Experimental Basis for the High Oral Toxicity of Dinophysistoxin 1: A Comparative Study of DSP
Abstract
:1. Introduction
2. Results
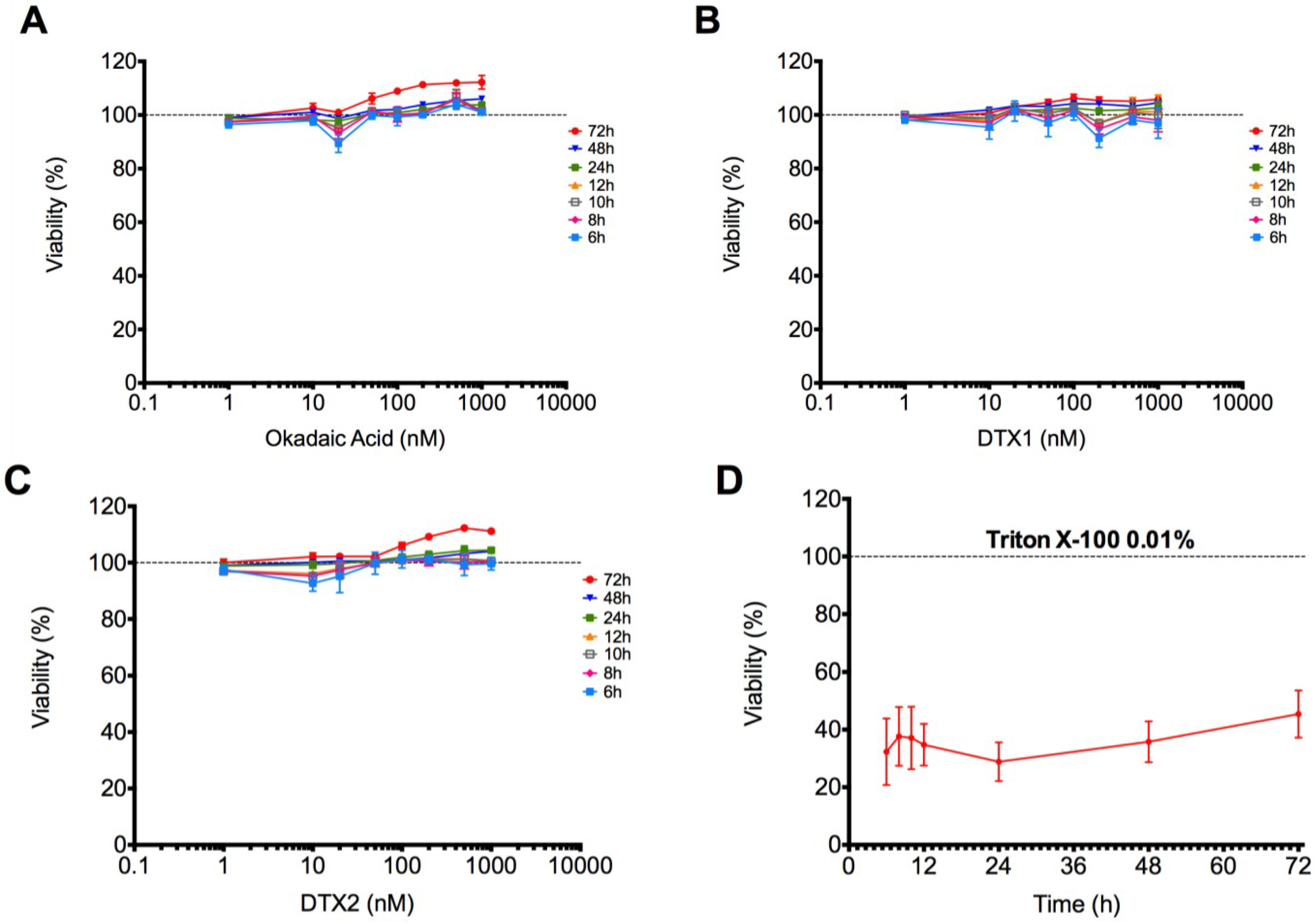
2.1. Cytotoxic Effect of OA, DTX1 and DTX2 in Differentiated Caco-2 Monolayers
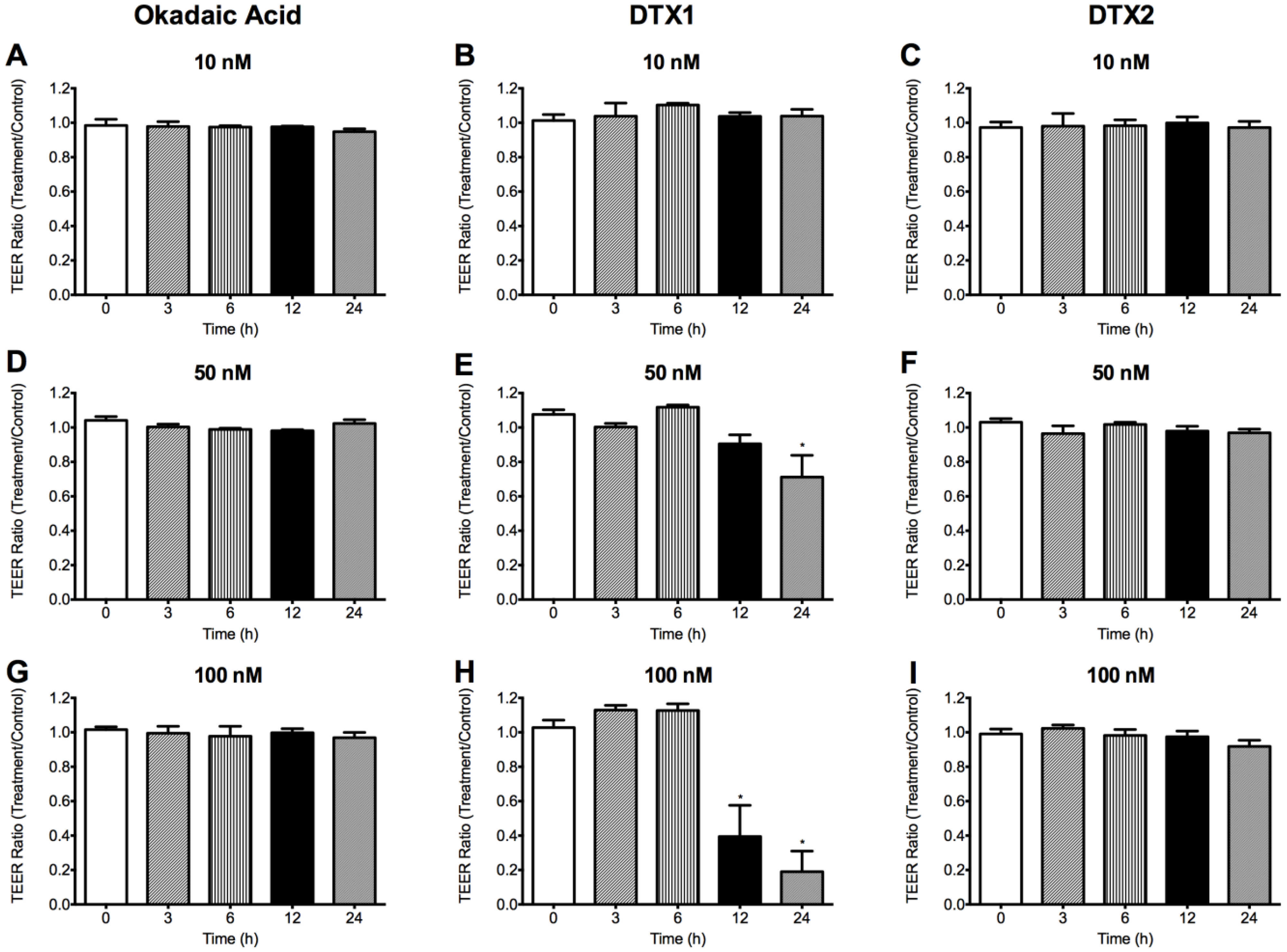
2.2. Effect of OA, DTX1 and DTX2 on the Trans-Epithelial Electrical Resistance (TEER) of Differentiated Caco-2 Monolayers
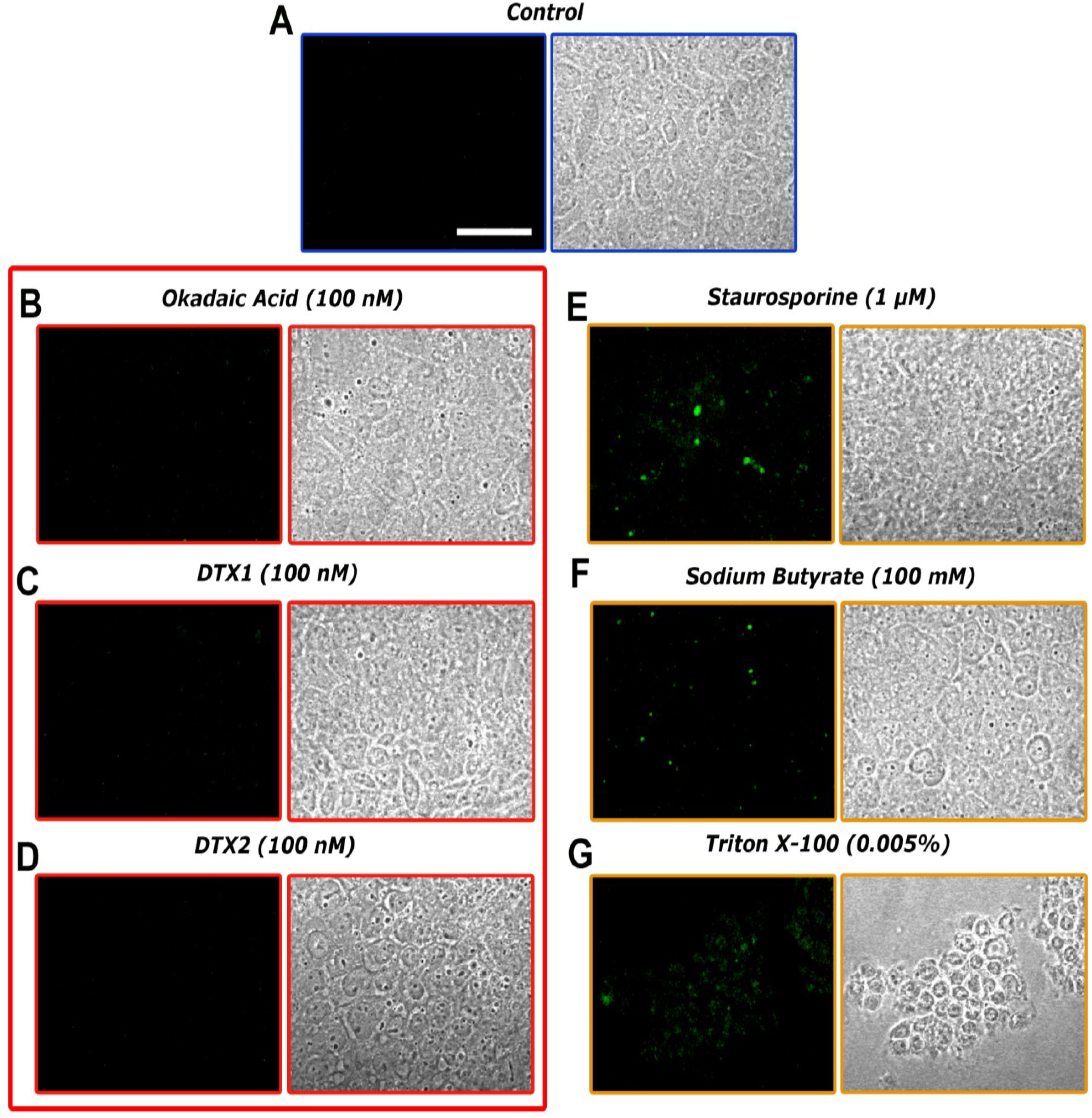
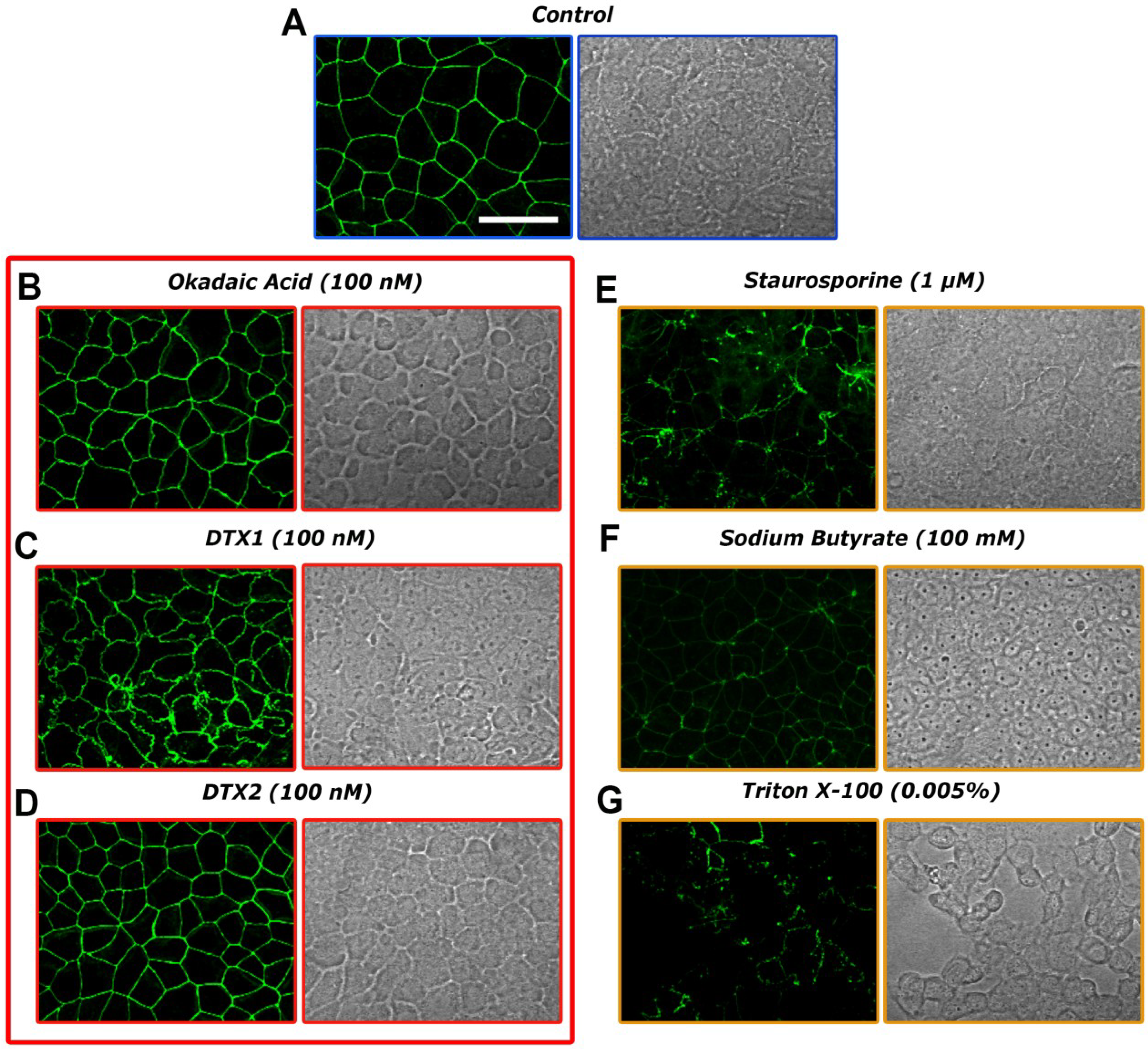
2.3. Confocal Microscopy Imaging for Visualizing Occludin in Caco-2 Cells Treated with OA, DTX1 and DTX2
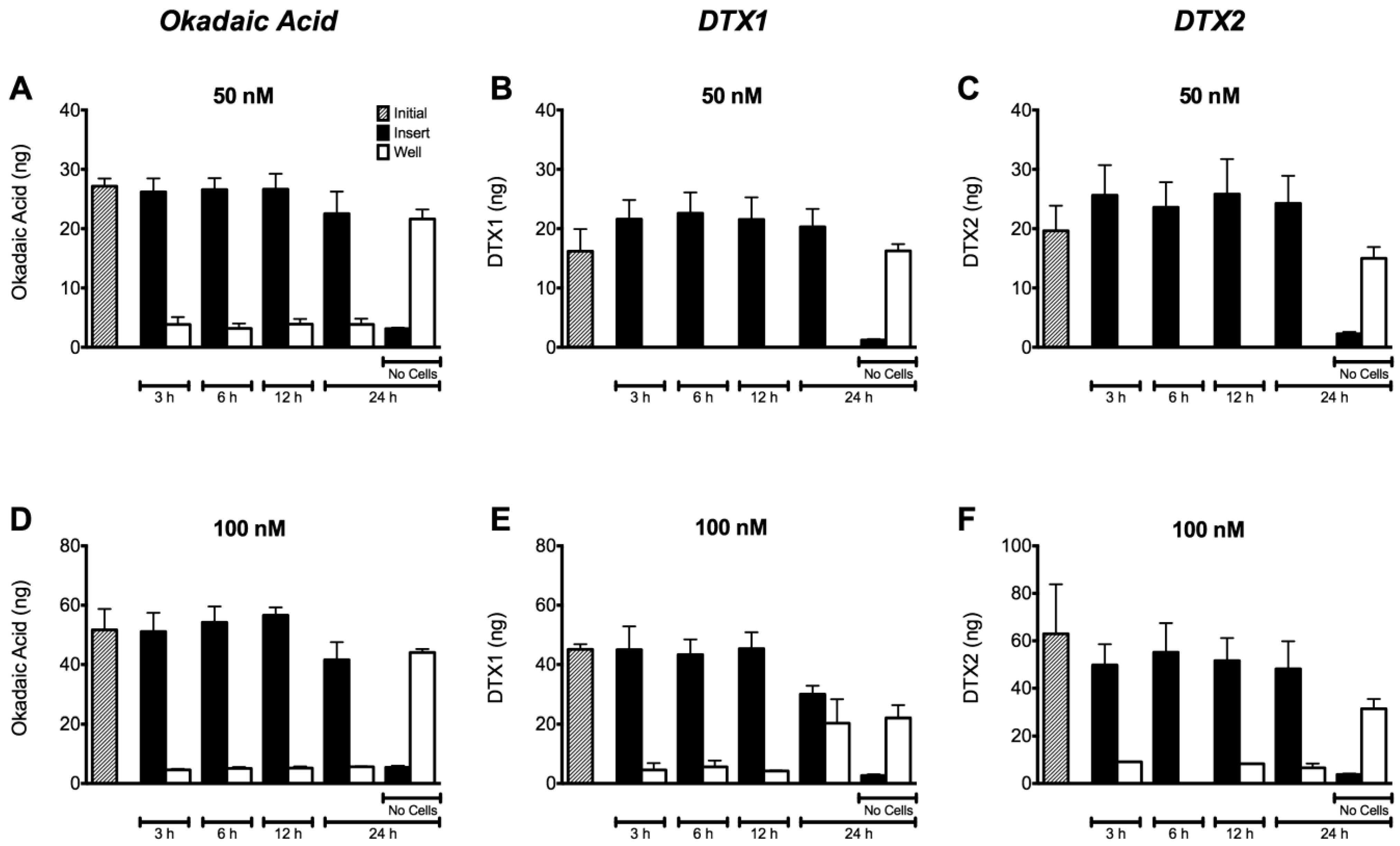
2.4. Permeability of Caco-2 Monolayers to OA, DTX1 and DTX2
3. Discussion
4. Experimental Section
4.1. Chemicals and Biological Materials
4.2. Cell Lines
4.3. Metabolic Activity Assay
4.4. Apoptosis Detection Assay with Annexin V-FITC
4.5. Trans Epithelial Electric Resistance Measurement
4.6. Confocal Microscopy Imaging for Visualizing Occludin
4.7. Permeability Assay
4.8. Microsphere-Based Immunoassay for DSPs Detection
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M.G. Harmful dinophysis species: A review. Harmful Algae 2012, 14, 87–106. [Google Scholar]
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of a new type of shellfish poisoning in the tohoku district. Nippon Suisan Gakkaishi 1978, 44, 1249–1255. [Google Scholar] [CrossRef]
- Dominguez, H.J.; Paz, B.; Daranas, A.H.; Norte, M.; Franco, J.M.; Fernandez, J.J. Dinoflagellate polyether within the yessotoxin, pectenotoxin and okadaic acid toxin groups: Characterization, analysis and human health implications. Toxicon 2010, 56, 191–217. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Marine Biotoxins: Food and Nutrition Paper 80; FAO: Rome, Italy, 2004; p. 295. [Google Scholar]
