Monoclonal Antibody Therapy and Renal Transplantation: Focus on Adverse Effects
Abstract
:1. Role and Biological Functions of Monoclonal Antibody Therapy in Renal Transplantation
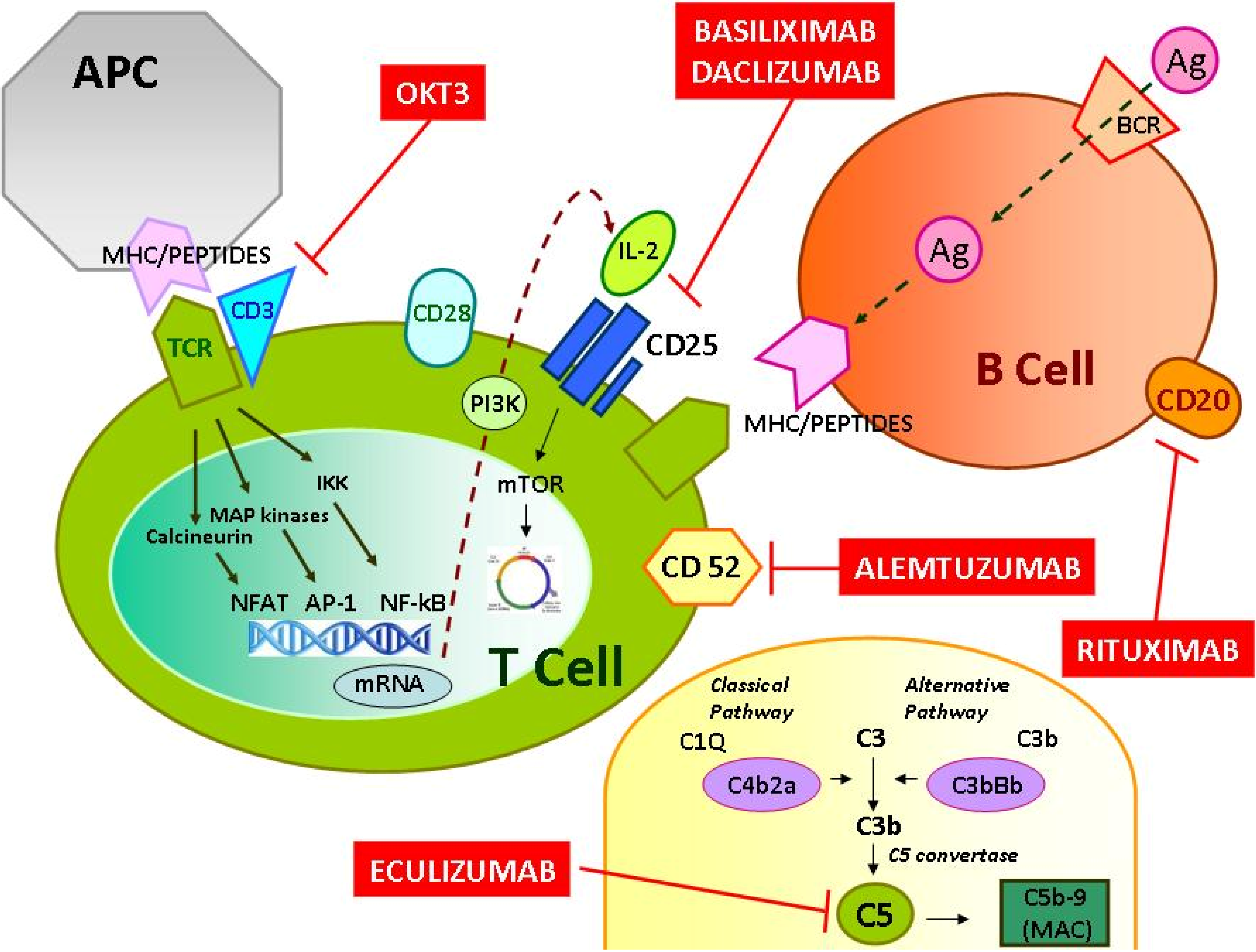
2. Adverse Effects/Toxicities of the Monoclonal Antibody Therapy in Renal Transplantation

3. Non-Depleting Antibodies
3.1. Anti-CD25 Mechanism of Action
3.2. Anti-CD25 Side Effects
3.3. Rabbit Antithymocyte Globulin (rATG) versus BASILIXIMAB: Just a Few Words
4. Depleting Antibodies
4.1. Mechanism of Action of Alemtuzumab (Anti-CD52 Antibody)
4.2. Alemtuzumab Side Effects
4.3. Rituximab (Anti-CD20/ Anti-B-Cell) Mechanism of Action
4.4. Rituximab Side Effects
4.5. Muromonab-CD3 (OKT3) Mechanism of Action
4.6. OKT3 Side Effects
5. Monoclonal Antibody Directed against Human Complement Protein
5.1. Mechanism of Action of Eculizumab (Anti-C5)
5.2. Eculizumab Side Effects
6. Conclusions
Conflict of Interest
References
- Van Der Woude, F.J. Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J. Am. Soc. Nephrol. 1998, 9, 2135–2141. [Google Scholar]
- Safinia, N.; Afzali, B.; Atalar, K.; Lombardi, G.; Lechler, R.I. T-cell alloimmunity and chronic allograft dysfunction. Kidney Int. Suppl. 2010, 119, S2–S12. [Google Scholar]
- Cecka, J.M. The UNOS renal transplant registry. Clin. Transpl. 2002, 16, 1–20. [Google Scholar] [CrossRef]
- Starzl, T.E.; Klintmalm, G.B.; Weil, R., 3rd; Porter, K.A.; Iwatsuki, S.; Schroter, G.P.; Fernandez-Bueno, C.; MacHugh, N. Cyclosporin A and steroid therapy in sixty-six cadaver kidney recipients. Surg. Gynecol. Obstet. 1981, 153, 486–494. [Google Scholar]
- Wolfe, R.A.; Roys, E.C.; Merion, R.M. Trends in organ donation and transplantation in the United States, 1999–2008. Am. J. Transplant. 2010, 10, 961–972. [Google Scholar] [CrossRef]
- Ciancio, G.; Burke, G.W.; Miller, J. Induction therapy in renal transplantation: An overview of current developments. Drugs 2007, 67, 2667–2680. [Google Scholar] [CrossRef]
- Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1998–2010; Annual Report of the U.S.; Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation: Rockville, MD, USA, 2011.
