Limited Stability of Microcystins in Oligopeptide Compositions of Microcystis aeruginosa (Cyanobacteria): Implications in the Definition of Chemotypes
Abstract
:1. Introduction
2. Results
2.1. Oligopeptide Compositions
2.2. Growth Rates
| Peptide Name | Protonated m/z [M + H+] (Da) | UAM254 | UAM264 | UAM265 |
|---|---|---|---|---|
| Desmethyl-Mcyst-LR | 981.5 | x | x | x |
| Mcyst-LR | 995.6 | x | x | x |
| Cyanopeptolin 1006A | 1007.5 | x | x | |
| Desmethyl-Mcyst-RR | 1024.6 | x | x | x |
| Mcyst-FR | 1029.5 | x | x | x |
| Desmethyl-Mcyst-YR | 1031.5 | x | x | x |
| Mcyst-RR | 1038.6 | x | x | x |
| Mcyst-YR | 1045.5 | x | x | x |
| Mcyst-H4YR | 1049.6 | x | x | x |
| Mcyst-WR | 1068.6 | x | x | x |
| Strain | Growth rates (day−1) | Relative intensity threshold (a) | |
|---|---|---|---|
| Strain UAM254 | NC | 0.146 | 0.04 |
| NN | 0.108 (*) | 0.34 | |
| NP | 0.181 | 0.04 | |
| LL | 0.135 | 0.08 | |
| LM | 0.060 (*) | 0.25 | |
| LH | 0.070 (*) | 0.25 | |
| Strain UAM264 | NC | 0.274 | 0 (stable) |
| NN | 0.205 | 0 (stable) | |
| NP | 0.275 | 0 (stable) | |
| LL | 0.206 | 0 (stable) | |
| LM | 0.117 (*) | 0.03 | |
| LH | 0.163 (*) | 0.03 | |
| Strain UAM265 | NC | 0.383 | 0 (stable) |
| NN | 0.172 (*) | 0.07 | |
| NP | 0.179 (*) | 0.06 | |
| LL | 0.190 | 0 (stable) | |
| LM | 0.103 (*) | 0.03 | |
| LH | ----- | ----- |
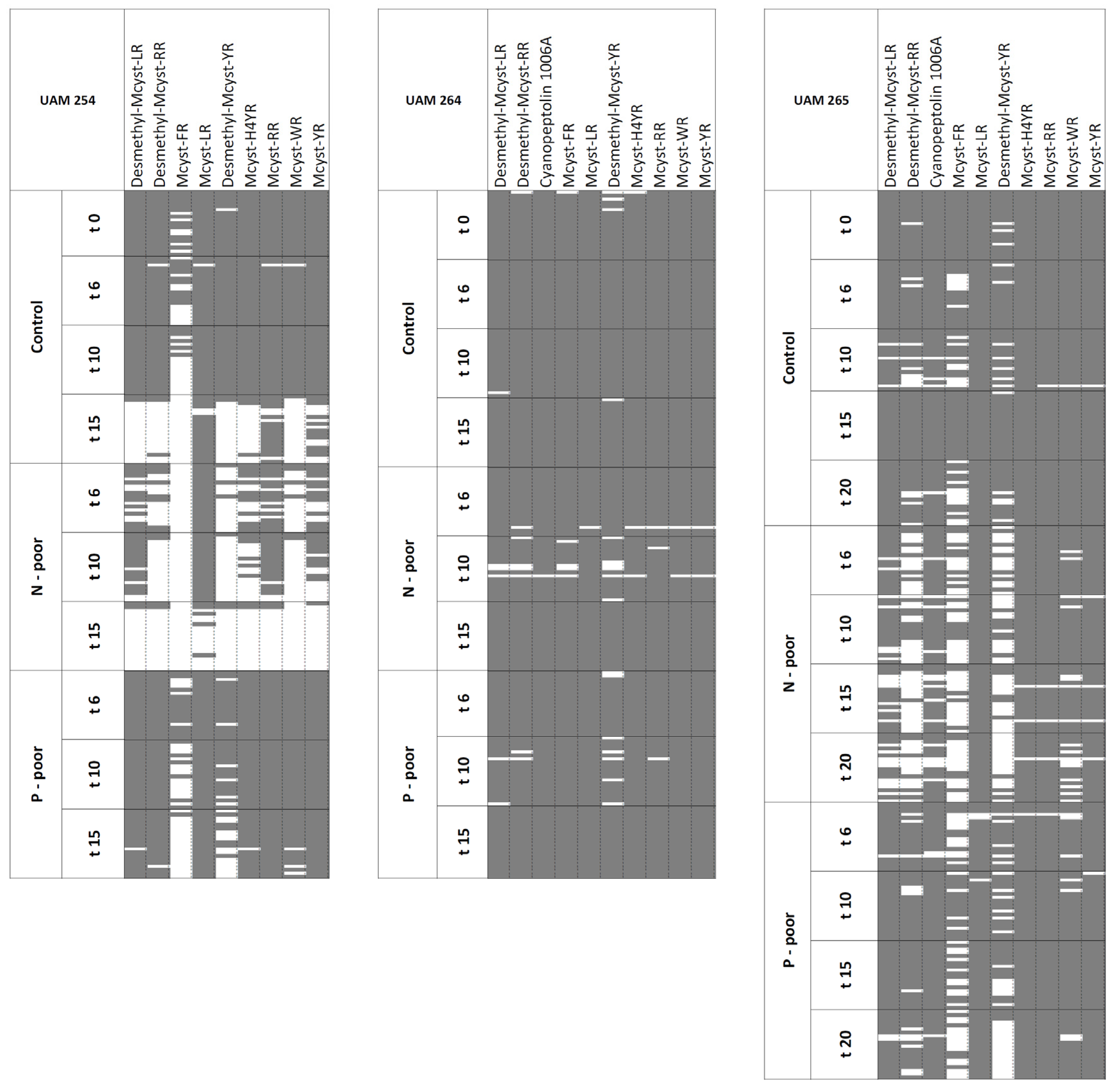
2.3. Stability under N- and P-Reduction
| IDF | Average relative intensities | IDF | Average relative intensities | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain UAM254 | Total | NC | NN | NP | Total | NC | NN | NP | Strain UAM254 | Total | LL | LM | LH | Total | LL | LM | LH | ||||||
| MC-LR | 91% | 96% | 75% | 100% | STABLE | 1.00 | 1.00 | 1.00 | 1.00 | MC-RR | 79% | 99% | 76% | 61% | STABLE | 0.97 | 1.00 | 0.89 | 0.98 | ||||
| MC-RR | 84% | 94% | 60% | 100% | STABLE | 0.63 | 0.73 | 0.39 | 0.65 | MC-LR | 87% | 97% | 91% | 70% | STABLE | 0.61 | 0.29 | 0.87 | 0.91 | ||||
| MC-YR | 78% | 89% | 42% | 100% | UNSTABLE | 0.34 | 0.39 | 0.24 | 0.31 | MC-YR | 59% | 91% | 37% | 34% | UNSTABLE | 0.25 | 0.24 | 0.23 | 0.28 | ||||