- Yasumoto, T.; Murata, M. Polyether Toxins Involved in Seafood Poisoning. In Marine toxins; American Chemical Society: Washington, DC, USA, 1990; Volume 418, pp. 120–132. [Google Scholar]
- Tachibana, K.; Scheuer, P.J.; Tsukitani, Y.; Kikuchi, H.; Van Engen, D.; Clardy, J.; Gopichand, Y.; Schmitz, F.J. Okadaic acid, a cytotoxic polyether from two marine sponges of the genus halichondria. J. Am. Chem. Soc. 1981, 103, 2469–2471. [Google Scholar] [CrossRef]
- Yasumoto, T.; Murata, M.; Oshima, Y.; Sano, M.; Matsumoto, G.K.; Clardy, J. Diarrhetic shellfish toxins. Tetrahedron 1985, 41, 1019–1025. [Google Scholar] [CrossRef]
- Gerssen, A.; Pol-Hofstad, I.E.; Poelman, M.; Mulder, P.P.; Van den Top, H.J.; De Boer, J. Marine toxins: Chemistry, toxicity, occurrence and detection, with special reference to the dutch situation. Toxins 2010, 2, 878–904. [Google Scholar] [CrossRef]
- Larsen, K.; Petersen, D.; Wilkins, A.L.; Samdal, I.A.; Sandvik, M.; Rundberget, T.; Goldstone, D.; Arcus, V.; Hovgaard, P.; Rise, F.; et al. Clarification of the c-35 stereochemistries of dinophysistoxin-1 and dinophysistoxin-2 and its consequences for binding to protein phosphatase. Chem. Res. Toxicol 2007, 20, 868–875. [Google Scholar] [CrossRef]
- Munday, R. Is protein phosphatase inhibition responsible for the toxic effects of okadaic acid in animals? Toxins 2013, 5, 267–285. [Google Scholar] [CrossRef]
- Takai, A.; Bialojan, C.; Troschka, M.; Ruegg, J.C. Smooth muscle myosin phosphatase inhibition and force enhancement by black sponge toxin. FEBS Lett. 1987, 217, 81–84. [Google Scholar] [CrossRef]
- Honkanen, R.E.; Codispoti, B.A.; Tse, K.; Boynton, A.L.; Honkanan, R.E. Characterization of natural toxins with inhibitory activity against serine/threonine protein phosphatases. Toxicon 1994, 32, 339–350. [Google Scholar] [CrossRef]
- Dawson, J.F.; Holmes, C.F. Molecular mechanisms underlying inhibition of protein phosphatases by marine toxins. Front. Biosci. J. Virtual Libr. 1999, 4, D646–D658. [Google Scholar] [CrossRef]
