Effects of Fraxinellone on the Midgut Enzyme Activities of the 5th Instar Larvae of Oriental Armyworm, Mythimna separata Walker
Abstract
:1. Introduction
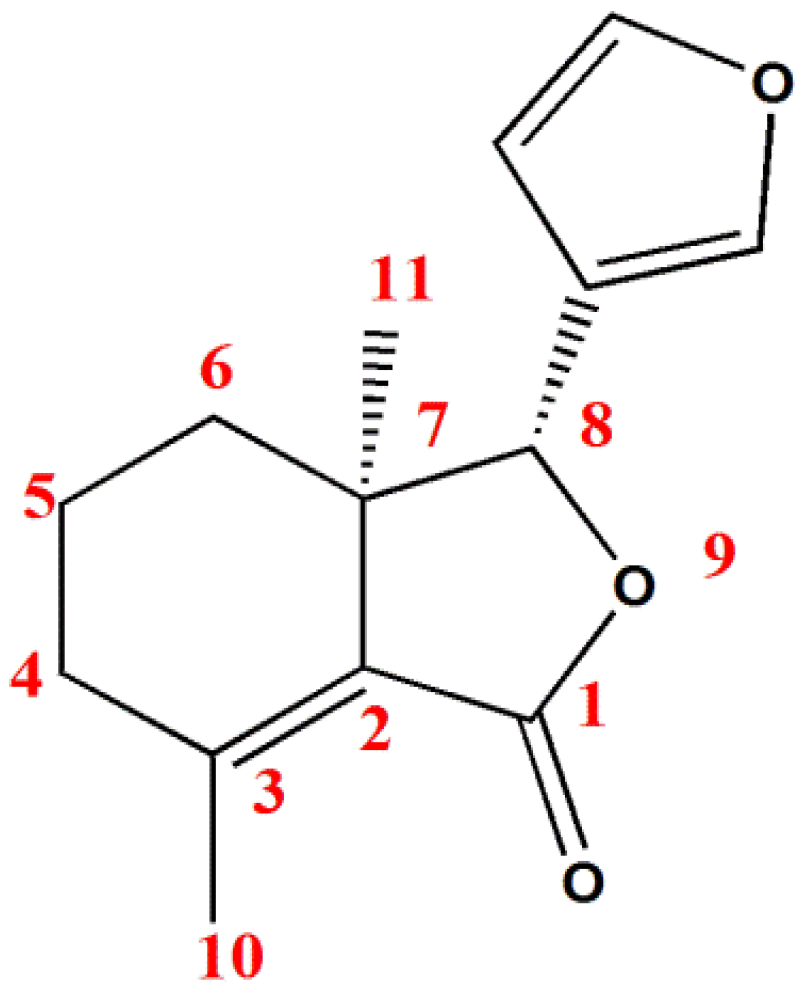
2. Results and Discussion
2.1. The Effects of Fraxinellone on the Midgut Digestive Enzymes

2.2. The Effects of Fraxinellone on the Specific Protease Activities
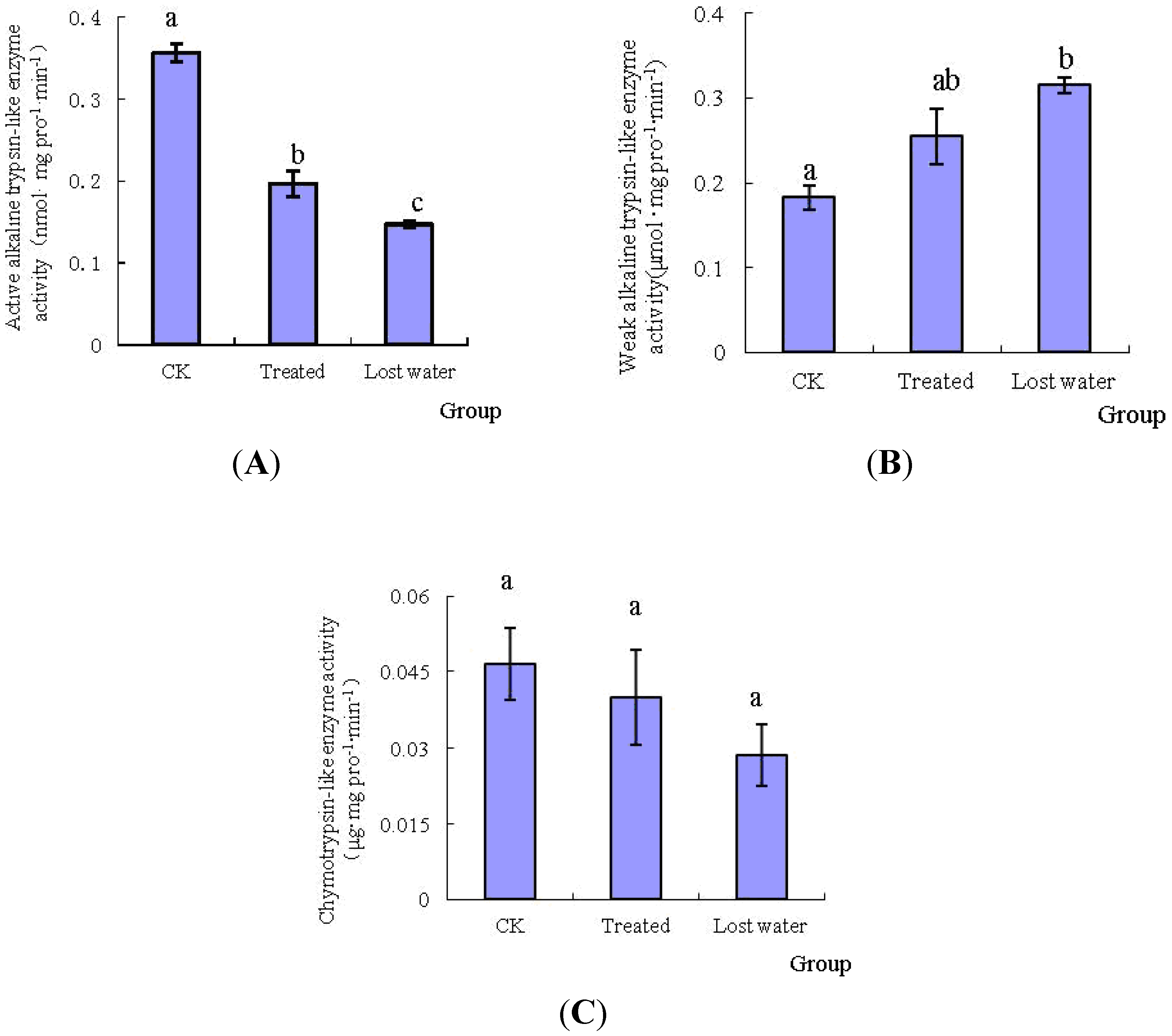
2.3. The Effects of Fraxinellone on the Midgut Detoxification Enzymes

3. Experimental Section
3.1. Insects
3.2. Chemicals
3.3. Enzyme Preparation
3.4. Digestive Enzyme Assays
3.5. Detoxification Enzyme Assays
3.6. Protein Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar]
- Senthil-Nathan, S. Effects of Melia azedarach on nutritional physiology and enzyme activities of the rice leaffolder Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2006, 84, 98–108. [Google Scholar]
- Hu, C.Q.; Han, J.W.; Zhao, J.G.; Song, G.Q.; Li, Y.H.; Yin, D.X. Limonoids from Dictamnus angustifolius. J. Integr. Plant Biol. 1989, 31, 453–458. [Google Scholar]
- Boustie, J.; Moulis, C.; Gleye, J.; Fouraste, I.; Servin, P.; Bon, M. A degraded limonoid from Fagaropsis glabra. Phytochemistry 1990, 29, 1699–1701. [Google Scholar] [CrossRef]
- Nakatani, M.; Huang, R.C.; Okamura, H.; Iwagawa, T.; Tadera, K. Degraded limonoids from Melia azedarach. Phytochemistry 1998, 49, 1773–1776. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Nakaoka, M.; Yamamoto, T.; Takahashi, H.; Minami, H. Degraded and oxotane-bearing limonoids from the roots of Melia azedarach. Chem. Pharm. Bull. 2006, 54, 1219–1222. [Google Scholar]
- Biavatti, M.W.; Vieira, P.C.; da Silva, M.F.G.F.; Fermamdes, J.B.; Albuquerque, S. Limonoids from the endemic Brazilian species Raulinoa echinata. Z. Naturforsch. 2001, 56, 570–574. [Google Scholar]