- Bunnapradist, S.; Takemoto, S.K. Multivariate analyses of antibody induction therapies. Clin. Transpl. 2003, 17, 405–417. [Google Scholar]
- Hariharan, S.; Johnson, C.P.; Bresnahan, B.A.; Taranto, S.E.; McIntosh, M.J.; Stablein, D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N. Engl. J. Med. 2000, 342, 605–612. [Google Scholar] [CrossRef]
- Gabardi, S.; Martin, S.T.; Roberts, K.L.; Grafals, M. Induction immunosuppressive therapies in renal transplantation. Am. J. Health Syst. Pharm. 2011, 68, 211–218. [Google Scholar] [CrossRef]
- Goggins, W.C.; Pascual, M.A.; Powelson, J.A.; Magee, C.; Tolkoff-Rubin, N.; Farrell, M.L.; Ko, D.S.; Williams, W.W.; Chandraker, A.; Delmonico, F.L.; et al. A prospective, randomized, clinical trial of intraoperative versus postoperative Thymoglobulin in adult cadaveric renal transplant recipients. Transplantation 2003, 76, 798–802. [Google Scholar] [CrossRef]
- Nashan, B. Antibody induction therapy in renal transplant patients receiving calcineurin-inhibitor immunosuppressive regimens: A comparative review. BioDrugs 2005, 19, 39–46. [Google Scholar] [CrossRef]
- Berard, J.L.; Velez, R.L.; Freeman, R.B.; Tsunoda, S.M. A review of interleukin-2 receptor antagonists in solid organ transplantation. Pharmacotherapy 1999, 19, 1127–1137. [Google Scholar] [CrossRef]
- Hardinger, K.L.; Koch, M.J.; Brennan, D.C. Current and future immunosuppressive strategies in renal transplantation. Pharmacotherapy 2004, 24, 1159–1176. [Google Scholar] [CrossRef]
- Kissmeyer-Nielsen, F.; Olsen, S.; Petersen, V.P.; Fjeldborg, O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet 1966, 288, 662–665. [Google Scholar] [CrossRef]
- Lefaucheur, C.; Loupy, A.; Hill, G.S.; Andrade, J.; Nochy, D.; Antoine, C.; Gautreau, C.; Charron, D.; Glotz, D.; Suberbielle-Boissel, C. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J. Am. Soc. Nephrol. 2010, 21, 1398–1406. [Google Scholar] [CrossRef]
- Fehr, T.; Rüsi, B.; Fischer, A.; Hopfer, H.; Wüthrich, R.P.; Gaspert, A. Rituximab and intravenous immunoglobulin treatment of chronic antibody-mediated kidney allograft rejection. Transplantation 2009, 87, 1837–1841. [Google Scholar] [CrossRef]
- Zaza, G.; Tomei, P.; Ria, P.; Granata, S.; Boschiero, L.; Lupo, A. Systemic and nonrenal adverse effects occurring in renal transplant patients treated with mTOR inhibitors. Clin. Dev. Immunol. 2013, 2013. [Google Scholar] [CrossRef]
- Casey, M.J.; Meier-Kriesche, H.U. Calcineurin inhibitors in kidney transplantation: Friend or foe? Curr. Opin. Nephrol. Hypertens. 2011, 20, 610–615. [Google Scholar] [CrossRef]
- Pascual, J.; Marcén, R.; Ortuño, J. Anti-interleukin-2 receptor antibodies: Basiliximab and daclizumab. Nephrol. Dial. Transplant. 2001, 16, 1756–1760. [Google Scholar] [CrossRef]
- Leonard, W.J.; Depper, J.M.; Uchiyama, T.; Smith, K.A.; Waldmann, T.A.; Greene, W.C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor: Partial characterization of the receptor. Nature 1982, 300, 267–269. [Google Scholar] [CrossRef]
- Ringheim, G.E.; Freimark, B.D.; Robb, R.J. Quantitative characterization of the intrinsic ligand-binding affinity of the interleukin 2 receptor beta chain and its modulation by the alpha chain and a second affinity-modulating element. Lymphokine. Cytokine Res. 1991, 10, 219–224. [Google Scholar]
- Robb, R.J.; Greene, W.C.; Rusk, C.M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J. Exp. Med. 1984, 160, 1126–1146. [Google Scholar] [CrossRef]
- Ferrara, J.L. Pathogenesis of acute graft-versus-host disease: Cytokines and cellular effectors. J. Hematother. Stem Cell. Res. 2000, 9, 299–306. [Google Scholar] [CrossRef]
- Kahan, B.D.; Rajagopalan, P.R.; Hall, M. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-interleukin-2-receptor monoclonal antibody. United States Simulect Renal Study Group. Transplantation 1999, 67, 276–284. [Google Scholar] [CrossRef]
- Nashan, B.; Moore, R.; Amlot, P.; Schmidt, A.G.; Abeywickrama, K.; Soulillou, J.P. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group. Lancet 1997, 350, 1193–1198. [Google Scholar] [CrossRef]
- Vincenti, F.; de Andrés, A.; Becker, T.; Choukroun, G.; Cole, E.; González-Posada, J.M.; Kumar, M.A.; Moore, R.; Nadalin, S.; Nashan, B.; et al. Interleukin-2 receptor antagonist induction in modern immunosuppression regimens for renal transplant recipients. Transpl. Int. 2006, 19, 446–457. [Google Scholar] [CrossRef]
