica Sin. 2006, 33, 374–378. [Google Scholar]
- Chang, X.; Liu, G.G.; He, K.L.; Shen, Z.C.; Peng, Y.F.; Ye, G.Y. Efficacy evaluation of two transgenic maize events expressing fused proteins to Cry1Ab-susceptible and -resistant Ostrinia fumacalis (Lepidoptera: Crambidae). J. Econ. Entomol. 2013, 106, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Z.; Zhang, J.; He, K.; Ferry, N.; Gatehouse, A.M.R. Cross-resistance of Cry1Ab-selected Asian corn borer to other Cry toxins. J. Appl. Entomol. 2010, 134, 429–438. [Google Scholar] [CrossRef]
- Han, H.L.; Li, G.T.; Wang, Z.Y.; Zhang, J.; He, K.L. Cross-resistance of Cry1Ac-selected Asian cornborer to other Bt toxins. Acta Phytophylacica Sin. 2009, 36, 329–334. [Google Scholar]
- Huang, F.; Higgins, R.A.; Buschman, L.L. Baseline susceptibility and changes in susceptibility to Bacillus thuringiensis subsp. kurstaki under selection pressure in European corn borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 1997, 90, 1137–1143. [Google Scholar]
- Bolin, P.C.; Hutchison, W.D.; Andow, D.A. Long-term selection for resistance to Bacillus thuringiensis Cry1Ac endotoxin in a Minnesota population of European corn borer (Lepidoptera: Crambidae). J. Econ. Entomol. 1999, 92, 1021–1030. [Google Scholar]
- Siqueira, H.A.A.; Moellenbeck, D.; Spencer, T.; Siegfried, B.D. Cross-resistance of Cry1Ab-selected Ostrinia nubilalis (Lepidoptera: Crambidae) to Bacillus thuringiensis δ-Endotoxins. J. Econ. Entomol. 2004, 97, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.J.G.; Lang, B.A.; Storer, N.P.; Siegfried, B.D. Selection for Cry1F resistance in the European corn borer and cross-resistance to other Cry toxins. Entomol. Exp. Appl. 2008, 126, 115–121. [Google Scholar] [CrossRef]
- Devos, Y.; Meihls, L.; Kiss, J.; Hibbard, B. Resistance evolution to the first generation of genetically modified Diabrotica-active Bt-maize events by western corn rootworm: Management and monitoring considerations. Transgenic Res. 2013, 22, 269–299. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Schwartz, J.M.; Finson, N.; Johnson, M.W. Inheritance of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 1992, 85, 1046–1055. [Google Scholar]
- Liu, Y.; Tabashnik, B.E. Inheritance of resistance to the Bacillus thuringiensis toxin Cry1C in the diamondback moth. Appl. Environ. Microbiol. 1997, 63, 2218–2223. [Google Scholar] [PubMed]
- Janmaat, A.F.; Wang, P.; Kain, W.; Zhao, J.-Z.; Myers, J. Inheritance of resistance to Bacillus thuringiensis subsp. kurstaki in Trichoplusia ni. Appl. Environ. Microbiol. 2004, 70, 5859–5867. [Google Scholar] [CrossRef]
- Huang, F.; Buschman, L.L.; Higgins, R.A.; McGaughey, W.H. Inheritance of resistance to Bacillus thuringiensis toxin (Dipel ES) in the European corn borer. Science 1999, 284, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Spencer, T.; Tabashnik, B.; Siegfried, B. Inheritance of resistance to the Cry1Ab Bacillus thuringiensis toxin in Ostrinia nubilalis (Lepidoptera:Crambidae). J. Econ. Entomol. 2006, 99, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.J.G.; Storer, N.P.; Siegfried, B.D. Inheritance of Cry1F resistance in laboratory-selected European corn borer and its survival on transgenic corn expressing the Cry1F toxin. Bull. Entomol. Res. 2008, 98, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.C.; Walton, W.E.; Federici, B.A. Inheritance patterns, dominance, stability, and allelism of insecticide resistance and cross-resistance in two colonies of Culex quinquefasciatus (Diptera: Culicidae) selected with Cry toxins from Bacillus thuringiensis subsp. israelensis. J. Med. Entomol. 2010, 47, 814–822. [Google Scholar] [CrossRef]
- Kranthi, K.R.; Dhawad, C.S.; Naidu, S.R.; Mate, K.; Behere, G.T.; Wadaskar, R.M.; Kranthi, S. Inheritance of resistance in indian Helicoverpa armigera (Hübner) to Cry1Ac toxin of Bacillus thuringiensis. Crop Prot. 2006, 25, 119–124. [Google Scholar] [CrossRef]