- Biavatti, M.W.; Westerlon, R.; Vieira, P.C.; Silva, M.F.G.F.; Fernandes, J.B.; Penaflor, M.F.G.V.; Bueno, O.C.; Ellena, J. Leaf-cutting ants toxicity of limonexic acid and degraded limonoids from Raulinon echinata. X-ray structure of epoxyfraxinellone. J. Braz. Chem. Soc. 2005, 16, 1443–1447. [Google Scholar] [CrossRef]
- Liu, Z.L.; Xu, Y.J.; Goh, S.H.; Ho, S.H. Feeding deterrents from Dictamnus dasycarpus against two stored-product insects. J. Agric. Food Chem. 2002, 50, 1447–1450. [Google Scholar] [CrossRef]
- Yoon, J.S.; Sung, S.H.; Kim, Y.C. Neuroprotective limonoids of root bark of Dictamnus dasycarpus. J. Nat. Prod. 2008, 71, 208–211. [Google Scholar] [CrossRef]
- Akhmedzhanova, V.I.; Bessonova, I.A.; Yunusov, S.Y. The roots of Dictamnus angustifolius. Chem. Nat. Compd. 1978, 14, 404–406. [Google Scholar] [CrossRef]
- Okamura, H.; Yamauchi, K.; Miyawaki, K.; Iwagawa, T.; Nakatani, M. Synthesis and biological activities of degraded limonoids, (±)-fraxinellonone and its related compounds. Tetrahedron Lett. 1997, 38, 263–266. [Google Scholar] [CrossRef]
- Heasley, B. Synthesis of Limonoid natural products. Eur. J. Org. Chem. 2011, 19–46. [Google Scholar] [CrossRef]
- Yu, S.M.; Ko, F.N.; Su, M.J.; Wu, T.S.; Wang, M.L.; Huang, T.F.; Teng, C.M. Vasorelaxing effect in rat thoracic aorta caused by fraxinellone and dictamine isolated from the Chinese herb Dictamnus dasycarpus Turcz: Comparison with cromakalim and Ca2+ channel blockers. Naunyn-Schmiedeberg’s Arch. Parmacol. 1992, 34, 5349–5355. [Google Scholar]
- Wu, T.S.; Wang, M.L.; Shyru, H.J.; Leu, Y.L.; Chan, Y.Y.; Teng, C.M.; Kuo, S.C. Chemical constituents and bioactive principle from the root bark of Dictamnus dasycarpus. Chin. Pharm. J. 1994, 46, 447–455. [Google Scholar]
- Yang, R.Z.; Tang, C.S. Plants used for pest control in China: A literature review. Econ. Bot. 1988, 42, 376–406. [Google Scholar]
