Influence of Carbohydrates on Secondary Metabolism in Fusarium avenaceum
Abstract
:1. Introduction

2. Results and Discussion
2.1. Quantification of Secondary Metabolites
| Compound | RT (min)a | Precursor ion (m/z) | Product ions (m/z)b | S-lens (eV) | CID (eV)c |
|---|---|---|---|---|---|
| Notes: a: Retention time; b: Quantifier/qualifier ions; c: Collision induced dissociation energy for quantifier/qualifier ions. | |||||
| Moniliformin | 0.75 | 97.0 [M − H]− | 41.1 | 38 | 18 |
| Chlamydosporol | 2.65 | 227.1 [M + H]+ | 167.7/77.1 | 63 | 36/15 |
| Antibiotic Y | 4.81 | 319.1 [M + H]+ | 287.1/175.1 | 79 | 22/30 |
| Fusarin C | 5.38–5.54 | 432.2 [M + H]+ | 185.1/213.2 | 64 | 29/25 |
| Aurofusarin | 6.19 | 571.1 [M + H]+ | 556.2/485.2 | 200 | 34/24 |
| 2-AOD-3-ol | 6.36 | 314.4 [M + H]+ | 69.1/97.1 | 76 | 20/27 |
| Enniatin B | 7.67 | 657.5 [M + NH4]+ | 196.2/214.2 | 136 | 30/31 |
| Enniatin B1 | 7.87 | 671.5 [M + NH4]+ | 196.2/214.2 | 110 | 30/24 |
| Enniatin A1 | 8.10 | 685.5 [M + NH4]+ | 210.1/228.2 | 113 | 30/30 |
| Enniatin A | 8.21 | 699.5 [M + NH4]+ | 210.1/228.2 | 115 | 24/25 |
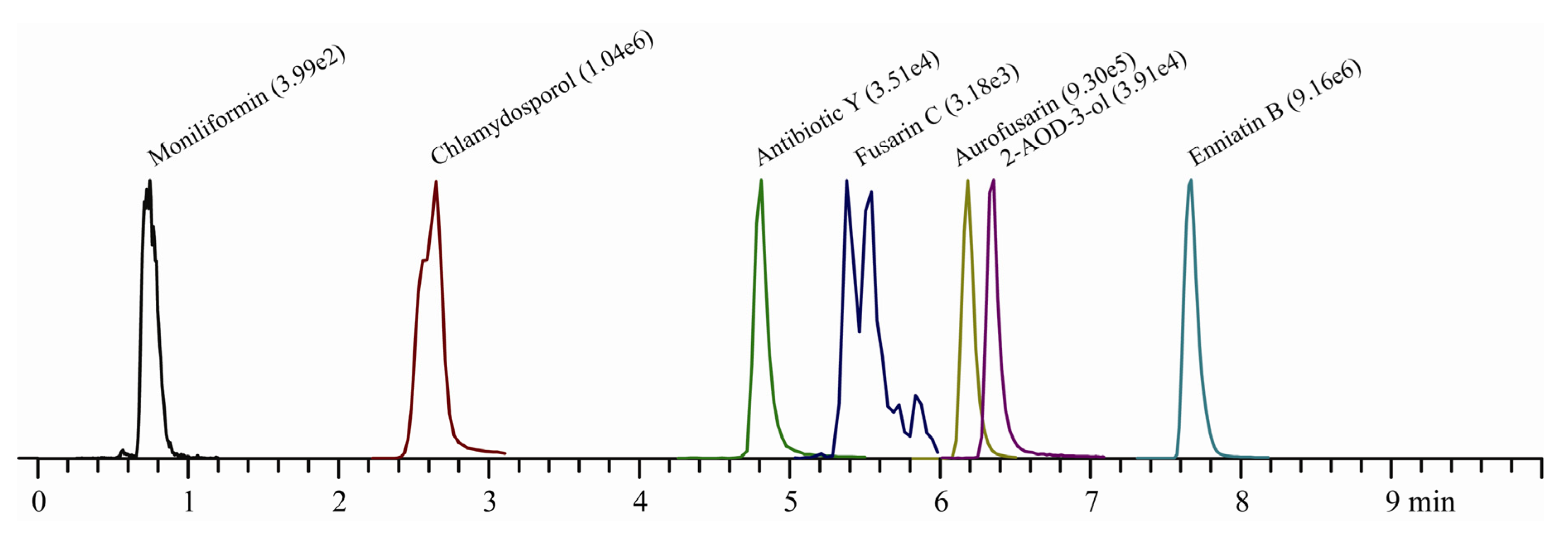
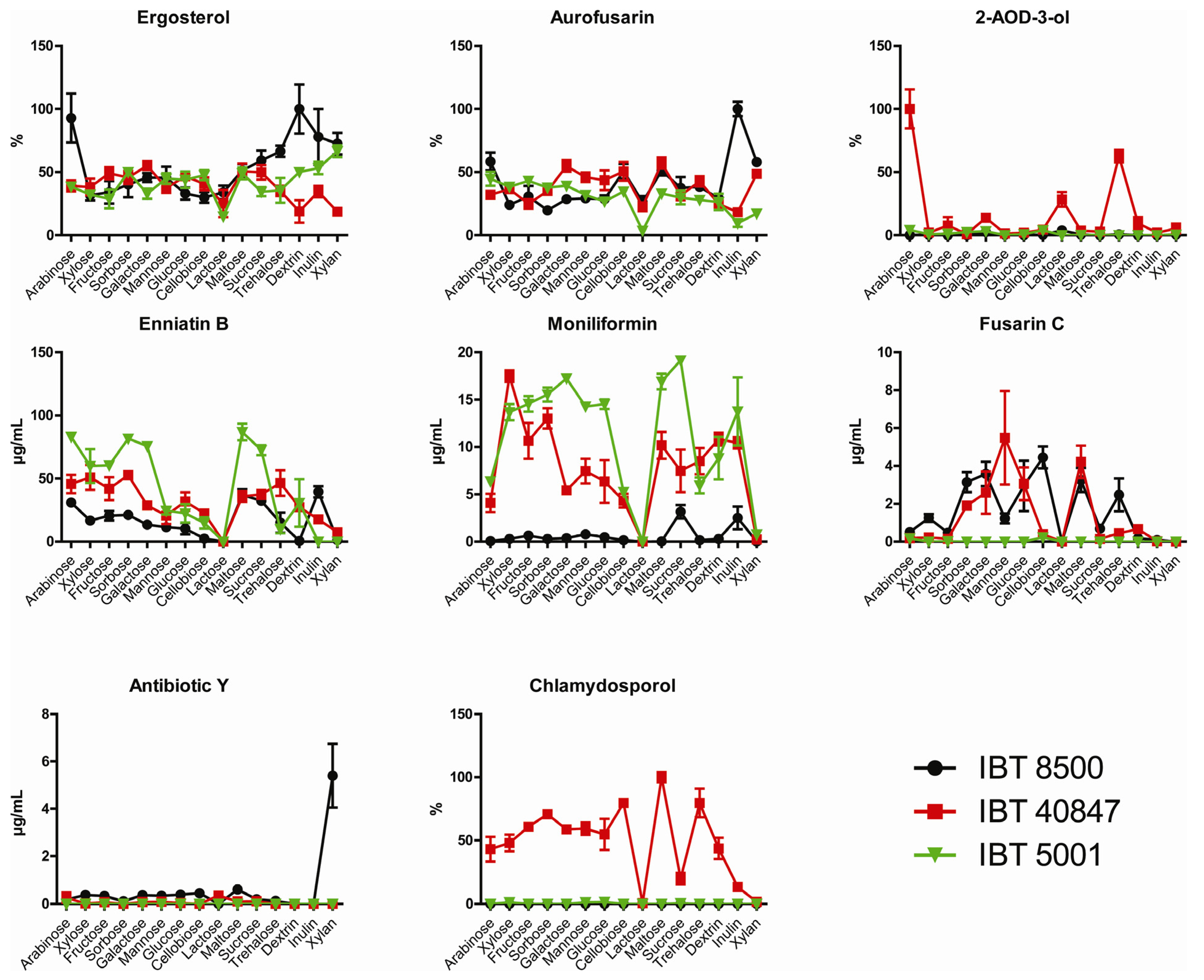
2.2. Growth and Production of Secondary Metabolites
3. Experimental Section
3.1. Chemicals
3.2. Fungal Isolates and Cultivation
3.3. Chemical Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sørensen, J.L.; Nielsen, K.F.; Thrane, U. Analysis of moniliformin in maize plants using hydrophilic interaction chromatography. J. Agric. Food Chem. 2007, 55, 9764–9768. [Google Scholar] [CrossRef]
- Kosiak, B.; Torp, M.; Skjerve, E.; Thrane, U. The prevalence and distribution of Fusarium species in Norwegian cereals: A survey. Acta Agric. Scand. Sect. B. Soil Plant Sci. 2003, 53, 168–176. [Google Scholar]
- Andersen, B.; Thrane, U.; Svendsen, A.; Rasmussen, I.A. Associated field mycobiota on malt barley. Can. J. Bot. 1996, 74, 854–858. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Parikka, P.; Konstantinova, P.; Gagkaeva, T.Y. Molecular and morphological diversity of Fusarium species in Finland and north-western Russia. Eur. J. Plant Pathol. 2004, 110, 573–585. [Google Scholar] [CrossRef]