| MC-H4YR | 71% | 80% | 33% | 98% | UNSTABLE | 0.18 | 0.20 | 0.17 | 0.16 | Desmethyl-MC-RR | 61% | 93% | 33% | 41% | UNSTABLE | 0.24 | 0.25 | 0.15 | 0.29 | ||||
| Desmethyl-MC-LR | 76% | 77% | 52% | 98% | UNSTABLE | 0.18 | 0.15 | 0.31 | 0.13 | MC-WR | 56% | 93% | 34% | 24% | UNSTABLE | 0.16 | 0.17 | 0.15 | 0.14 | ||||
| Desmethyl-MC-RR | 66% | 77% | 20% | 98% | UNSTABLE | 0.14 | 0.15 | 0.15 | 0.13 | Desmethyl-MC-LR | 32% | 47% | 20% | 23% | UNSTABLE | 0.08 | 0.05 | 0.08 | 0.15 | ||||
| MC-WR | 61% | 75% | 10% | 95% | UNSTABLE | 0.11 | 0.13 | 0.08 | 0.10 | Desmethyl-MC-YR | 8% | 18% | 1% | 1% | UNSTABLE | 0.06 | 0.06 | 0.04 | 0.07 | ||||
| Desmethyl-MC-YR | 53% | 76% | 10% | 67% | UNSTABLE | 0.08 | 0.09 | 0.11 | 0.06 | MC-FR | 14% | 28% | 7% | 1% | UNSTABLE | 0.03 | 0.03 | 0.03 | 0.04 | ||||
| MC-FR | 26% | 37% | 0% | 37% | UNSTABLE | 0.04 | 0.04 | ND | 0.04 | MC-H4YR | 13% | 25% | 5% | 3% | UNSTABLE | 0.03 | 0.03 | 0.04 | 0.05 | ||||
| Strain UAM264 | Total | NC | NN | NP | Total | NC | NN | NP | Strain UAM264 | Total | LL | LM | LH | Total | LL | LM | LH | ||||||
| MC-LR | 99% | 100% | 97% | 100% | STABLE | 0.99 | 0.97 | 0.99 | 1.00 | MC-RR | 100% | 100% | 100% | 100% | STABLE | 0.80 | 1.00 | 0.71 | 0.60 | ||||
| MC-RR | 98% | 100% | 95% | 98% | STABLE | 0.81 | 0.85 | 0.79 | 0.77 | MC-LR | 100% | 100% | 100% | 100% | STABLE | 0.79 | 0.51 | 0.99 | 0.99 | ||||
| MC-YR | 99% | 100% | 95% | 100% | STABLE | 0.47 | 0.53 | 0.39 | 0.47 | Cyanopeptolin 1006A | 100% | 99% | 100% | 100% | STABLE | 0.33 | 0.15 | 0.36 | 0.56 | ||||
| MC-H4YR | 98% | 99% | 95% | 100% | STABLE | 0.34 | 0.32 | 0.26 | 0.43 | MC-YR | 100% | 100% | 100% | 100% | STABLE | 0.33 | 0.29 | 0.38 | 0.33 | ||||
| Cyanopeptolin 1006A | 99% | 100% | 97% | 100% | STABLE | 0.30 | 0.26 | 0.37 | 0.30 | MC-WR | 100% | 99% | 100% | 100% | STABLE | 0.20 | 0.17 | 0.25 | 0.18 | ||||
| MC-WR | 99% | 100% | 95% | 100% | STABLE | 0.23 | 0.27 | 0.19 | 0.22 | MC-FR | 100% | 99% | 100% | 100% | STABLE | 0.16 | 0.10 | 0.17 | 0.23 | ||||
| MC-FR | 97% | 99% | 92% | 100% | STABLE | 0.10 | 0.10 | 0.09 | 0.11 | MC-H4YR | 98% | 96% | 99% | 100% | STABLE | 0.12 | 0.06 | 0.17 | 0.13 | ||||