- Liu, Z.L.; Ho, S.H.; Goh, S.H. Effect of fraxinellone on growth and digestive physiology of Asian corn borer, Ostrinia furnacalis Guenee. Pestic. Biochem. Physiol. 2008, 91, 122–127. [Google Scholar] [CrossRef]
- Liu, Z.L.; Ho, S.H.; Goh, S.H. Modes of action of fraxinellone against the tobacco budworm, Heliothis virescens. Insect Sci. 2009, 16, 147–155. [Google Scholar] [CrossRef]
- Lü, M.; Wu, W.J.; Liu, H.X. Insecticidal and feeding deterrent effects of fraxinellone from Dictamnus dasycarpus against four major pests. Molecules 2013, 18, 2754–2762. [Google Scholar] [CrossRef]
- Lü, M.; Wu, W.J.; Liu, H.X. Effects of fraxinellone on the midgut ultrastructural changes of Mythimna separata Walker. Pestic. Biochem. Physiol. 2010, 98, 263–268. [Google Scholar] [CrossRef]
- Dauterman, W.C. Insect metabolism extramicrosomal. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon: Oxford, UK, 1985; Volume 12, p. 713. [Google Scholar]
- He, S.; Shao, Y.; Fan, L.; Che, Z.; Xu, H.; Zhi, X.; Wang, J.; Yao, X.; Qu, H. Synthesis and quantitative structure—Activity relationship (QSAR) study of novel 4-acyloxypodophyllotoxin derivatives modified in the A and C rings as insecticidal agents. J. Agric. Food Chem. 2013, 61, 618–625. [Google Scholar]
- Haq, S.K.; Atif, S.M.; Khan, R.H. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: Natural and engineered phytoprotection. Arch. Biochem. Biophys. 2004, 431, 145–159. [Google Scholar]
- Bernhardt, R. Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 2006, 124, 128–145. [Google Scholar]
- Feng, G.Z.; Wan, S.Q.; Pan, D.J. Isolation of antifeeding components from wild rice Oryza punctata and their effect on digestivie enzymes of Spodoptera litura (Fabricius) (Leipidoptera: Noctuidae). Acta Entomol. Sin. 2006, 49, 556–561. [Google Scholar]
- Chen, C.K. Experiment Handbook of Insect Physiology and Biochemistry; Agriculture Press: Beijing, China, 1993; pp. 15–25. [Google Scholar]