- Cai, J.; Terasaki, P.I. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: An analysis of United Network for Organ Sharing registry data. Transplantation 2010, 90, 1511–1515. [Google Scholar] [CrossRef]
- Waldmann, T.A. Immunotherapy: Past, present and future. Nat. Med. 2003, 9, 269–277. [Google Scholar] [CrossRef]
- Kidney Disease Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9, S1–S155. [Google Scholar]
- Ren, Q.; Paramesh, A.; Yau, C.L.; Killackey, M.; Slakey, D.; Florman, S.; Buell, J.; Alper, B.; Simon, E.; Hamm, L.L. Long-term outcome of highly sensitized African American patients transplanted with deceased donor kidneys. Transpl. Int. 2011, 24, 259–265. [Google Scholar] [CrossRef]
- Zhang, R.; Florman, S.; Devidoss, S.; Zarifian, A.; Yau, C.L.; Paramesh, A.; Killackey, M.; Alper, B.; Fonseca, V.; Slakey, D. A comparison of long-term survivals of simultaneous pancreas-kidney transplant between African American and Caucasian recipients with basiliximab induction therapy. Am. J. Transpl. 2007, 7, 1815–1821. [Google Scholar] [CrossRef]
- Zhang, R.; Paramesh, A.; Florman, S.; Yau, C.L.; Balamuthusamy, S.; Krane, N.K.; Slakey, D. Long-term outcome of adults who undergo transplantation with single pediatric kidneys: How young is too young. Clin. J. Am. Soc. Nephrol. 2009, 4, 1500–1506. [Google Scholar] [CrossRef]
- Ramirez, C.B.; Marino, I.R. The role of basiliximab induction therapy in organ transplantation. Expert Opin. Biol. Ther. 2007, 7, 137–148. [Google Scholar] [CrossRef]
- Novartis Pharmaceutical Corporation. Simulect® (basiliximab): Summary of product characteristics. Available online: www.pharma.us.novartis.com (accessed on 11 November 2013).
- Mottershead, M.; Neuberger, J. Daclizumab. Expert Opin. Biol. Ther. 2007, 7, 1583–1596. [Google Scholar] [CrossRef]
- Penfornis, A.; Kury-Paulin, S. Immunosuppressive drug-induced diabetes. Diabetes MeTable 2006, 32, 539–546. [Google Scholar] [CrossRef]
- Brennan, D.C.; Daller, J.A.; Lake, K.D.; Cibrik, D.; Del Castillo, D.; Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N. Engl. J. Med. 2006, 355, 1967–1977. [Google Scholar] [CrossRef]
- Lebranchu, Y.; Bridoux, F.; Büchler, M.; Le Meur, Y.; Etienne, I.; Toupance, O.; Hurault de Ligny, B.; Touchard, G.; Moulin, B.; Le Pogamp, P.; et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am. J. Transpl. 2002, 2, 48–56. [Google Scholar] [CrossRef]
- Mourad, G.; Rostaing, L.; Legendre, C.; Garrigue, V.; Thervet, E.; Durand, D. Sequential protocols using basiliximab versus antithymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation 2004, 78, 584–590. [Google Scholar]
- Kim, J.M.; Jang, H.R.; Kwon, C.H.; Huh, W.S.; Kim, G.S.; Kim, S.J.; Joh, J.W.; Oh, H.Y. Rabbit antithymocyte globulin compared with basiliximab in kidney transplantation: A single-center study. Transpl. Proc. 2012, 44, 167–170. [Google Scholar] [CrossRef]
- Chapman, T.M.; Keating, G.M. Basiliximab: A review of its use as induction therapy in renal transplantation. Drugs 2003, 63, 2803–2835. [Google Scholar] [CrossRef]
- Sollinger, H.; Kaplan, B.; Pescovitz, M.D.; Philosophe, B.; Roza, A.; Brayman, K.; Somberg, K. Basiliximab versus antithymocyte globulin for prevention of acute renal allograft rejection. Transplantation 2001, 72, 1915–1919. [Google Scholar] [CrossRef]
- Wang, W.; Yin, H.; Li, X.B.; Hu, X.P.; Yang, X.Y.; Liu, H.; Ren, L.; Wang, Y.; Zhang, X.D. A retrospective comparison of the efficacy and safety in kidney transplant recipients with basiliximab and anti-thymocyte globulin. Chin. Med. J. 2012, 125, 1135–1140. [Google Scholar]
- Deeks, E.D.; Keating, G.M. Rabbit antithymocyte globulin (thymoglobulin): A review of its use in the prevention and treatment of acute renal allograft rejection. Drugs 2009, 69, 1483–1512. [Google Scholar] [CrossRef]
- Mourad, G.; Garrigue, V.; Squifflet, J.P.; Besse, T.; Berthoux, F.; Alamartine, E.; Durand, D.; Rostaing, L.; Lang, P.; Baron, C.; et al. Induction versus noninduction in renal transplant recipients with tacrolimus based immunosuppression. Transplantation 2001, 72, 1050–1055. [Google Scholar] [CrossRef]
- Charpentier, B.; Rostaing, L.; Berthoux, F.; Lang, P.; Civati, G.; Touraine, J.L.; Squifflet, J.P.; Vialtel, P.; Abramowicz, D.; Mourad, G.; et al. A three-arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation 2003, 75, 844–851. [Google Scholar] [CrossRef]
