Ochratoxin A Management in Vineyards by Lobesia botrana Biocontrol
Abstract
:1. Introduction
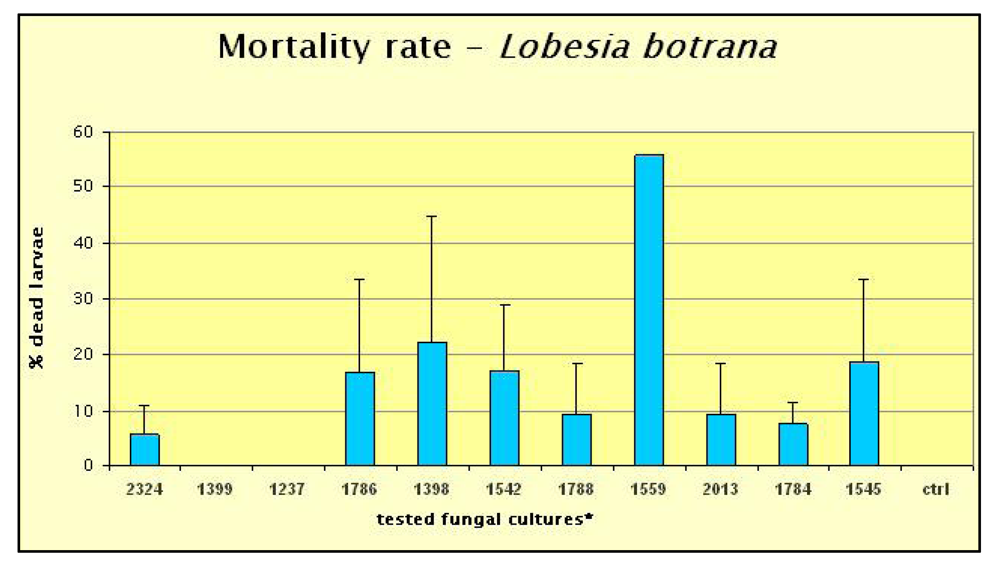
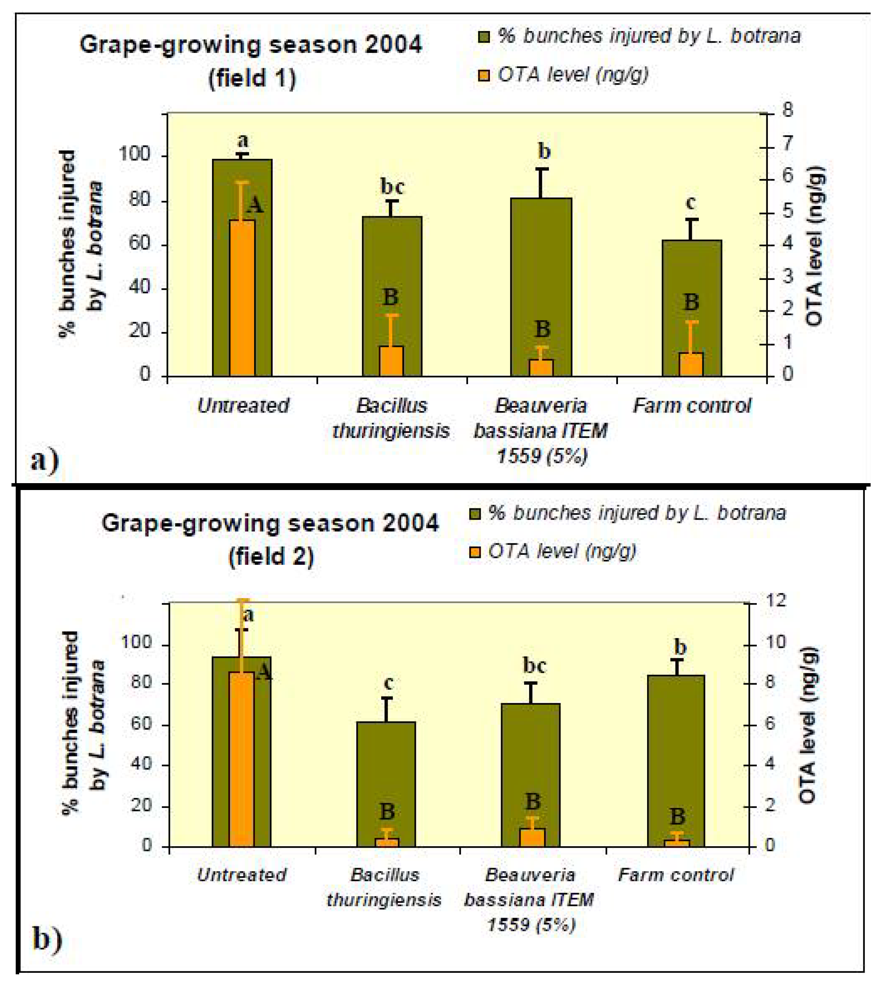
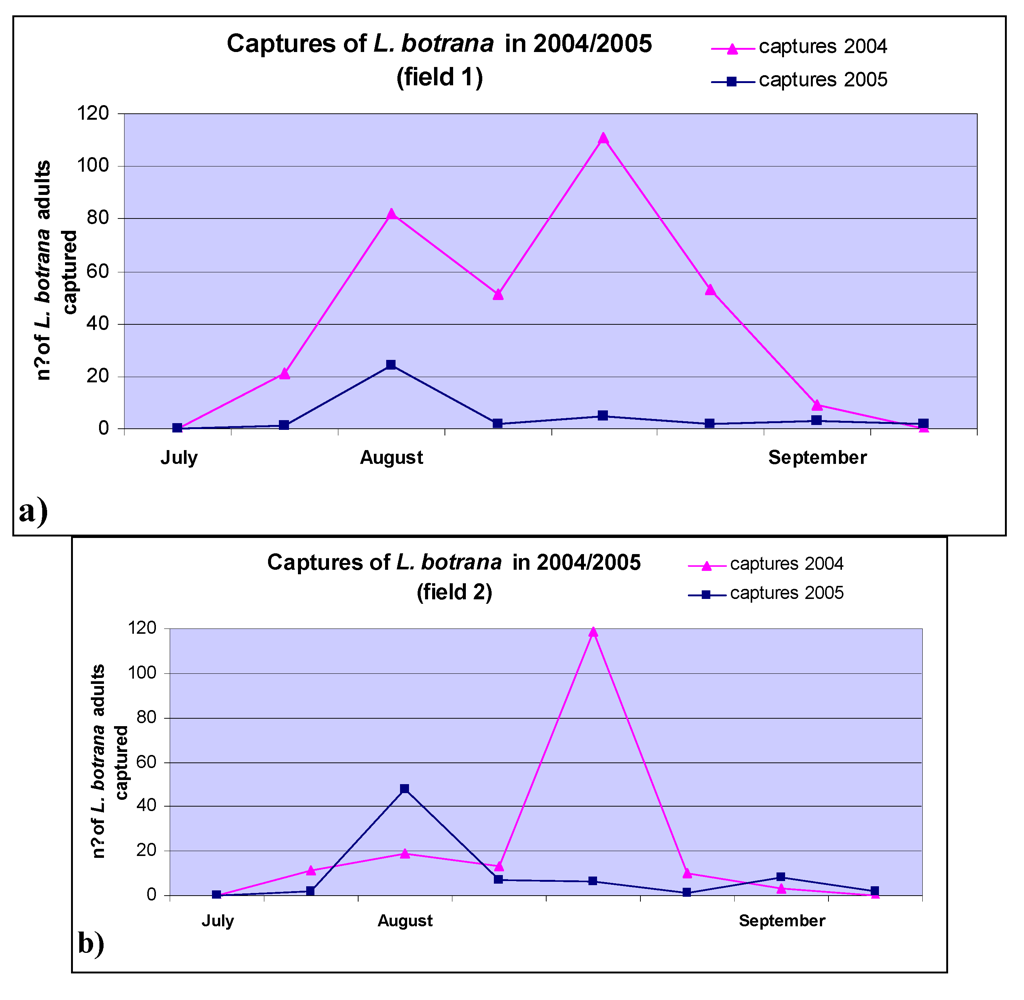
2. Results and Discussion

3. Experimental Section
3.1. Entomopathogenic Fungal Strains Selection
3.2. Formulation with B. bassiana ITEM-1559 Preparation for Field Trials
3.3. Field Trials
3.4. Lobesia Botrana
3.5. Sampling of Grapes
3.6. Determination of Ochratoxin A (OTA)
3.7. Statistics
4. Conclusions
Conflict of Interest
Acknowledgements
References
- El Khoury, A.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef]
- Haighton, L.A.; Lynch, B.S.; Magnuson, B.A.; Nestmann, E.R. A reassessment of risk associated with dietary intake of ochratoxin A based on a lifetime exposure model. Crit. Rev. Toxicol. 2012, 42, 147–168. [Google Scholar]
- International Agency for Research on Cancer, Monographs on Evaluation of Carcinogenic Risks to Humans. In Some Naturally Occurring Substances: Food Items and cConstituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; Volume 56, pp. 489–521.