- Mahon, R.J.; Olsen, K.M.; Garsia, K.A.; Young, S.R. Resistance to Bacillus thuringiensis toxin Cry2Ab in a strain of Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. J. Econ. Entomol. 2007, 100, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Liu, Y.B.; Dennehy, T.J.; Sims, M.A.; Sisterson, M.S.; Biggs, R.W.; Carrière, Y. Inheritance of resistance to Bt toxin Cry1Ac in a field-derived strain of pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 2002, 95, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.-M.; Wu, K.-M.; Yu, H.-K.; Li, K.-K.; Feng, X.; Guo, Y.-Y. Changes of inheritance mode and fitness in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) along with its resistance evolution to Cry1Ac toxin. J. Invertebr. Pathol. 2008, 97, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Vassal, J.M.; Royer, M.; Pieretti, I. A single linkage group confers dominant resistance to Bacillus thuringiensis δ-endotoxin Cry1Ac in Helicoverpa armigera. J. Appl. Entomol. 2009, 133, 375–380. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Liu, Y.-B.; de Maagd, R.A.; Dennehy, T.J. Cross-resistance of pink bollworm (Pectinophora gossypiella) to Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 2000, 66, 4582–4584. [Google Scholar] [CrossRef] [PubMed]
- Crespo, A.L.B.; Rodrigo-Simón, A.; Siqueira, H.A.A.; Pereira, E.J.G.; Ferré, J.; Siegfried, B.D. Cross-resistance and mechanism of resistance to Cry1Ab toxin from Bacillus thuringiensis in a field-derived strain of European corn borer, Ostrinia nubilalis. J. Invertebr. Pathol. 2011, 107, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Rajamohan, F.; Gould, F.; Dean, D.H. Resistance to Bacillus thuringiensis CryIA delta-endotoxins in a laboratory-selected Heliothis virescens strain is related to receptor alteration. Appl. Environ. Microbiol. 1995, 61, 3836–3842. [Google Scholar] [PubMed]
- Tabashnik, B.E. Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 1994, 39, 47–79. [Google Scholar] [CrossRef]
- Wirth, M.C.; Walton, W.E.; Federici, B.A. Inheritance, stability, and dominance of Cry resistance in Culex quinquefasciatus (Diptera: Culicidae) selected with the three Cry toxins of Bacillus thuringiensis subsp. israelensis. J. Med. Entomol. 2012, 49, 886–894. [Google Scholar] [CrossRef]
- Petzold-Maxwell, J.L.; Cibils-Stewart, X.; French, B.W.; Gassmann, A.J. Adaptation by western corn rootworm (Coleoptera: Chrysomelidae) to Bt maize: Inheritance, fitness costs, and feeding preference. J. Econ. Entomol. 2012, 105, 1407–1418. [Google Scholar]
- Song, F.; Zhang, J.; Gu, A.; Wu, Y.; Han, L.; He, K.; Chen, Z.; Yao, J.; Hu, Y.; Li, G.; et al. Identification of cry1I-Type genes from Bacillus thuringiensis strains and characterization of a novel cry1I-type gene. Appl. Environ. Microbiol. 2003, 69, 5207–5211. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E. Determining the mode of inheritance of pesticide resistance with backcross experiments. J. Econ. Entomol. 1991, 84, 703–712. [Google Scholar]
- Robertson, J.; Preisler, H. Pesticide Bioassays with Arthropods; CRC: Boca Raton, FL, USA, 1992. [Google Scholar]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, T.; He, M.; Gatehouse, A.M.R.; Wang, Z.; Edwards, M.G.; Li, Q.; He, K. Inheritance Patterns, Dominance and Cross-Resistance of Cry1Ab- and Cry1Ac-Selected Ostrinia furnacalis (Guenée). Toxins 2014, 6, 2694-2707. https://doi.org/10.3390/toxins6092694
Zhang T, He M, Gatehouse AMR, Wang Z, Edwards MG, Li Q, He K. Inheritance Patterns, Dominance and Cross-Resistance of Cry1Ab- and Cry1Ac-Selected Ostrinia furnacalis (Guenée). Toxins. 2014; 6(9):2694-2707. https://doi.org/10.3390/toxins6092694
Chicago/Turabian StyleZhang, Tiantao, Mingxia He, Angharad M. R. Gatehouse, Zhenying Wang, Martin G. Edwards, Qing Li, and Kanglai He. 2014. "Inheritance Patterns, Dominance and Cross-Resistance of Cry1Ab- and Cry1Ac-Selected Ostrinia furnacalis (Guenée)" Toxins 6, no. 9: 2694-2707. https://doi.org/10.3390/toxins6092694
APA StyleZhang, T., He, M., Gatehouse, A. M. R., Wang, Z., Edwards, M. G., Li, Q., & He, K. (2014). Inheritance Patterns, Dominance and Cross-Resistance of Cry1Ab- and Cry1Ac-Selected Ostrinia furnacalis (Guenée). Toxins, 6(9), 2694-2707. https://doi.org/10.3390/toxins6092694