- Brennan, D.C.; Schnitzler, M.A. Long-term results of rabbit antithymocyte globulin and basiliximab induction. N. Engl. J. Med. 2008, 359, 1736–1738. [Google Scholar] [CrossRef]
- Jamil, B.; Nicholls, K.M.; Becker, G.J.; Walker, R.G. Influence of anti-rejection therapy on the timing of cytomegalovirus disease and other infections in renal transplant recipients. Clin. Transpl. 2000, 14, 14–18. [Google Scholar] [CrossRef]
- Zamora, M.R. Controversies in lung transplantation: Management of cytomegalovirus infections. J. Heart Lung Transpl. 2002, 21, 841–849. [Google Scholar] [CrossRef]
- Huurman, V.A.; Kalpoe, J.S.; van de Linde, P.; Vaessen, N.; Ringers, J.; Kroes, A.C.; Roep, B.O.; De Fijter, J.W. Choice of antibody immunotherapy influences cytomegalovirus viremia in simultaneous pancreas-kidney transplant recipients. Diabetes Care 2006, 29, 842–847. [Google Scholar] [CrossRef]
- Ozaki, K.S.; Pestana, J.O.; Granato, C.F.; Pacheco-Silva, A.; Camargo, L.F. Sequential cytomegalovirus antigenemia monitoring in kidney transplant patients treated with antilymphocyte antibodies. Transpl. Infect. Dis. 2004, 6, 63–68. [Google Scholar] [CrossRef]
- Hale, G.; Xia, M.Q.; Tighe, H.P.; Dyer, M.J.; Waldmann, H. The CAMPATH-1 antigen (CDw52). Tissue Antigens. 1990, 35, 118–127. [Google Scholar] [CrossRef]
- Weaver, T.A.; Kirk, A.D. Alemtuzumab. Transplantation 2007, 84, 1545–1547. [Google Scholar] [CrossRef]
- Xia, M.Q.; Hale, G.; Lifely, M.R.; Ferguson, M.A.; Campbell, D.; Packman, L.; Waldmann, H. Structure of the CAMPATH-1 antigen, a GPI-anchored glycoprotein which is an exceptionally good target for complement lysis. Biochem. J. 1993, 29, 633–640. [Google Scholar]
- Magliocca, J.F.; Knechtle, S.J. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl. Int. 2006, 19, 705–714. [Google Scholar] [CrossRef]
- Huang, E.; Cho, Y.W.; Hayashi, R.; Bunnapradist, S. Alemtuzumab induction in deceased donor kidney transplantation. Transplantation 2007, 84, 821–828. [Google Scholar] [CrossRef]
- Margreiter, R.; Klempnauer, J.; Neuhaus, P.; Muehlbacher, F.; Boesmueller, C.; Calne, R.Y. Alemtuzumab (Campath-1H) and tacrolimus monotherapy after renal transplantation: Results of a prospective randomized trial. Am. J. Transplant. 2008, 8, 1480–1485. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Han, S.; Fu, S.; Wang, L. Alemtuzumab induction in renal transplantation: A meta-analysis and systemic review. Transpl. Immunol. 2012, 27, 63–68. [Google Scholar] [CrossRef]
- Ortiz, J.; Palma-Vargas, J.; Wright, F.; Bingaman, A.; Agha, I.; Rosenblatt, S.; Foster, P. Campath induction for kidney transplantation: Report of 297 cases. Transplantation 2008, 85, 1550–1556. [Google Scholar] [CrossRef]
- Tan, H.P.; Donaldson, J.; Basu, A.; Unruh, M.; Randhawa, P.; Sharma, V.; Morgan, C.; McCauley, J.; Wu, C.; Shah, N.; et al. Two hundred living donor kidney transplantations under alemtuzumab induction and tacrolimus monotherapy: 3-year follow-up. Am. J. Transpl. 2009, 9, 355–366. [Google Scholar] [CrossRef]
- Hillmen, P.; Skotnicki, A.B.; Robak, T.; Jaksic, B.; Dmoszynska, A.; Wu, J.; Sirard, C.; Mayer, J. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J. Clin. Oncol. 2007, 25, 5616–5623. [Google Scholar] [CrossRef]
- Ferrajoli, A.; O’Brien, S.; Keating, M.J. Alemtuzumab: A novel monoclonal antibody. Expert Opin. Biol. Ther. 2001, 1, 1059–1065. [Google Scholar] [CrossRef]
- Puttarajappa, C.; Yabes, J.; Bei, L.; Shah, N.; Bernardo, J.; McCauley, J.; Basu, A.; Tan, H.; Shapiro, R.; Unruh, M.; et al. Cancer risk with alemtuzumab following kidney transplantation. Clin. Transpl. 2013, 27, E264–E271. [Google Scholar] [CrossRef]
- Hanaway, M.J.; Woodle, E.S.; Mulgaonkar, S.; Peddi, V.R.; Kaufman, D.B.; First, M.R.; Croy, R.; Holman, J. Alemtuzumab induction in renal transplantation. N. Engl. J. Med. 2011, 364, 1909–1919. [Google Scholar] [CrossRef]
- Kirk, A.D.; Cherikh, W.S.; Ring, M.; Burke, G.; Kaufman, D.; Knechtle, S.J.; Potdar, S.; Shapiro, R.; Dharnidharka, V.R.; Kauffman, H.M. Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am. J. Transpl. 2007, 7, 2619–2625. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Husain, S.; Kwak, E.J.; Silveira, F.P.; Tran, M.N.J.; Shutt, K.A.; Shapiro, R.; Thai, N.; Abu-Elmagd, K.; McCurry, K.R.; et al. Opportunistic infections in 547 organ transplant recipients receiving Alemtuzumab, a humanized monoclonal CD-52 antibody. Clin. Infect. Dis. 2007, 44, 204–212. [Google Scholar] [CrossRef]