| Desmethyl-MC-RR | 96% | 99% | 90% | 97% | STABLE | 0.09 | 0.09 | 0.10 | 0.09 | Desmethyl-MC-RR | 97% | 99% | 95% | 95% | STABLE | 0.11 | 0.14 | 0.09 | 0.08 | ||||
| Desmethyl-MC-LR | 97% | 99% | 93% | 97% | STABLE | 0.09 | 0.08 | 0.11 | 0.09 | Desmethyl-MC-LR | 95% | 95% | 94% | 96% | STABLE | 0.06 | 0.04 | 0.06 | 0.07 | ||||
| Desmethyl-MC-YR | 91% | 95% | 88% | 88% | STABLE | 0.05 | 0.05 | 0.05 | 0.05 | Desmethyl-MC-YR | 52% | 75% | 47% | 23% | UNSTABLE | 0.03 | 0.03 | 0.03 | 0.04 | ||||
| Strain UAM265 | Total | NC | NN | NP | Total | NC | NN | NP | Strain UAM265 | Total | LL | LM | LH | Total | LL | LM | LH | ||||||
| MC-LR | 99% | 100% | 100% | 96% | STABLE | 1.00 | 1.00 | 1.00 | 1.00 | MC-RR | 100% | 100% | 100% | STABLE | 0.88 | 1.00 | 0.71 | ||||||
| MC-H4YR | 98% | 100% | 96% | 99% | STABLE | 0.39 | 0.32 | 0.28 | 0.58 | MC-LR | 100% | 100% | 100% | STABLE | 0.74 | 0.57 | 0.99 | ||||||
| MC-RR | 98% | 99% | 96% | 99% | STABLE | 0.33 | 0.38 | 0.26 | 0.34 | MC-YR | 100% | 100% | 100% | STABLE | 0.42 | 0.38 | 0.48 | ||||||
| MC-YR | 98% | 99% | 95% | 99% | STABLE | 0.33 | 0.38 | 0.28 | 0.32 | MC-WR | 100% | 100% | 100% | STABLE | 0.23 | 0.20 | 0.27 | ||||||
| Cyanopeptolin 1006A | 92% | 96% | 82% | 96% | STABLE | 0.17 | 0.15 | 0.13 | 0.23 | Cyanopeptolin 1006A | 100% | 100% | 100% | STABLE | 0.23 | 0.13 | 0.36 | ||||||
| MC-WR | 90% | 99% | 78% | 91% | STABLE | 0.14 | 0.16 | 0.13 | 0.13 | MC-H4YR | 99% | 99% | 100% | STABLE | 0.15 | 0.09 | 0.23 | ||||||
| Desmethyl-MC-LR | 88% | 97% | 69% | 96% | STABLE | 0.13 | 0.12 | 0.09 | 0.17 | Desmethyl-MC-RR | 100% | 100% | 100% | STABLE | 0.12 | 0.14 | 0.10 | ||||||
| Desmethyl-MC-RR | 67% | 86% | 27% | 84% | UNSTABLE | 0.07 | 0.06 | 0.04 | 0.08 | MC-FR | 99% | 99% | 100% | STABLE | 0.10 | 0.08 | 0.14 | ||||||
| Desmethyl-MC-YR | 58% | 85% | 26% | 56% | UNSTABLE | 0.06 | 0.06 | 0.04 | 0.07 | Desmethyl-MC-LR | 98% | 99% | 96% | STABLE | 0.06 | 0.04 | 0.08 | ||||||
| MC-FR | 53% | 74% | 29% | 50% | UNSTABLE | 0.05 | 0.05 | 0.04 | 0.05 | Desmethyl-MC-YR | 77% | 88% | 60% | UNSTABLE | 0.03 | 0.03 | 0.05 | ||||||