- Louzao, M.C.; Vieytes, M.R.; Botana, L.M. Effect of okadaic acid on glucose regulation. Mini Rev. Med. Chem. 2005, 5, 207–215. [Google Scholar] [CrossRef]
- Takai, A.; Murata, M.; Torigoe, K.; Isobe, M.; Mieskes, G.; Yasumoto, T. Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem. J. 1992, 284, 539–544. [Google Scholar]
- Holmes, C.F.; Luu, H.A.; Carrier, F.; Schmitz, F.J. Inhibition of protein phosphatases-1 and -2a with acanthifolicin. Comparison with diarrhetic shellfish toxins and identification of a region on okadaic acid important for phosphatase inhibition. FEBS Lett. 1990, 270, 216–218. [Google Scholar] [CrossRef]
- Rivas, M.; Garcia, C.; Liberona, J.L.; Lagos, N. Biochemical characterization and inhibitory effects of dinophysistoxin-1, okadaic acid and microcystine 1-r on protein phosphatase 2a purified from the mussel Mytilus chilensis. Biol. Res. 2000, 33, 197–206. [Google Scholar]
- Garibo, D.; De la Iglesia Gonzalez, P.; Diogene, J.; Campas, M. Inhibition equivalency factors for dinophysistoxin-1 and dinophysistoxin-2 in protein phosphatase assays, applicability to the analysis of shellfish samples and comparison with LC-MS/MS. J. Agric. Food Chem. 2013, 61, 2572–2579. [Google Scholar] [CrossRef]
- Aune, T.; Larsen, S.; Aasen, J.A.; Rehmann, N.; Satake, M.; Hess, P. Relative toxicity of dinophysistoxin-2 (dtx-2) compared with okadaic acid, based on acute intraperitoneal toxicity in mice. Toxicon 2007, 49, 1–7. [Google Scholar] [CrossRef]
- Hu, T.; Curtis, J.M.; Walter, J.A.; Wright, J.L.C. Identification of dtx-4, a new water-soluble phosphatase inhibitor from the toxic dinoflagellate prorocentrum lima. J. Chem. Soc. Chem. Commun. 1995. [Google Scholar] [CrossRef]
- Yanagi, T.; Murata, M.; Torigoe, K.; Yasumoto, T. Biological activities of semisynthetic analogs of dinophysistoxin-3, the major diarrhetic shellfish toxin. Agric. Biol. Chem. 1989, 53, 525–529. [Google Scholar] [CrossRef]