- Turkington, T.K.; Clear, R.M.; Burnett, P.A.; Patrick, S.K.; Orr, D.D.; Xi, K. Fungal plant pathogens infecting barley and wheat seed from Alberta, 1995–1997. Can. J. Plant Pathol. 2002, 24, 302–308. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Phipps, R.K.; Nielsen, K.F.; Frank, J.; Schroers, H.J.; Thrane, U. Analysis of Fusarium avenaceum metabolites produced during wet apple core rot. J. Agric. Food Chem. 2009, 57, 1632–1639. [Google Scholar] [CrossRef]
- Peters, R.D.; Barasubiye, T.; Driscoll, J. Dry rot of rutabaga caused by Fusarium avenaceum. Hortscience 2007, 42, 737–739. [Google Scholar]
- Peters, J.C.; Lees, A.K.; Cullen, D.W.; Sullivan, L.; Stroud, G.P.; Cunnington, A.C. Characterization of Fusarium spp. responsible for causing dry rot of potato in Great Britain. Plant Pathol. 2008, 57, 262–271. [Google Scholar] [CrossRef]
- Tseng, T.C.; Tu, J.C. Mycoflora and mycotoxins in adzuki and mung beans produced in Ontario, Canada. Microbios 1997, 90, 87–95. [Google Scholar]
- Uhlig, S.; Jestoi, M.; Knutsen, A.K.; Heier, B.T. Multiple regression analysis as a tool for the identification of relations between semi-quantitative LC-MS data and cytotoxicity of extracts of the fungus Fusarium avenaceum (syn. F. arthrosporioides). Toxicon 2006, 48, 567–579. [Google Scholar] [CrossRef]
- Uhlig, S.; Jestoi, M.; Parikka, P. Fusarium avenaceum—The North European situation. Int. J. Food Microbiol. 2007, 119, 17–24. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging Fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef]
- Jestoi, M.; Rokka, M.; Yli-Mattila, T.; Parikka, P.; Rizzo, A.; Peltonen, K. Presence and concentrations of the Fusarium-related mycotoxins beauvericin, enniatins and moniliformin in Finnish grain samples. Food Addit. Contam. 2004, 21, 794–802. [Google Scholar] [CrossRef]
- Keller, N.P.; Hohn, T.M. Metabolic pathway gene clusters in filamentous fungi. Fungal Genet. Biol. 1997, 21, 17–29. [Google Scholar] [CrossRef]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Kazan, K.; Manners, J.M. Nutrient profiling reveals potent inducers of trichothecene biosynthesis in Fusarium graminearum. Fungal Genet. Biol. 2009, 46, 604–613. [Google Scholar] [CrossRef]
