Pharmacological Aspects of Vipera xantina palestinae Venom
Abstract
:1. Introduction
2. V.x.p. Venom Active Components
2.1. Neurotoxins
2.2. Hemorrhagins
2.3. Proteomics
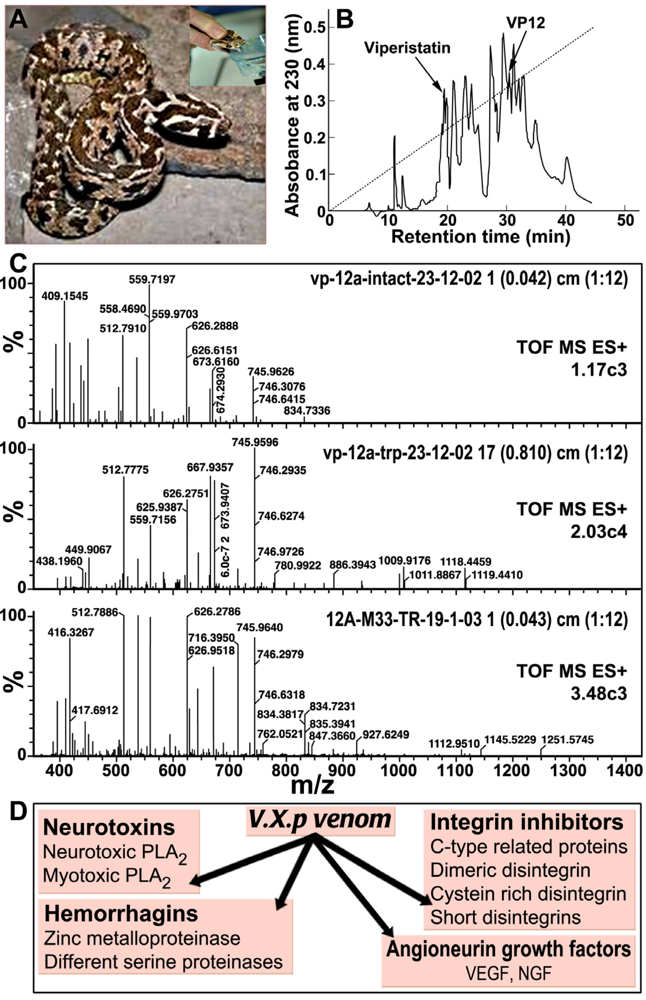
3. V.x.p. Growth Factors: Vascular Endothelial Growth Factors (VEGF) and Nerve Growth Factor (NGF)
4. V.x.p. Integrin Inhibitors
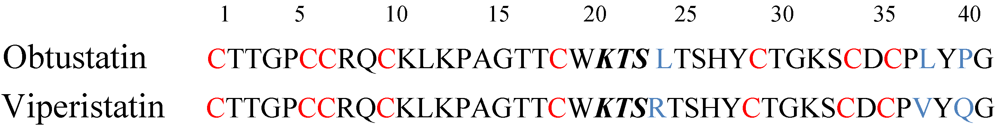
5. Antivenom Therapy of V.x.p. Envenomation
6. Conclusions
Conflict of Interest
Acknowledgements
References
- Paret, G.; Ben-Abraham, R.; Ezra, D.; Shrem, D.; Eshel, G.; Vardi, A.; Winkler, E.; Barzilay, Z. Vipera palaestinae snake envenomations: Experience in children. Hum. Exp. Toxicol. 1997, 16, 683–687. [Google Scholar] [PubMed]
- Coppola, M.; Hogan, D.E. Venomous snakes of south-west Asia. Am. J. Emerg. Med. 1992, 10, 230–236. [Google Scholar]
- Mendelssohn, H. On the biology of venomous snakes of Israel. Isr. J. Zool. 1963, 12, 143–170. [Google Scholar]
- Thwin, M.-M.; Gopalakrishnakone, P. Snake envenomation and protective natural endogenous proteins: A mini review of the recent developments (1991-1997). Toxicon 1998, 36, 1471–1482. [Google Scholar]
- Winkler, E.; Chovers, M.; Almog, S.; Pri-Chen, S.; Rotenberg, M.; Tirosh, M.; Ezra, D.; Halkin, H. Decreased serum cholesterol level after snake bite (Vipera palestinae) as a marker of severity of envenomation. J. Lab. Clin. Med. 1993, 121, 774–778. [Google Scholar] [PubMed]
- Bentur, Y.; Raikhlin-Eisenkraft, B.; Galperin, M. Evaluation of antivenom therapy in Vipera palaestinae bites. Toxicon 2004, 44, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Aroch, I.; Harrus, S. Retrospective study of the epidemiological, clinical, haematological and biochemical findings in 109 dogs poisoned by Vipera xanthina palestina. Vet. Rec. 1999, 144, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Gitter, S.; de Vries, A. Symptomatology, Pathology and Treatment of Bites by Near Eastern, European and North African Snakes. In Symptomatology, Pathology and Treatment of Bites by Near Eastern, European and North African Snakes; Bucherl, W., Buckley, E.E., Deulofeu, V., Eds.; Academic Press: New York, NY, USA, 1968; pp. 359–401. [Google Scholar]
- Moroz-Perlmutter, C.; Goldblum, N.; de Vries, A. Biochemical and antigenic properties of a purified neurotoxin of Vipera palestinae venom. J. Immunol. 1965, 94, 164–171. [Google Scholar] [PubMed]
- Ovadia, M.; Kochva, E.; Moav, B. Purification and partial characterization of lethal synergistic components from the venom of Vipera palaestinae. Toxicon 1977, 15, 549–560. [Google Scholar] [PubMed]
- Simon, T.; Bdolah, A.; Kochva, E. The two-component toxin of Vipera palaestinae: Contribution of phospholipase A to its activity. Toxicon 1980, 18, 249–259. [Google Scholar] [PubMed]
- Krizaj, I.; Bdolah, A.; Gubensek, F.; Bencina, P.; Pungercar, J. Protein and cDNA structures of an acidic phospholipase A2, the enzymatic part of an unusual, two-component toxin from Vipera palaestina. Biochem. Biophys. Res. Commun. 1996, 227, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Grotto, L.; Jerushalmy, Z.; de Vries, A. Effect of purified Vipera paletinae hemorrhagin on blood coagulation and platelet function. Thromb. Diath. Haemorrh. 1969, 22, 482–495. [Google Scholar] [PubMed]
- Grotto, L.; Moroz, C.; de Vries, A.; Goldblum, N. Isolation of Vipera palestinae hemorrhagin and distinction between its hemorrhagic and proteolytic activities. Biochim. Biophys. Acta 1967, 133, 356–362. [Google Scholar] [PubMed]