- Nishiwaki, S.; Fujiki, H.; Suganuma, M.; Furuya-Suguri, H.; Matsushima, R.; Iida, Y.; Ojika, M.; Yamada, K.; Uemura, D.; Yasumoto, T.; et al. Structure-activity relationship within a series of okadaic acid derivatives. Carcinogenesis 1990, 11, 1837–1841. [Google Scholar]
- Hu, T.; Curtis, J.M.; Walter, J.A.; McLachlan, J.L.; Wright, J.L.C. Two new water-soluble dsp toxin derivatives from the dinoflagellate prorocentrum maculosum: Possible storage and excretion products. Tetrahedron Letters 1995, 36, 9273–9276. [Google Scholar]
- Artursson, P. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (caco-2) cells. J. Pharm. Sci. 1990, 79, 476–482. [Google Scholar] [CrossRef]
- Vignoli, A.L.; Srivastava, R.C.; Stammati, A.; Turco, L.; Tanori, M.; Zucco, F. Nitric oxide production in caco-2 cells exposed to different inducers, inhibitors and natural toxins. Toxicol. In Vitro 2001, 15, 289–295. [Google Scholar] [CrossRef]
- Pinto, M.; Robine-Leon, S.; Appay, M.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simon-Assmann, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line caco-2 in culture. Biol. Cell. 1983, 47, 323–330. [Google Scholar]
- Tripuraneni, J.; Koutsouris, A.; Pestic, L.; De Lanerolle, P.; Hecht, G. The toxin of diarrheic shellfish poisoning, okadaic acid, increases intestinal epithelial paracellular permeability. Gastroenterology 1997, 112, 100–108. [Google Scholar] [CrossRef]
- Ehlers, A.; Scholz, J.; These, A.; Hessel, S.; Preiss-Weigert, A.; Lampen, A. Analysis of the passage of the marine biotoxin okadaic acid through an in vitro human gut barrier. Toxicology 2011, 279, 196–202. [Google Scholar] [CrossRef]
- Okada, T.; Narai, A.; Matsunaga, S.; Fusetani, N.; Shimizu, M. Assessment of the marine toxins by monitoring the integrity of human intestinal caco-2 cell monolayers. Toxicol. In Vitro 2000, 14, 219–226. [Google Scholar] [CrossRef]