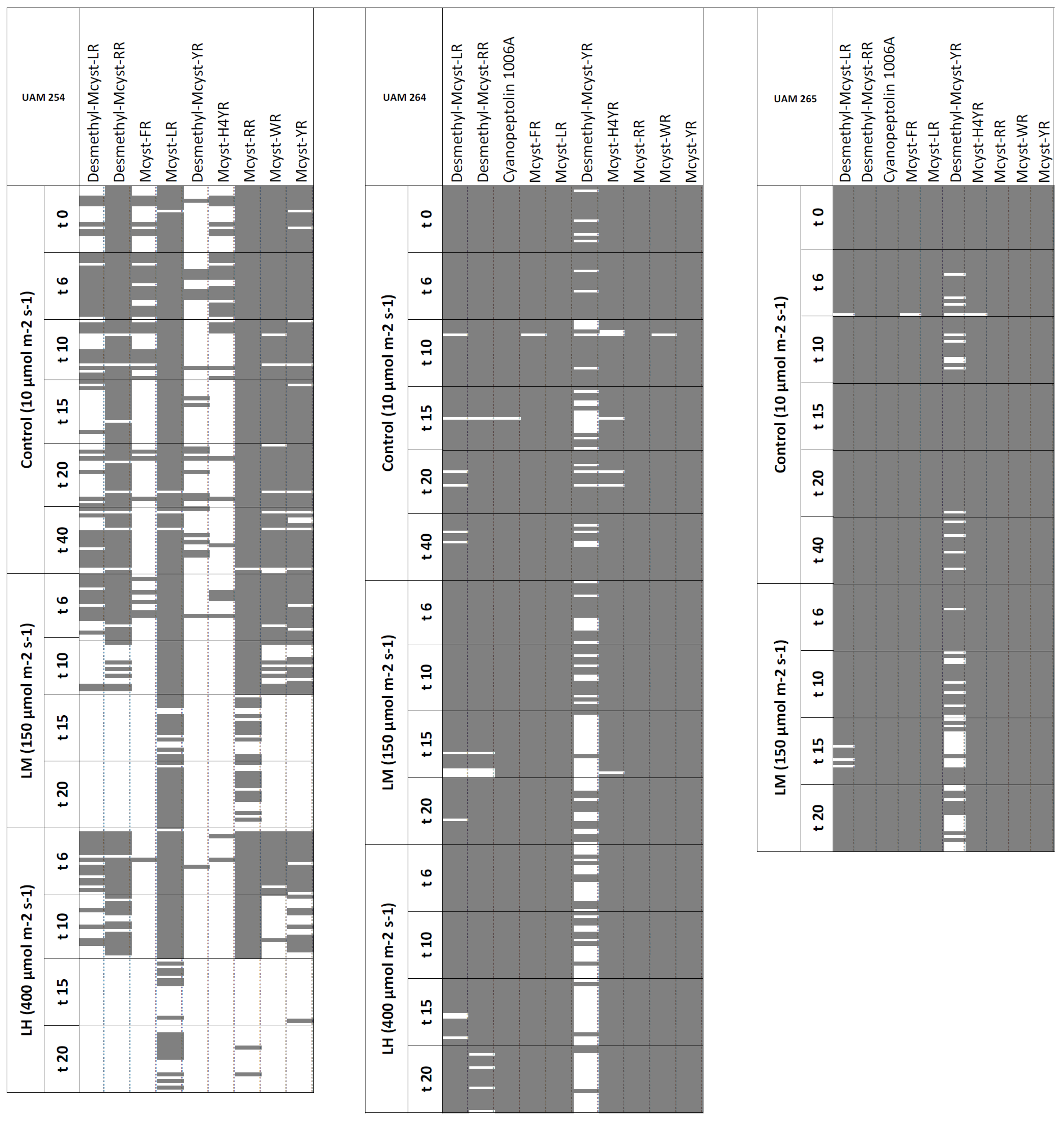
2.4. Stability under High Light Intensities
2.5. Extent of the Peptide Fingerprint Stability
3. Discussion
4. Experimental Section
4.1. Microcystis aeruginosa Strains
4.2. Experimental Setup
4.3. MALDI-TOF MS Analysis
4.4. Data Processing
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar]
- Komarek, J.; Kling, H. Variation in 6 planktonic cyanophyte genera in lake Victoria (East-Africa). Arch. Hydrobiol. 1991, 88, 21–45. [Google Scholar]
- Laamanen, M.J.; Gugger, M.F.; Lehtimaki, J.M.; Haukka, K.; Sivonen, K. Diversity of toxic and nontoxic Nodularia isolates (Cyanobacteria) and filaments from the Baltic Sea. Appl. Environ. Microbiol. 2001, 67, 4638–4647. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; E & FN Spoon: London, UK, 1999; pp. 41–111. [Google Scholar]
- Neilan, B.A.; Dittmann, E.; Rouhiainen, L.; Bass, R.A.; Schaub, V.; Sivonen, K.; Borner, T. Nonribosomal peptide synthesis and toxigenicity of cyanobacteria. J. Bacteriol. 1999, 181, 4089–4097. [Google Scholar]
- Welker, M.; von Dohren, H. Cyanobacterial peptides—Nature’s own combinatorial biosynthesis. Fems Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef]
- Dittmann, E.; Neilan, B.A.; Erhard, M.; von Dohren, H.; Borner, T. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol. Microbiol. 1997, 26, 779–787. [Google Scholar] [CrossRef]
- Fastner, J.; Erhard, M.; von Dohren, H. Determination of oligopeptide diversity within a natural population of Microcystis spp. (Cyanobacteria) by typing single colonies by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2001, 67, 5069–5076. [Google Scholar] [CrossRef]
- Welker, M.; Sejnohova, L.; Nemethova, D.; von Dohren, H.; Jarkovsky, J.; Marsalek, B. Seasonal shifts in chemotype composition of Microcystis sp communities in the pelagial and the sediment of a shallow reservoir. Limnol. Oceanogr. 2007, 52, 609–619. [Google Scholar] [CrossRef]