- Somma, S.; Perrone, G.; Logrieco, A.F. Diversity of black Aspergilli and mycotoxin risks in grape, wine and dried vine fruits. Phytopathol. Mediterr. 2012, 51, 131–147. [Google Scholar]
- Abarca, M.L.; Accensi, F.; Bragulat, M.R.; Cabañes, F.J. Current importance of ochratoxin A-producing Aspergillus spp. J. Food Prot. 2001, 64, 903–906. [Google Scholar]
- Sage, L.; Krivobok, S.; Delbos, E.; Seigle-Murandi, F.; Creppy, E.E. Fungal flora and ochratoxin A production in grapes and musts from France. J. Agric. Food Chem. 2002, 50, 1306–1311. [Google Scholar] [CrossRef]
- Da Rocha Rosa, C.A.; Palacios, V.; Combina, M.; Fraga, M.E.; de Oliveira Rekson, A.; Magnoli, C.E.; Dalcero, A.M. Potential ochratoxin A producers from wine grapes in Argentina and Brazil. Food Addit. Contam. 2002, 19, 408–414. [Google Scholar] [CrossRef]
- Leong, S.L.; Hocking, A.D.; Pitt, J.I.; Kazi, B.A.; Emmett, R.W.; Scott, E.S. Australian research on ochratoxigenic fungi and ochratoxin A. Int. J. Food Microb. 2006, 111, S10–S17. [Google Scholar] [CrossRef]
- Perrone, G.; Mulè, G.; Susca, A.; Battilani, P.; Pietri, A.; Logrieco, A. Ochratoxin A production and amplified fragment lenght polymorphism analysis of Apergillus carbonarius, Aspergillus tubingensis and Aspergillus niger strains isolated from grapes in Italy. Appl. Environ. Microbiol. 2006, 72, 680–685. [Google Scholar] [CrossRef]
- Tremblay, E. Entomologia Applicata V2; Liguori, Liguori: Napoli, Italy, 1986. [Google Scholar]
- Cozzi, G.; Pascale, M.; Perrone, G.; Visconti, A.; Logrieco, A. Effect of Lobesia botrana damages on black aspergilli rot and ochratoxin A content in grapes. Int. J. Food Microb. 2006, 111, S88–S92. [Google Scholar] [CrossRef]
- Rousseau, J.; Blateyron, L.; Drouillard, J.B. Incidence of Harvest Conditions of Grapes on Ochratoxin A in Wine. In Proceedings of International Workshop “Ochratoxin A in Grapes and Wine: Prevention and Control”, Marsala, Italy, 20–21 October 2005.