- Cuker, A.; Coles, A.J.; Sullivan, H.; Fox, E.; Goldberg, M.; Oyuela, P.; Purvis, A.; Beardsley, D.S.; Margolin, D.H. A distinctive form of immune thrombocytopenia in a phase 2 study of alemtuzumab for the treatment of relapsing-remitting multiple sclerosis. Blood 2011, 118, 6299–6305. [Google Scholar] [CrossRef]
- Reda, G.; Maura, F.; Gritti, G.; Gregorini, A.; Binda, F.; Guidotti, F.; Piciocchi, A.; Visco, C.; Rodeghiero, F.; Cortelezzi, A. Low-dose alemtuzumab-associated immune thrombocytopenia in chronic lymphocytic leukemia. Am. J. Hematol. 2012, 87, 936–937. [Google Scholar] [CrossRef]
- Barnett, A.N.; Hadjianastassiou, V.G.; Mamode, N. Rituximab in renal transplantation. Transpl. Int. 2013, 26, 563–575. [Google Scholar] [CrossRef]
- Nadler, L.M.; Ritz, J.; Hardy, R.; Pesando, J.M.; Schlossman, S.F.; Stashenko, P. A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J. Clin Invest. 1981, 67, 134–140. [Google Scholar] [CrossRef]
- Markasz, L.; Vanherberghen, B.; Flaberg, E.; Otvös, R.; Stuber, G.; Gustafsson, A.J.; Olah, E.; Skribek, H.; Szekely, L. NK cell-mediated lysis is essential to kill Epstein-Barr virus transformed lymphoblastoid B cells when using rituximab. Biomed. Pharmacother. 2009, 63, 413–420. [Google Scholar] [CrossRef]
- Di Gaetano, N.; Cittera, E.; Nota, R.; Vecchi, A.; Grieco, V.; Scanziani, E.; Botto, M.; Introna, M.; Golay, J. Complement activation determines the therapeutic activity of rituximab in vivo. J. Immunol. 2003, 171, 1581–1587. [Google Scholar]
- Vo, A.A.; Lukovsky, M.; Toyoda, M.; Wang, J.; Reinsmoen, N.L.; Lai, C.H.; Peng, A.; Villicana, R.; Jordan, S.C. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N. Engl. J. Med. 2008, 359, 242–251. [Google Scholar] [CrossRef]
- Takagi, T.; Ishida, H.; Shirakawa, H.; Shimizu, T.; Tanabe, K. Evaluation of low-dose rituximab induction therapy in living related kidney transplantation. Transplantation 2010, 89, 1466–1470. [Google Scholar] [CrossRef]
- Kaposztas, Z.; Podder, H.; Mauiyyedi, S.; Illoh, O.; Kerman, R.; Reyes, M.; Pollard, V.; Kahan, B.D. Impact of rituximab therapy for treatment of acute humoral rejection. Clin. Transpl. 2009, 23, 63–73. [Google Scholar] [CrossRef]
- Hong, Y.A.; Kim, H.G.; Choi, S.R.; Sun, I.O.; Park, H.S.; Chung, B.H.; Choi, B.S.; Park, C.W.; Kim, Y.S.; Yang, C.W. Effectiveness of rituximab and intravenous immunoglobulin therapy in renal transplant recipients with chronic active antibody-mediated rejection. Transpl. Proc. 2012, 44, 182–184. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Andreadis, C.; Aqui, N.A.; Ahya, V.N.; Bloom, R.D.; Brozena, S.C.; Olthoff, K.M.; Schuster, S.J.; Nasta, S.D.; Stadtmauer, E.A.; et al. Treatment of PTLD with rituximab or chemotherapy. Am. J. Transpl. 2006, 6, 569–576. [Google Scholar] [CrossRef]
- Gea-Banacloche, J.C. Rituximab-associated infections. Semin. Hematol. 2010, 47, 187–198. [Google Scholar] [CrossRef]
- Kamar, N.; Milioto, O.; Puissant-Lubrano, B.; Esposito, L.; Pierre, M.C.; Mohamed, A.O.; Lavayssière, L.; Cointault, O.; Ribes, D.; Cardeau, I.; et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am. J. Transpl. 2010, 10, 89–98. [Google Scholar] [CrossRef]
- Genberg, H.; Kumlien, G.; Wennberg, L.; Berg, U.; Tydén, G. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: A 3-year follow-up. Transplantation 2008, 85, 1745–1754. [Google Scholar] [CrossRef]
- Mitka, M. FDA: Increased HBV reactivation risk with ofatumumab or rituximab. JAMA 2013, 310, 1664. [Google Scholar] [CrossRef]
- Dong, H.J.; Ni, L.N.; Sheng, G.F.; Song, H.L.; Xu, J.Z.; Ling, Y. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: A meta-analysis. J. Clin. Virol. 2013, 57, 209–214. [Google Scholar] [CrossRef]
- Kusumoto, S.; Tanaka, Y.; Ueda, R.; Mizokami, M. Reactivation of hepatitis B virus following rituximab-plus-steroid combination chemotherapy. J. Gastroenterol. 2011, 46, 9–16. [Google Scholar] [CrossRef]
- Hui, C.K.; Cheung, W.W.; Zhang, H.; Au, W.Y.; Yueng, Y.H.; Leung, A.Y.H.; Leung, N.; Luk, J.M.C.; Lie, A.K.W.; Kwong, Y.L.; et al. Rituximab increases the risk of de novo hepatitis B infection in hepatitis B surface antigen negative patients undergoing cytotoxic chemotherapy. J. Gastroenterol. Hepatol. 2006, 21, A73–A74. [Google Scholar]