- Jiao, F.; Kawakami, A.; Nakajima, T. Effects of different carbon sources on trichothecene production and Tri gene expression by Fusarium graminearum in liquid culture. FEMS Microbiol. Lett. 2008, 285, 212–219. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Akk, E.; Thrane, U.; Giese, H.; Sondergaard, T.E. Production of fusarielins by Fusarium. Int. J. Food Microbiol. 2013, 160, 206–211. [Google Scholar] [CrossRef]
- Min, K.; Shin, Y.; Son, H.; Lee, J.; Kim, J.-C.; Choi, G.J.; Lee, Y.-W. Functional analyses of the nitrogen regulatory gene areA in Gibberella zeae. FEMS Microbiol. Lett. 2012, 334, 66–73. [Google Scholar] [CrossRef]
- Linnemannstöns, P.; Schulte, J.; Prado, M.D.; Proctor, R.H.; Avalos, J.; Tudzynski, B. The polyketide synthase gene pks4 from Gibberella fujikuroi encodes a key enzyme in the blosynthesis of the red pigment bikaverin. Fungal Genet. Biol. 2002, 37, 134–148. [Google Scholar] [CrossRef]
- Mihlan, M.; Homann, V.; Liu, T.W.D.; Tudzynski, B. AREA directly mediates nitrogen regulation of gibberellin biosynthesis in Gibberella fujikuroi, but its activity is not affected by NMR. Mol. Microbiol. 2003, 47, 975–991. [Google Scholar] [CrossRef]
- Macios, M.; Caddick, M.X.; Weglenski, P.; Scazzocchio, C.; Dzikowska, A. The GATA factors AREA and AREB together with the co-repressor NMRA, negatively regulate arginine catabolism in Aspergillus nidulans in response to nitrogen and carbon source. Fungal Genet. Biol. 2012, 49, 189–198. [Google Scholar] [CrossRef]
- Brakhage, A.A. Molecular regulation of beta-lactam biosynthesis in filamentous fungi. Microbiol. Mol. Biol. Rev. 1998, 62, 547–585. [Google Scholar]
- Kokkonen, M.; Ojala, L.; Parikka, P.; Jestoi, M. Mycotoxin production of selected Fusarium species at different culture conditions. Int. J. Food Microbiol. 2010, 143, 17–25. [Google Scholar] [CrossRef]