- Rohrlack, T.; Edvardsen, B.; Skulberg, R.; Halstvedt, C.B.; Utkilen, H.C.; Ptacnik, R.; Skulberg, O.M. Oligopeptide chemotypes of the toxic freshwater cyanobacterium Planktothrix can form subpopulations with dissimilar ecological traits. Limnol. Oceanogr. 2008, 53, 1279–1293. [Google Scholar] [CrossRef]
- Carrasco, D.; Moreno, E.; Sanchis, D.; Wormer, L.; Paniagua, T.; del Cueto, A.; Quesada, A. Cyanobacterial abundance and microcystin occurrence, in Mediterranean water reservoirs in Central Spain: microcystins in the Madrid area. Eur. J. Phycol. 2006, 41, 281–291. [Google Scholar] [CrossRef]
- Orr, P.T.; Jones, G.J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 1998, 43, 1604–1614. [Google Scholar] [CrossRef]
- Agha, R.; Cires, S.; Woermer, L.; Antonio Dominguez, J.; Quesada, A. Multi-scale strategies for the monitoring of freshwater cyanobacteria: Reducing the sources of uncertainty. Water Res. 2012, 46, 3043–3053. [Google Scholar] [CrossRef]
- Halstvedt, C.B.; Rohrlack, T.; Ptacnik, R.; Edvardsen, B. On the effect of abiotic environmental factors on production of bioactive oligopeptides in field populations of Planktothrix spp. (Cyanobacteria). J. Plankton Res. 2008, 30, 607–617. [Google Scholar] [CrossRef]
- Repka, S.; Koivula, M.; Harjunpa, V.; Rouhiainen, L.; Sivonen, K. Effects of phosphate and light on growth of and bioactive peptide production by the cyanobacterium Anabaena strain 90 and its anabaenopeptilide mutant. Appl. Environ. Microbiol. 2004, 70, 4551–4560. [Google Scholar] [CrossRef]
- Rohrlack, T.; Utkilen, H. Effects of nutrient and light availability on production of bioactive anabaenopeptins and microviridin by the cyanobacterium Planktothrix agardhii. Hydrobiologia 2007, 583, 231–240. [Google Scholar] [CrossRef]
- Cook, C.M.; Vardaka, E.; Lanaras, T. Toxic cyanobacteria in Greek freshwaters, 1987–2000: Occurrence, toxicity, and impacts in the Mediterranean region. Acta Hydrochim. Hydrobiol. 2004, 32, 107–124. [Google Scholar] [CrossRef]
- Welker, M.; Marsalek, B.; Sejnohova, L.; von Dohren, H. Detection and identification of oligopeptides in Microcystis (cyanobacteria) colonies: Toward an understanding of metabolic diversity. Peptides 2006, 27, 2090–2103. [Google Scholar] [CrossRef]