- De Angelis, I.; Vincentini, O.; Brambilla, G.; Stammati, A.; Zucco, F. Characterization of furazolidone apical-related effects to human polarized intestinal cells. Toxicol. Appl. Pharmacol. 1998, 152, 119–127. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.Y.; Lin, L.; Gao, Y.; Hong, H.S.; Wang, D.Z. Quantitative proteomic analysis of okadaic acid treated mouse small intestines reveals differentially expressed proteins involved in diarrhetic shellfish poisoning. J. Proteomics 2012, 75, 2038–2052. [Google Scholar] [CrossRef]
- Pinto da Silva, P.; Kachar, B. On tight-junction structure. Cell 1982, 28, 441–450. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995, 269, G467–G475. [Google Scholar]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell. Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef]
- Andreeva, A.Y.; Piontek, J.; Blasig, I.E.; Utepbergenov, D.I. Assembly of tight junction is regulated by the antagonism of conventional and novel protein kinase c isoforms. Int. J. Biochem. Cell Biol. 2006, 38, 222–233. [Google Scholar]
- Dunagan, M.; Chaudhry, K.; Samak, G.; Rao, R.K. Acetaldehyde disrupts tight junctions in caco-2 cell monolayers by a protein phosphatase 2a-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1356–G1364. [Google Scholar] [CrossRef]
- Turner, J.R. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef]
- Seth, A.; Sheth, P.; Elias, B.C.; Rao, R. Protein phosphatases 2a and 1 interact with occludin and negatively regulate the assembly of tight junctions in the caco-2 cell monolayer. J. Biol. Chem. 2007, 282, 11487–11498. [Google Scholar] [CrossRef]
- Rao, R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann. N.Y. Acad. Sci. 2009, 1165, 62–68. [Google Scholar]