- European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B. J. Hepatol. 2012, 57, 167–185. [Google Scholar]
- Hadjinicolaou, A.V.; Nisar, M.K.; Parfrey, H.; Chilvers, E.R.; Ostor, A.J.K. Non-infectious pulmonary toxicity of rituximab: A systematic review. Rheumatology 2012, 51, 653–662. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, Y.; Xu, J.; Shuai, X.; Wu, Y. Fatal interstitial lung disease induced by rituximab-containing chemotherapy, treatment with TNF-a antagonist and cytokine profiling: A case-report and review of the literature. J. Clin. Pharm. Ther. 2013, 38, 249–253. [Google Scholar] [CrossRef]
- Biehn, S.E.; Kirk, D.; Rivera, M.P.; Martinez, A.E.; Khandani, A.H.; Orlowski, R.Z. Bronchiolitis obliterans with organizing pneumonia after rituximab therapy for non-Hodgkin’s lymphoma. Hematol. Oncol. 2006, 24, 234–237. [Google Scholar] [CrossRef]
- Macartney, C.; Burke, E.; Elborn, S.; Magee, N.; Noone, P.; Gleadhill, I.; Allen, D.; Kettle, P.; Drake, M. Bronchiolitis obliterans organizing pneumonia in a patient with non-Hodgkin’s lymphoma following R-CHOP and pegylated filgrastim. Leuk. Lymphoma 2005, 46, 1523–1526. [Google Scholar] [CrossRef]
- Agarwal, A.; Vieira, C.A.; Book, B.K.; Sidner, R.A.; Fineberg, N.S.; Pescovitz, M.D. Rituximab, anti-CD20, induces in vivo cytokine release but does not impair ex vivo T-cell responses. Am. J. Transpl. 2004, 4, 1357–1360. [Google Scholar] [CrossRef]
- Pahl, M.V.; Gollapudi, S.; Sepassi, L.; Gollapudi, P.; Elahimehr, R.; Vaziri, N.D. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol. Dial. Transpl. 2010, 25, 205–212. [Google Scholar] [CrossRef]
- Wolach, O.; Shpilberg, O.; Lahav, M. Neutropenia after rituximab treatment: New insights on a late complication. Curr. Opin. Hematol. 2012, 19, 32–38. [Google Scholar] [CrossRef]
- Giezen, T.J.; Mantel-Teeuwisse, A.K.; ten Berg, M.J.; Straus, S.M.; Leufkens, H.G.; van Solinge, W.W.; Egberts, T.C. Rituximab-induced thrombocytopenia: A cohort study. Eur. J. Haematol. 2012, 89, 256–266. [Google Scholar] [CrossRef]
- Mitsuhata, N.; Fujita, R.; Ito, S.; Mannami, M.; Keimei, K. Delayed onset neutropenia in a patient receiving rituximab as treatment for refractory kidney transplantation. Transplantation 2005, 80, 1355. [Google Scholar] [CrossRef]
- Grant, C.; Wilson, W.H.; Dunleavy, K. Neutropenia associated with rituximab therapy. Curr. Opin. Hematol. 2011, 18, 49–54. [Google Scholar] [CrossRef]
- Ishida, H.; Inui, M.; Furusawa, M.; Tanabe, K. Late-onset neutropenia (LON) after low-dose rituximab treatment in living related kidney transplantation--single-center study. Transpl. Immunol. 2013, 28, 93–99. [Google Scholar] [CrossRef]
- Ram, R.; Bonstein, L.; Gafter-Gvili, A.; Ben-Bassat, I.; Shpilberg, O.; Raanani, P. Rituximab-associated acute thrombocytopenia: An under-diagnosed phenomenon. Am. J. Hematol. 2009, 84, 247–250. [Google Scholar] [CrossRef]
- Winkler, U.; Jensen, M.; Manzke, O.; Schulz, H.; Diehl, V.; Engert, A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an Anti-CD20 monoclonal antibody (Rituximab, IDEC-C2B8). Blood 1999, 94, 2217–2224. [Google Scholar]
- Yi, J.H.; Kim, S.J.; Ahn, H.K.; Lee, S.J.; Chang, M.H.; Kim, W.S. Rituximab-induced acute thrombocytopenia: A case report and review of the literature. Med.Oncol. 2009, 26, 45–48. [Google Scholar] [CrossRef]
- Carson, K.R.; Evens, A.M.; Richey, E.A.; Habermann, T.M.; Focosi, D.; Seymour, J.F.; Laubach, J.; Bawn, S.D.; Gordon, L.I.; Winter, J.N.; et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: A report of 57 cases from the research on adverse drug events and reports project. Blood 2009, 113, 4834–4840. [Google Scholar] [CrossRef]
- Novak, J.; Mocikova, H.; Pavlicek, P.; Gaherova, L.; Kozak, T. Rituximab-induced coagulopathy. Leuk. Lymphoma. 2012, 53, 2299–2301. [Google Scholar] [CrossRef]
- Tydén, G.; Ekberg, H.; Tufveson, G.; Mjörnstedt, L. A randomized, double-blind, placebo-controlled study of single dose rituximab as induction in renal transplantation: A 3-year follow-up. Transplantation 2012, 94, e21–e22. [Google Scholar] [CrossRef]
- Parlevliet, K.J.; Schellekens, P.T. Monoclonal antibodies in renal transplantation: A review. Transpl. Int. 1992, 5, 234–246. [Google Scholar] [CrossRef]