- Erhard, M.; von Dohren, H.; Jungblut, P. Rapid typing and elucidation of new secondary metabolites of intact cyanobacteria using MALDI-TOF mass spectrometry. Nat. Biotechnol. 1997, 15, 906–909. [Google Scholar] [CrossRef]
- Welker, M.; Fastner, J.; Erhard, M.; von Dohren, H. Applications of MALDI-TOF MS analysis in cyanotoxin research. Environ. Toxicol. 2002, 17, 367–374. [Google Scholar] [CrossRef]
- Martins, J.; Saker, M.L.; Moreira, C.; Welker, M.; Fastner, J.; Vasconcelos, V.M. Peptide diversity in strains of the cyanobacterium Microcystis aeruginosa isolated from Portuguese water supplies. Appl. Microbiol. Biotechnol. 2009, 82, 951–961. [Google Scholar] [CrossRef]
- Bister, B.; Keller, S.; Baumann, H.I.; Nicholson, G.; Weist, S.; Jung, G.; Sussmuth, R.D.; Juttner, F. Cyanopeptolin 963A, a chymotrypsin inhibitor of Microcystis PCC 7806. J. Nat. Prod. 2004, 67, 1755–1757. [Google Scholar] [CrossRef]
- Tillett, D.; Dittmann, E.; Erhard, M.; von Dohren, H.; Borner, T.; Neilan, B.A. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: An integrated peptide-polyketide synthetase system. Chem. Biol. 2000, 7, 753–764. [Google Scholar] [CrossRef]
- Kosol, S.; Schmidt, J.; Kurmayer, R. Variation in peptide net production and growth among strains of the toxic cyanobacterium Planktothrix spp. Eur. J. Phycol. 2009, 44, 49–62. [Google Scholar] [CrossRef]
- Wu, Z.X.; Song, L.R. Physiological comparison between colonial and unicellular forms of Microcystis aeruginosa Kutz. (Cyanobacteria). Phycologia 2008, 47, 98–104. [Google Scholar] [CrossRef]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Borner, T.; Dittmann, E. The Cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS One 2011, 6, e17615. [Google Scholar] [CrossRef]
- Kurmayer, R.; Dittmann, E.; Fastner, J.; Chorus, I. Diversity of microcystin genes within a population of the toxic cyanobacterium Microcystis spp. in Lake Wannsee (Berlin, Germany). Microb. Ecol. 2002, 43, 107–118. [Google Scholar]