- Sheth, P.; Samak, G.; Shull, J.A.; Seth, A.; Rao, R. Protein phosphatase 2a plays a role in hydrogen peroxide-induced disruption of tight junctions in caco-2 cell monolayers. Biochem. J. 2009, 421, 59–70. [Google Scholar] [CrossRef]
- Espina, B.; Louzao, M.C.; Cagide, E.; Alfonso, A.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. The methyl ester of okadaic acid is more potent than okadaic acid in disrupting the actin cytoskeleton and metabolism of primary cultured hepatocytes. Br. J. Pharmacol. 2010, 159, 337–344. [Google Scholar] [CrossRef]
- Vale, C.; Botana, L.M. Marine toxins and the cytoskeleton: Okadaic acid and dinophysistoxins. FEBS J. 2008, 275, 6060–6066. [Google Scholar] [CrossRef]
- Vilarino, N.; Ares, I.R.; Cagide, E.; Louzao, M.C.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Induction of actin cytoskeleton rearrangement by methyl okadaate—Comparison with okadaic acid. FEBS J. 2008, 275, 926–934. [Google Scholar] [CrossRef]
- Martin-Lopez, A.; Gallardo-Rodriguez, J.J.; Sanchez-Miron, A.; Garcia-Camacho, F.; Molina-Grima, E. Cytotoxicity of yessotoxin and okadaic acid in mouse t lymphocyte cell line el-4. Toxicon 2012, 60, 1049–1056. [Google Scholar] [CrossRef]
- Small, J.V.; Kaverina, I. Microtubules meet substrate adhesions to arrange cell polarity. Curr. Opin. Cell. Biol. 2003, 15, 40–47. [Google Scholar] [CrossRef]
- Mountfort, D.O.; Suzuki, T.; Truman, P. Protein phosphatase inhibition assay adapted for determination of total dsp in contaminated mussels. Toxicon 2001, 39, 383–390. [Google Scholar] [CrossRef]
- Ikehara, T.; Imamura, S.; Yoshino, A.; Yasumoto, T. PP2A inhibition assay using recombinant enzyme for rapid detection of okadaic acid and its analogs in shellfish. Toxins 2010, 2, 195–204. [Google Scholar] [CrossRef]
- Smienk, H.G.; Calvo, D.; Razquin, P.; Dominguez, E.; Mata, L. Single laboratory validation of a ready-to-use phosphatase inhibition assay for detection of okadaic acid toxins. Toxins 2012, 4, 339–352. [Google Scholar] [CrossRef]