- Ovadia, M. Isolation and characterization of three hemorrhagic factors from the venom of Vipera palaestinae. Toxicon 1978, 16, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Nakar, O.; Ovadia, M.; Kochva, E. Isolation and characterization of a proteolytic factor from the venom of Vipera palaestinae. Toxicon 1986, 24, 293–304. [Google Scholar] [CrossRef] [PubMed]
- UniProtKB/Swiss-Prot v56.5. Available online: http://us.expasy.org/sprot/ (accessed on 11 November 2011).
- Brown, M.C.; Calvete, J.J.; Staniszewska, I.; Walsh, E.M.; Georgina, P.-L.; Dell Valle, L.; Lazarovici, P.; Marcinkiewicz, C. VEGF-related protein isolated from Vipera palestinae venom, promotes angiogenesis. Growth Factors 2007, 25, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Kostiza, T.; Dahinden, C.A.; Rihs, S.; Otten, U.; Meier, J. Nerve growth factor from the venom of the Chinese cobra Naja naja atra: Purification and description of non-neuronal activities. Toxicon 1995, 33, 1249–1261. [Google Scholar]
- Weis, S.M.; Cheresh, D.A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 2005, 437, 497–504. [Google Scholar]
- Kisiel, D.G.; Calvete, J.J.; Katzhendler, J.; Fertala, A.; Lazarovici, P.; Marcinkiewicz, C. Structural determinants of the selectivity of KTS-disintegrins for the α1β1 integrin. FEBS Lett. 2004, 577, 478–482. [Google Scholar]
- Calvete, J.J.; Juárez, P.; Sanz, L. Snake venomics. Strategy and applications. J. Mass Spectrom. 2007, 42, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Molecular and functional diversity of vascular endothelial growth factors. Mol. Divers. 2006, 10, 515–527. [Google Scholar]
- Yamane, A.; Seetharam, L.; Yamaguchi, S.; Gotoh, N.; Takahashi, T.; Neufeld, G.; Shibuya, M. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1). Oncogene 1994, 9, 2683–2690. [Google Scholar]
- de Vries, C.; Escobedo, J.A.; Ueno, H.; Houck, K.; Ferrara, N.; Williams, L.T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255, 989–991. [Google Scholar]
- Terman, B.I.; Dougher-Vermazen, M.; Carrion, M.E.; Dimitrov, D.; Armellino, D.C.; Gospodarowicz, D.; Böhlen, P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 1992, 187, 1579–1586. [Google Scholar]
- Quinn, T.P.; Peters, K.G.; de Vries, C.; Ferrara, N.; Williams, L.T. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc. Natl. Acad. Sci. USA 1993, 90, 7533–7537. [Google Scholar]
- Joukov, V.; Pajusola, K.; Kaipainen, A.; Chilov, D.; Lahtinen, I.; Kukk, E.; Saksela, O.; Kalkkinen, N.; Alitalo, K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinase. EMBO J. 1996, 15, 290–298. [Google Scholar]
- Lee, J.; Gray, A.; Yuan, J.; Luoh, S.M.; Avraham, H.; Wood, W.I. Vascular endothelial growth factor-related protein: A ligand and specific activator of the tyrosine kinase receptor Flt4. Proc. Natl. Acad. Sci. USA 1996, 93, 1988–1992. [Google Scholar]
- Achen, M.G.; Jeltsch, M.; Kukk, E.; Makinen, T.; Vitali, A.; Wilks, A.F.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 1998, 95, 548–553. [Google Scholar]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar]
- Gluzman-Poltorak, Z.; Cohen, T.; Herzog, Y.; Neufeld, G. europilin-2 and neuropilin-1 are receptors for the 165-amino acid form of vascular endothelial growth factor (VEGF) and of placenta growth actor-2, but only neuropilin-2 functions as a receptor for the 145-amino acid form of VEGF. J. Biol. Chem. 2000, 275, 18040–18045. [Google Scholar]