- Van de Waal, D.B.; Ferreruela, G.; Tonk, L.; van Donk, E.; Huisman, J.; Visser, P.M.; Matthijs, H.C.P. Pulsed nitrogen supply induces dynamic changes in the amino acid composition and microcystin production of the harmful cyanobacterium Planktothrix agardhii. FEMS Microbiol. Ecol. 2010, 74, 430–438. [Google Scholar] [CrossRef]
- Tonk, L.; van de Waal, D.B.; Slot, P.; Huisman, J.; Matthijs, H.C.P.; Visser, P.M. Amino acid availability determines the ratio of microcystin variants in the cyanobacterium Planktothrix agardhii. FEMS Microbiol. Ecol. 2008, 65, 383–390. [Google Scholar] [CrossRef]
- Krause, E.; Wenschuh, H.; Jungblut, P.R. The dominance of arginine-containing peptides in MALDI-derived tryptic mass fingerprints of proteins. Anal. Chem. 1999, 71, 4160–4165. [Google Scholar] [CrossRef]
- Sanchis, D.; Carrasco, D.; Padilla, C.; Leganes, F.; Fernandez-Valiente, E.; del Campo, F.F.; Quesada, A. Spatial and temporal heterogeneity in succession of cyanobacterial blooms in a Spanish reservoir. Ann. Limnol. Int. J. Limnol. 2002, 38, 173–183. [Google Scholar] [CrossRef]
- Sanchis, D.; Carrasco, D.; Quesada, A. The genus Microcystis (Microcystaceae/Cyanobacteria) from a Spanish reservoir: A contribution to the definition of morphological variations. Nova Hedwig. 2004, 79, 479–495. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Agha, R.; Cirés, S.; Wörmer, L.; Quesada, A. Limited Stability of Microcystins in Oligopeptide Compositions of Microcystis aeruginosa (Cyanobacteria): Implications in the Definition of Chemotypes. Toxins 2013, 5, 1089-1104. https://doi.org/10.3390/toxins5061089
Agha R, Cirés S, Wörmer L, Quesada A. Limited Stability of Microcystins in Oligopeptide Compositions of Microcystis aeruginosa (Cyanobacteria): Implications in the Definition of Chemotypes. Toxins. 2013; 5(6):1089-1104. https://doi.org/10.3390/toxins5061089
Chicago/Turabian StyleAgha, Ramsy, Samuel Cirés, Lars Wörmer, and Antonio Quesada. 2013. "Limited Stability of Microcystins in Oligopeptide Compositions of Microcystis aeruginosa (Cyanobacteria): Implications in the Definition of Chemotypes" Toxins 5, no. 6: 1089-1104. https://doi.org/10.3390/toxins5061089