- Dickey, R.W.; Bobzin, S.C.; Faulkner, D.J.; Bencsath, F.A.; Andrzejewski, D. Identification of okadaic acid from a caribbean dinoflagellate, prorocentrum concavum. Toxicon 1990, 28, 371–377. [Google Scholar] [CrossRef]
- Tubaro, A.; Sosa, S.; Carbonatto, M.; Altinier, G.; Vita, F.; Melato, M.; Satake, M.; Yasumoto, T. Oral and intraperitoneal acute toxicity studies of yessotoxin and homoyessotoxins in mice. Toxicon 2003, 41, 783–792. [Google Scholar] [CrossRef]
- Murata, M.; Shimatani, M.; Sugitani, H.; Oshima, Y.; Yasumoto, T. Isolation and structural elucidation of the causative toxin of the diarrhetic shellfish poisoning [from the mussel mytilus edulis]. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 549–552. [Google Scholar] [CrossRef]
- The European Food Safety Authority. Opinion of the scientific panel on contaminants in the food chain on a request from the european commission on marine biotoxins in shellfish—Okadaic acid and analogues. EFSA J. 2008, 510, 1–62. [Google Scholar]
- Ito, E.; Yasumoto, T.; Takai, A.; Imanishi, S.; Harada, K. Investigation of the distribution and excretion of okadaic acid in mice using immunostaining method. Toxicon 2002, 40, 159–165. [Google Scholar] [CrossRef]
- Aune, T.; Espenes, A.; Aasen, J.A.; Quilliam, M.A.; Hess, P.; Larsen, S. Study of possible combined toxic effects of azaspiracid-1 and okadaic acid in mice via the oral route. Toxicon 2012, 60, 895–906. [Google Scholar] [CrossRef]
- Le Hegarat, L.; Jacquin, A.G.; Bazin, E.; Fessard, V. Genotoxicity of the marine toxin okadaic acid, in human caco-2 cells and in mice gut cells. Environ. Toxicol. 2006, 21, 55–64. [Google Scholar] [CrossRef]
- Ito, E.; Terao, K. Injury and recovery process of intestine caused by okadaic acid and related compounds. Nat. Toxins 1994, 2, 371–377. [Google Scholar]