- Yamazaki, Y.; Takani, K.; Atoda, H.; Morita, T. Snake venom vascular endothelial growth factors (VEGFs) exhibit potent activity through their specific recognition of KDR (VEGF Receptor 2). J. Biol. Chem. 2003, 278, 51985–51988. [Google Scholar]
- Paalme, V.; Trummal, K.; Samel, M.; Tõnismägi, K.; Järvekülg, L.; Vija, H.; Subbi, J.; Siigur, J.; Siigur, E. Nerve growth factor from Vipera lebetina venom. Toxicon 2009, 54, 329–336. [Google Scholar] [PubMed]
- Levi-Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar]
- Kaplan, D.R.; Miller, F.D. Signal transduction by the neutrophin receptors. Curr. Opin. Cell Biol. 1997, 9, 213–221. [Google Scholar]
- Selby, M.J.; Edwards, R.H.; Rutter, W.J. Cobra nerve growth factor: Structure and evolutionary comparison. J. Neurosci. Res. 1987, 18, 293–298. [Google Scholar]
- Siigur, E.; Neuman, T.; Järve, V.; Tara, A.; Siigur, J. Isolation and characterization of nerve growth factor from Vipera lebetina (snake) venom. Comp. Biochem. Physiol. B 1985, 81, 211–215. [Google Scholar] [PubMed]
- Hogue-Angeletti, R.A.; Bradshaw, R.A. Nerve Growth Factors in Snake Venoms. In Snake Venoms; Lee, C.Y., Ed.; Springer: Berlin, Germany, 1979; pp. 276–294. [Google Scholar]
- Dolle, J.-P.; Rezvan, A.; Allen, F.D.; Lazarovici, P.; Lelkes, P.I. Nerve growth factor-induced migration of endothelial cells. J. Pharmacol. Exp. Ther. 2005, 315, 1220–1227. [Google Scholar]
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. Cross talk between the cardiovascular and nervous systems: Neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr. Pharm. Des. 2006, 12, 2609–2622. [Google Scholar]
- Clark, E.; Brugge, J. Integrins and signal transduction pathways: The road taken. Science 1995, 268, 233–239. [Google Scholar]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar]
- Liddington, R.C.; Ginsberg, M.H. Integrin activation takes shape. J. Cell Biol. 2002, 158, 833–839. [Google Scholar]
- Schaller, M. Biochemical signals and biological response elicited by the focal adhesion kinase. Biochim. Biophys. Acta 2001, 1540, 1–21. [Google Scholar]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1033. [Google Scholar]
- Stupack, D.G.; Cheresh, D.A. Get a ligand, get a life: Integrins, signaling and cell surviva. J. Cell Sci. 2002, 115, 3729–3738. [Google Scholar]
- Marcinkiewicz, C. Functional characteristic of snake venom disintegrins: Potential therapeutic implication. Curr. Pharm. Des. 2005, 11, 815–827. [Google Scholar]
- Plow, E.F.; Haas, T.A.; Zhang, L.; Loftus, J.; Smith, J.W. Ligand binding to integrins. J. Biol. Chem. 2000, 275, 21785–21788. [Google Scholar]
- Marcinkiewicz, C.; Calvete, J.J.; Marcinkiewicz, M.M.; Raida, M.; Vijay-Kumar, S.; Huang, Z.; Lobb, R.R.; Niewiarowski, S. EC3, a novel heterodimeric disintegrin from Echis carinatus venom, inhibits alpha4 and alpha5 integrins in an RGD-independent manne. J. Biol. Chem. 1999, 274, 12468–12473. [Google Scholar] [PubMed]
- Moreno-Murciano, M.P.; Monleón, D.; Calvete, J.J.; Celda, B.; Marcinkiewicz, C. Amino acid sequence and homology modeling of obtustatin, a novel non-RGD-containing short disintegrin isolated from the venom of Vipera lebetina obtuse. Protein Sci. 2003, 12, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Eble, J.A.; Calvete, J.J.; Marcinkiewicz, C. Structural requirements of KTS-disintegrins for inhibition of α1β1 integrin. Biochem. J. 2009, 417, 95–101. [Google Scholar]