- Jaffers, G.J.; Fuller, T.C.; Cosimi, A.B.; Russell, P.S.; Winn, H.J.; Colvin, R.B. Monoclonal antibody therapy. Anti-idiotypic and non-anti-idiotypic antibodies to OKT3 arising despite intense immunosuppression. Transplantation 1986, 41, 572–578. [Google Scholar] [CrossRef]
- Chatenoud, L.; Baudrihaye, M.F.; Chkoff, N.; Kreis, H.; Goldstein, G.; Bach, J.F. Restriction of the human in vivo immune response against the mouse monoclonal antibody OKT3. J. Immunol. 1986, 137, 830–838. [Google Scholar]
- Norman, D.J. Mechanisms of action and overview of OKT3. Ther. Drug Monit. 1995, 17, 615–620. [Google Scholar] [CrossRef]
- Sgro, C. Side-effects of a monoclonal antibody, muromonab CD3/orthoclone OKT3: Bibliographic review. Toxicology 1995, 105, 23–29. [Google Scholar] [CrossRef]
- Kuypers, D.R.; Vanrenterghem, Y.F. Monoclonal antibodies in renal transplantation: Old and new. Nephrol. Dial. Transpl. 2004, 19, 297–300. [Google Scholar] [CrossRef]
- Breslin, S. Cytokine-release syndrome: Overview and nursing implications. Clin. J. Oncol. Nurs. 2007, 11, 37–42. [Google Scholar] [CrossRef]
- First, M.R.; Schroeder, T.J.; Hariharan, S. OKT3-induced cytokine-release syndrome: Renal effects (cytokine nephropathy). Transpl. Proc. 1993, 25, 25–26. [Google Scholar]
- Raasveld, M.H.; Bemelman, F.J.; Schellekens, P.T.; van Diepen, F.N.; van Dongen, A.; van Royen, E.A.; Hack, C.E.; ten Berge, I.J. Complement activation during OKT3 treatment: A possible explanation for respiratory side effects. Kidney Int. 1993, 43, 1140–1149. [Google Scholar] [CrossRef]
- Ten Berge, R.J.; Raasveld, M.H.; van Diepen, F.N.; Hack, C.E. Activation of coagulation and fibrinolysis during treatment with OKT3. Transpl. Proc. 1993, 25, 566–567. [Google Scholar]
- Abramowicz, D.; Pradier, O.; Marchant, A.; Florquin, S.; De Pauw, L.; Vereerstraeten, P.; Kinnaert, P.; Vanherweghem, J.L.; Goldman, M. Induction of thromboses within renal grafts by high-dose prophylactic OKT3. Lancet 1992, 339, 777–778. [Google Scholar] [CrossRef]
- Raasveld, M.H.; Hack, C.E.; ten Berge, I.J. Activation of coagulation and fibrinolysis following OKT3 administration to renal transplant recipients: Association with distinct mediators. Thromb. Haemost. 1992, 68, 264–267. [Google Scholar]
- Martin, M.A.; Massanari, R.M.; Nghiem, D.D.; Smith, J.L.; Corry, R.J. Nosocomial aseptic meningitis associated with administration of OKT3. JAMA 1988, 259, 2002–2005. [Google Scholar] [CrossRef]
- Shihab, F.S.; Barry, J.M.; Norman, D.J. Encephalopathy following the use of OKT3 in renal allograft transplantation. Transpl. Proc. 1993, 25, 31–34. [Google Scholar]
- Morgan, J.D.; Horsburgh, T.; Simpson, A.; Donnelly, P.K.; Veitch, P.S.; Bell, P.R. Cytomegalovirus infection during OKT3 treatment for renal allograft rejection. Transpl. Proc. 1992, 24, 2634–2635. [Google Scholar]
- Swinnen, L.J.; Costanzo-Nordin, M.R.; Fisher, S.G.; O’Sullivan, E.J.; Johnson, M.R.; Heroux, A.L.; Dizikes, G.J.; Pifarre, R.; Fisher, R.I. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N. Engl. J. Med. 1990, 323, 1723–1728. [Google Scholar] [CrossRef]
- Melosky, B.; Karim, M.; Chui, A.; McBride, M.; Cameron, E.C.; Yeung, C.K.; Landsberg, D.; Shackleton, C.; Keown, P.A. Lymphoproliferative disorders after renal transplantation in patients receiving triple or quadruple immunosuppression. J. Am. Soc. Nephrol. 1992, 2, S290–S294. [Google Scholar]
- Opelz, G.; Henderson, R. Incidence of non-Hodgkin’s lymphoma in kidney and heart transplant recipients. Lancet 1993, 342, 1514–1516. [Google Scholar] [CrossRef]
- Stratta, R.J.; Shaefer, M.S.; Cushing, K.A.; Markin, R.S.; Reed, E.C.; Langnas, A.N.; Pillen, T.J.; Shaw, B.W., Jr. A randomized prospective trial of acyclovir and immune globulin prophylaxis in liver transplant recipients receiving OKT3 therapy. Arch. Surg. 1992, 127, 55–63. [Google Scholar] [CrossRef]
- Abramowicz, D.; Crusiaux, A.; Goldman, M. Anaphylactic shock after retreatment with OKT3 monoclonal antibody. N. Engl. J. Med. 1992, 327, 736. [Google Scholar] [CrossRef]
- Legendre, C.; Kreis, H.; Bach, J.F.; Chatenoud, L. Prediction of successful allograft rejection retreatment with OKT3. Transplantation 1992, 53, 87–90. [Google Scholar] [CrossRef]
- Rother, R.P.; Rollins, S.A.; Mojcik, C.F.; Brodsky, R.A.; Bell, L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat. Biotechnol. 2007, 25, 1256–1264. [Google Scholar] [CrossRef]