- Ogino, H.; Kumagai, M.; Yasumoto, T. Toxicologic evaluation of yessotoxin. Nat. Toxins 1997, 5, 255–259. [Google Scholar] [CrossRef]
- Botana, L.M.; Vilariño, N.; Alfonso, A.; Vale, C.; Louzao, C.; Elliott, C.T.; Campbell, K.; Botana, A.M. The problem of toxicity equivalent factors in developing alternative methods to animal bioassays for marine-toxin detection. TrAC Trends Anal. Chem. 2010, 29, 1316–1325. [Google Scholar] [CrossRef]
- Fernandez, D.A.; Louzao, M.C.; Vilarino, N.; Espina, B.; Fraga, M.; Vieytes, M.R.; Roman, A.; Poli, M.; Botana, L.M. The kinetic, mechanistic and cytomorphological effects of palytoxin in human intestinal cells (caco-2) explain its lower-than-parenteral oral toxicity. FEBS J. 2013, 280, 3906–3919. [Google Scholar] [CrossRef]
- Fraga, M.; Vilarino, N.; Louzao, M.C.; Rodriguez, P.; Campbell, K.; Elliott, C.T.; Botana, L.M. Multidetection of paralytic, diarrheic, and amnesic shellfish toxins by an inhibition immunoassay using a microsphere-flow cytometry system. Anal. Chem. 2013, 85, 7794–7802. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fernández, D.A.; Louzao, M.C.; Fraga, M.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Experimental Basis for the High Oral Toxicity of Dinophysistoxin 1: A Comparative Study of DSP. Toxins 2014, 6, 211-228. https://doi.org/10.3390/toxins6010211
Fernández DA, Louzao MC, Fraga M, Vilariño N, Vieytes MR, Botana LM. Experimental Basis for the High Oral Toxicity of Dinophysistoxin 1: A Comparative Study of DSP. Toxins. 2014; 6(1):211-228. https://doi.org/10.3390/toxins6010211
Chicago/Turabian StyleFernández, Diego A., M. Carmen Louzao, María Fraga, Natalia Vilariño, Mercedes R. Vieytes, and Luis M. Botana. 2014. "Experimental Basis for the High Oral Toxicity of Dinophysistoxin 1: A Comparative Study of DSP" Toxins 6, no. 1: 211-228. https://doi.org/10.3390/toxins6010211