- Kallech-Ziri, O.; Luis, J.; Faljoun, Z.; Sabatier, J.-M.; Lehmann, M.; El Ayeb, M.; Marrakchi, N.; Loret, E. Structure function relationships of KTS disintegrins and design of antiangiogenic drugs Lett. Drug Des. Discov. 2010, 7, 36–40. [Google Scholar]
- Staniszewska, I.; Walsh, E.M.; Rothman, V.L.; Gaathon, A.; Tuszynski, G.P.; Calvete, J.J.; Lazarovici, P.; Marcinkiewicz, C. Effect of VP12 and viperistatin on inhibition of collagen receptors: Dependent melanoma metastasis. Cancer Biol. Ther. 2009, 8, 1507–1516. [Google Scholar]
- Horii, K.; Okuda, D.; Morita, T.; Mizuno, H. Crystal structure of EMS16 in complex with the integrin α2-I domain. J. Mol. Biol. 2004, 341, 519–527. [Google Scholar]
- Eble, J.A.; Niland, S.; Bracht, T.; Mormann, M.; Peter-Katalinic, J.; Pohlentz, G.; Stetefeld, J. The α2β1integrin-specific antagonist rhodocetin is a cruciform, heterotetrameric molecule. FASEB J. 2009, 23, 2917–2927. [Google Scholar]
- Morita, T. Structure-function relationship of C-type lectin-related proteins. Pathophysiol. Haemost. Thromb. 2005, 34, 156–159. [Google Scholar]
- Arlinghaus, F.; Marcinkiewicz, C.; Lazarovici, P.; Eble, J.A. Snake Venoms Contain Important Anti-Thrombocit Antagonists Selectively Targeting Collagen α2β1. In Proceedings of the 33th World Congress of the International Society of Hematology, Jerusalem, Palestine, 10-14 October 2010.
- Walsh, E.M.; Marcinkiewicz, C. Non-RGD-containing snake venom disintegrins, functional and structural relations. Toxicon 2011, 58, 355–362. [Google Scholar]
- Moroz, C. Vipera palestinae antivenin. Public Health Rev. 1998, 26, 233–236. [Google Scholar]
- Moroz, C.; de Vries, A.; Goldblum, N. Preparation of an antivenin against Vipera palestinae venom with high antineurotoxic potency. Toxicon 1966, 4, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Moroz, C.; Hahn, J.; de Vries, A. Neutralization of Vipera palestinae hemorrhagin by antibody fragments. Toxicon 1971, 9, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.A.; Dart, R.C.; Staples, A.; White, J. Envenomations: An overview of clinical toxinology for the primary care physician. Am. Fam. Physician 2009, 80, 793–802. [Google Scholar]
- Williams, D.J.; Gutiérrez, J.-M.; Calvete, J.J.; Wüster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; O’Shea, M.; Jensen, S.D.; Winkel, K.D.; Warrell, D.A. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteomics 2011, 74, 1735–1767. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Momic, T.; Arlinghaus, F.T.; Arien-Zakay, H.; Katzhendler, J.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Pharmacological Aspects of Vipera xantina palestinae Venom. Toxins 2011, 3, 1420-1432. https://doi.org/10.3390/toxins3111420
Momic T, Arlinghaus FT, Arien-Zakay H, Katzhendler J, Eble JA, Marcinkiewicz C, Lazarovici P. Pharmacological Aspects of Vipera xantina palestinae Venom. Toxins. 2011; 3(11):1420-1432. https://doi.org/10.3390/toxins3111420
Chicago/Turabian StyleMomic, Tatjana, Franziska T. Arlinghaus, Hadar Arien-Zakay, Jeoshua Katzhendler, Johannes A. Eble, Cezary Marcinkiewicz, and Philip Lazarovici. 2011. "Pharmacological Aspects of Vipera xantina palestinae Venom" Toxins 3, no. 11: 1420-1432. https://doi.org/10.3390/toxins3111420