- Kleigrewe, K.; Sohnel, A.C.; Humpf, H.U. A new high-performance liquid chromatography-tandem mass spectrometry method based on dispersive solid phase extraction for the determination of the mycotoxin fusarin C in corn ears and processed corn samples. J. Agric. Food Chem. 2011, 59, 10470–10476. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Visconti, A.; Savard, M.E.; Blackwell, B.A.; Nelson, P.E. Isolation and characterization of new chlamydosporol related metabolites of Fusarium chlamydosporum and Fusarium tricinctum. Mycopathologia 1994, 127, 95–101. [Google Scholar] [CrossRef]
- Strauss, J.; Horvath, H.K.; Abdallah, B.M.; Kindermann, J.; Mach, R.L.; Kubicek, C.P. The function of CreA, the carbon catabolite repressor of Aspergillus nidulans, is regulated at the transcriptional and post-transcriptional level. Mol. Microbiol. 1999, 32, 169–178. [Google Scholar] [CrossRef]
- Flipphi, M.; van de Vondervoort, P.J.I.; Ruijter, G.J.G.; Visser, J.; Arst, H.N.; Felenbok, A. Onset of carbon catabolite repression in Aspergillus nidulans—Parallel involvement of hexokinase and glucokinase in sugar signaling. J. Biol. Chem. 2003, 278, 11849–11857. [Google Scholar]
- Hajjaj, H.; Niederberger, P.; Duboc, P. Lovastatin biosynthesis by Aspergillus terreus in a chemically defined medium. Appl. Environ. Microbiol. 2001, 67, 2596–2602. [Google Scholar] [CrossRef]
- Sondergaard, T.E.; Hansen, F.T.; Purup, S.; Nielsen, A.K.; Giese, H.; Sørensen, J.L. Fusarin C acts like an estrogenic agonist and stimulates breast cancer cells in vitro. Toxicol. Lett. 2011, 205, 116–121. [Google Scholar] [CrossRef]
- Yoder, W.T.; Christianson, L.M. Species-specific primers resolve members of Fusarium section Fusarium—Taxonomic status of the edible “Quorn” fungus reevaluated. Fungal Genet. Biol. 1998, 23, 68–80. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sørensen, J.L.; Giese, H. Influence of Carbohydrates on Secondary Metabolism in Fusarium avenaceum. Toxins 2013, 5, 1655-1663. https://doi.org/10.3390/toxins5091655
Sørensen JL, Giese H. Influence of Carbohydrates on Secondary Metabolism in Fusarium avenaceum. Toxins. 2013; 5(9):1655-1663. https://doi.org/10.3390/toxins5091655
Chicago/Turabian StyleSørensen, Jens Laurids, and Henriette Giese. 2013. "Influence of Carbohydrates on Secondary Metabolism in Fusarium avenaceum" Toxins 5, no. 9: 1655-1663. https://doi.org/10.3390/toxins5091655