- Hillmen, P.; Young, N.S.; Schubert, J.; Brodsky, R.A.; Socié, G.; Muus, P.; Röth, A.; Szer, J.; Elebute, M.O.; Nakamura, R.; et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2006, 355, 1233–1243. [Google Scholar] [CrossRef]
- McKeage, K. Eculizumab: A review of its use in paroxysmal nocturnal haemoglobinuria. Drugs 2011, 71, 2327–2345. [Google Scholar] [CrossRef]
- Mache, C.J.; Acham-Roschitz, B.; Fre´meaux-Bacchi, V.; Kirschfink, M.; Zipfel, P.F.; Roedl, S.; Vester, U.; Ring, E. Complement inhibitor Eculizumab in atypical hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. 2009, 4, 1312–1316. [Google Scholar] [CrossRef]
- McCaughan, J.A.; O’Rourke, D.M.; Courtney, A.E. Recurrent dense deposit disease after renal transplantation: An emerging role for complementary therapies. Am. J. Transpl. 2012, 12, 1046–1051. [Google Scholar] [CrossRef]
- Bomback, A.S.; Smith, R.J.; Barile, G.R.; Zhang, Y.; Heher, E.C.; Herlitz, H.L.; Stokes, M.B.; Markowitz, G.S.; D’Agati, V.D.; Canetta, P.A.; et al. Eculizumab for dense deposit disease and C3 Glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2012, 7, 748–756. [Google Scholar] [CrossRef]
- Legendre, C.; Sberro-Soussan, R.; Zuber, J.; Loupy, M.R.A.; Timsit, M.; Anglicheau, D. Eculizumab in renal transplantation. Transpl. Rev. 2013, 27, 90–92. [Google Scholar] [CrossRef]
- Stegall, M.D.; Diwan, T.; Raghavaiah, S.; Cornell, L.D.; Burns, J.; Dean, P.G.; Cosio, F.G.; Gandhi, M.J.; Kremers, W.; Gloor, J.M. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am. J. Transpl. 2011, 11, 2405–2413. [Google Scholar] [CrossRef]
- Stegall, M.D.; Chedid, M.F.; Cornell, L.D. The role of complement in antibody-mediated rejection in kidney transplantation. Nat. Rev. Nephrol. 2012, 8, 670–678. [Google Scholar] [CrossRef]
- Zuber, J.; Le Quintrec, M.; Morris, H.; Frémeaux-Bacchi, V.; Loirat, C.; Legendre, C. Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transpl. Rev. 2013, 27, 117–125. [Google Scholar] [CrossRef]
- Dmytrijuk, A.; Robie-Suh, K.; Cohen, M.H.; Rieves, D.; Weiss, K.; Pazdur, R. FDA report: Eculizumab (Soliris) for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Oncologist 2008, 13, 993–1000. [Google Scholar] [CrossRef]
- Barnett, A.N.R.; Asgari, E.; Chowdhury, P.; Sacks, S.H.; Dorling, A.; Mamode, N. The use of eculizumab in renal transplantation. Clin. Transpl. 2013, 27, E216–E229. [Google Scholar] [CrossRef]
- Haeney, M.R.; Thompson, R.A.; Faulkner, J.; Mackintosh, P.; Ball, A.P. Recurrent bacterial meningitis in patients with genetic defects of terminal complement components. Clin. Exp. Immunol. 1980, 40, 16–24. [Google Scholar]
- Platonov, A.E.; Vershinina, I.V.; Kuijper, E.J.; Borrow, R.; Kayhty, H. Long term effects of vaccination of patients deficient in a late complement component with a tetravalent meningococcal polysaccharide vaccine. Vaccine 2003, 21, 4437–4447. [Google Scholar] [CrossRef]
- Struijk, G.H.; Bouts, A.H.; Rijkers, G.T.; Kuin, E.A.; ten Berge, I.J.; Bemelman, F.J. Meningococcal sepsis complicating eculizumab treatment despite prior vaccination. Am. J. Transpl. 2013, 13, 819–820. [Google Scholar] [CrossRef]
- Bouts, A.; Monnens, L.; Davin, J.C.; Struijk, G.; Spanjaard, L. Insufficient protection by Neisseria meningitidis vaccination alone during eculizumab therapy. Pediatr. Nephrol. 2011, 26, 1919–1920. [Google Scholar] [CrossRef]
- Zaza, G.; Granata, S.; Sallustio, F.; Grandaliano, G.; Schena, F.P. Pharmacogenomics: A new paradigm to personalize treatments in nephrology patients. Clin. Exp. Immunol. 2010, 159, 268–280. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zaza, G.; Tomei, P.; Granata, S.; Boschiero, L.; Lupo, A. Monoclonal Antibody Therapy and Renal Transplantation: Focus on Adverse Effects. Toxins 2014, 6, 869-891. https://doi.org/10.3390/toxins6030869
Zaza G, Tomei P, Granata S, Boschiero L, Lupo A. Monoclonal Antibody Therapy and Renal Transplantation: Focus on Adverse Effects. Toxins. 2014; 6(3):869-891. https://doi.org/10.3390/toxins6030869
Chicago/Turabian StyleZaza, Gianluigi, Paola Tomei, Simona Granata, Luigino Boschiero, and Antonio Lupo. 2014. "Monoclonal Antibody Therapy and Renal Transplantation: Focus on Adverse Effects" Toxins 6, no. 3: 869-891. https://doi.org/10.3390/toxins6030869