- Wang, C.Z.; Qin, J.D. Partial characterization of protease activity in the midgut of Helicoverpa armigera larvae. Acta Entomol. Sin. 1996, 39, 7–14. [Google Scholar]
- Zhang, S.Y.; Li, D.M.; Xie, B.Y. Effects of Bt toxic protein on development and activities of several relative enzymes in Helicoverpa armigera. Entomol. Knowl. 2004, 41, 536–540. [Google Scholar]
- Liang, P.; Cui, J.Z.; Yang, X.Q.; Gao, X.W. Effects of host plants on insecticide susceptibility and carboxylesterase activity in Bemisia tabaci biotype B and greenhouse whitefly, Trialeurodes vaporariorum. Pest Manag. Sci. 2007, 63, 365–371. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Yasukochi, Y.; Masters, B.S.S. Some properties of a detergent solubilized NADPH-cytochrome c (cytochrome P-450): Reductase purified by biospecific affinity chromatography. J. Biol. Chem. 1976, 251, 5337–5344. [Google Scholar]
- Qiu, X.H.; Li, W.; Tian, Y.; Leng, X.F. Cytochrome P450 Monooxygenases in the cotton bollworm (Lepidoptera: Noctuidae): Tissue difference and induction. J. Econ. Entomol. 2003, 96, 1283–1289. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lv, M.; Wu, W.; Liu, H. Effects of Fraxinellone on the Midgut Enzyme Activities of the 5th Instar Larvae of Oriental Armyworm, Mythimna separata Walker. Toxins 2014, 6, 2708-2718. https://doi.org/10.3390/toxins6092708
Lv M, Wu W, Liu H. Effects of Fraxinellone on the Midgut Enzyme Activities of the 5th Instar Larvae of Oriental Armyworm, Mythimna separata Walker. Toxins. 2014; 6(9):2708-2718. https://doi.org/10.3390/toxins6092708
Chicago/Turabian StyleLv, Min, Wenjun Wu, and Huixia Liu. 2014. "Effects of Fraxinellone on the Midgut Enzyme Activities of the 5th Instar Larvae of Oriental Armyworm, Mythimna separata Walker" Toxins 6, no. 9: 2708-2718. https://doi.org/10.3390/toxins6092708