- Cozzi, G.; Haidukowski, M.; Perrone, G.; Visconti, A.; Logrieco, A. Influence of Lobesia botrana field control on black aspergilli rot and ochratoxin A contamination in grapes. J. Food Prot. 2009, 72, 894–897. [Google Scholar]
- Butt, T.M.; Jackson, C.W.; Magan, N. Introduction—Fungal Biological Control Agents: Progress, Problems and Potential. In Fungi as Biocontrol Agents: Progress, Problems and Potential; Butt, T.M., Jackson, C.W., Magan, N., Eds.; CABI Publishing: Wallingford, Oxon, UK, 2001; pp. 1–8. [Google Scholar]
- Valero, A.; Marín, S.; Ramos, A.J.; Sanchis, V. Ochratoxin A-producing species in grapes and sun-dried grapes and their relation to ecophysiological factors. Lett. Appl. Microbiol. 2005, 41, 196–201. [Google Scholar] [CrossRef]
- Battilani, P.; Pietri, A.; Logrieco. Risk assessment and management in practice: Ochratoxin in grapes and wine: Mycotoxin in food: Detection and control. Woodhead 2004, 244–261. [Google Scholar]
- Assocodipuglia Associazione Regionale Consorzi Difesa Puglia. Available online: http://www.agrometeopuglia.it/opencms/opencms/Agrometeo/home_agro (accessed on 20 November 2012).
- Visconti, A.; Perrone, G.; Cozzi, G.; Solfrizzo, M. Managing ochratoxin A risk in the grape-wine food chain. Food Addit. Contam. A 2008, 25, 193–202. [Google Scholar] [CrossRef]
- Bleve, G.; Greco, F.; Cozzi, G.; Logrieco, A.; Visconti, A. Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. Int. J. Food Microbiol. 2006, 108, 204–209. [Google Scholar] [CrossRef]
- Ponsone, M.L.; Chiotta, M.L.; Combina, M.; Dalcero, A.; Chulze, S. Biocontrol as a strategy to reduce the impact of ochratoxin A and Aspergillus section Nigri in grapes. Int. J. Food Microbiol. 2011, 151, 70–77. [Google Scholar] [CrossRef]
- Hamill, R.L.; Higgens, C.E.; Boaz, H.E.; Gorman, M. The structure of beauvericin, a new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Lett. 1969, 49, 4255–4258. [Google Scholar]
- Sanchis, V.; Bourget, D. Bacillus thuringiensis: Applications in agriculture and insect resistance management. A review. Agron. Sustain. 2008, 28, 11–20. [Google Scholar] [CrossRef]
- ITEM Collection. Available online: http://server.ispa.cnr.it/ITEM/Collection/ (accessed on 26 September 2012).
- Fermaud, M. Cultivar susceptibility of grape berry clusters to larvae of Lobesia botrana (Lepidoptera: Tortricidae). J. Econ. Entomol. 1998, 91, 974–980. [Google Scholar]
- Battilani, P.; Logrieco, A.; Giorni, P.; Cozzi, G.; Bertuzzi, T.; Pietri, A. Ochratoxin A production by Aspergillus carbonarius on some grape varieties grown in Italy. J. Sci. Food Agric. 2004, 84, 1736–1740. [Google Scholar] [CrossRef]
- Visconti, A.; Pascale, M.; Centonze, G. Determination of ochratoxin A in wine by means of immunoaffinity column clean-up and hig-performance liquid chromatography. J. Chromatogr. A 1999, 864, 89–101. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cozzi, G.; Somma, S.; Haidukowski, M.; Logrieco, A.F. Ochratoxin A Management in Vineyards by Lobesia botrana Biocontrol. Toxins 2013, 5, 49-59. https://doi.org/10.3390/toxins5010049
Cozzi G, Somma S, Haidukowski M, Logrieco AF. Ochratoxin A Management in Vineyards by Lobesia botrana Biocontrol. Toxins. 2013; 5(1):49-59. https://doi.org/10.3390/toxins5010049
Chicago/Turabian StyleCozzi, Giuseppe, Stefania Somma, Miriam Haidukowski, and Antonio F. Logrieco. 2013. "Ochratoxin A Management in Vineyards by Lobesia botrana Biocontrol" Toxins 5, no. 1: 49-59. https://doi.org/10.3390/toxins5010049




