Targeting Inflammatory Pathways by Triterpenoids for Prevention and Treatment of Cancer
Abstract
:1. Introduction


| Chemical Compound | Common Name | Botanical Name |
|---|---|---|
| Tetracyclic triterpenoid | ||
| Astragaloside | Chinese milk vetch | Astragalus membranaceus |
| Cucurbitacin | White bryony | Bryonia alba |
| Diosgenin | Fenugreek | Trigonella foenum graecum |
| Ganoderic acid | Reishi | Ganoderma lucidum |
| Ginsenoside | Ginseng | Panax ginseng |
| Gypenoside | Jiaogulan | Gynostemma pentaphyllum |
| Oleandrin | Oleander | Nerium oleander |
| Pentacyclic triterpenoid | ||
| Amyrin | Japanese persimmon | Diospyros kaki |
| Asiatic acid | Indian pennywort | Centella asiatica |
| Avicin | Elegant wattle | Acacia victoriae |
| Betulinic acid | Indian jujube | Ziziphus mauritiana |
| Anemone | Anemone raddeana | |
| Club mosses | Lycopodium cernuum | |
| Trumpet satinash | Syzygium claviflorum | |
| Boswellic acid | Boswellia, | Boswellia serrata |
| Frankincense, salai guggal | Boswellia carteri | |
| Celastrol | Thunder god vine | Tripterygium wilfordii |
| Escin | Horse chestnut | Aesculus hippocastanum |
| Glycyrrhizin | Licorice | Glycyrrhiza glabra |
| 18-β-Glycyrrhetinic acid | Licorice | Glycyrrhizia glabra |
| Lupeol | Mango | Mangifera indica |
| Three leaved caper | Crataeva nurvala | |
| Madecassic acid | Indian pennywort, gotu kola | Centella asiatica |
| Momordin | Burning bush | Kochia scoparia |
| Oleanolic acid | Bearberry | Arctostaphyllos uva-ursi |
| Heather | Calluna vulgaris | |
| Three leaved caper | Crataeva nurvala | |
| Reishi | Ganoderma lucidum | |
| Chinese elder | Sambucus chinensis | |
| Sodom's apple | Solanum incanum | |
| Platycodon D | Balloon flower | Platycodon grandiflorum |
| Pristimerin | Espinheira santa | Maytenus ilicifolia |
| Pale Bittersweet | Celastrus hypoleucus | |
| Thunder god vine | Tripterygium wilfordii | |
| Saikosaponins | Hare's ear root, sickle-leaf | Bupleurum falcatum L. |
| Ursolic acid | Holy basil, tulsi | Ocimum sanctum L. |
| Thyme | Thymus vulgaris L. | |
| Lavender | Lavandula augustifolia | |
| Catnip | Nepeta sibthorpii | |
| Peppermint leaves | Mentha piperita L. | |
| Withanolide | Indian ginseng, ashwagandha | Withania somnifera |
| Triterpenoid | Targets | References |
|---|---|---|
| Amyrin | NF-κB, IL-1β, COX-2, CREB, ERK, PKC, P38 MAPK | [9,10] |
| Avicin | NF-κB, Fas, STAT3, caspase-8, Bcl-2, Bcl-xL | [11,12,13,14,15,16] |
| Asiatic acid | NF-κB, caspases-2, -3, -8 and -9, PARP, Bcl-2 | [17,18,19,20,21,22,23,24] |
| Astragaloside | NF-κB, VCAM-1 | [25] |
| Betulinic acid | NF-κB, STAT3, Bax, Bcl-2, Bcl-xL, FAK | [26,27,28,29,30,31,32,33,34,35,36,37,38] |
| Boswellic acid | NF-κB, STAT3, AR, p21, DR5, caspase-3 and -8 | [32,39,40,41,42,43,44,45,46,47,48] |
| Celastrol | NF-κB, IAP1, IAP2, Bcl-2, Bcl-xL, c-FLIP, COX-2, survivin, cyclin D1, MMP9, VEGF, iNOS, Hsp90, cdc37, VEGFR | [49,50,51,52,53] |
| Cucurbitacin | Cyclin B1, cyclin D1, Mcl-1, cdc25C, STAT3, p53 | [54,55,56,57,58,59] |
| Diosgenin | NF-κB, survivin, XIAP, cyclin D1, cdk-2, cdk-4, mTOR, JNK, HMG-CoA reductase, p53, AIF, p21 ras, β-catenin | [60,61,62,63,64,65] |
| Escin | NF-κB, STAT3, JAK2, cyclin D1, Bcl-2, Bcl-xL, survivin, Mcl-1, VEGF, COX-2, MMP9 | [66,67] |
| Ganoderic acid | NF-κB, AP-1, NFATc1, cdk4, uPA, MMP2, MMP9, | [68,69,70,71,72] |
| Ginsenosides | NF-κB, Bax, caspase-3, caspase-8, Bcl-2, IAP, XIAP, cyclin B1, cyclin D, cdk2, cdk4, VEGF, MAPK, IL-1β, TNF-α, ICAM-1, JNK | [73,74,75,76] |
| Glycyrrhizin | NF-κB, AP-1, TLR2, COX-2, IL-1α, TNF-α | [77,78,79,80,81,82,83] |
| Glycyrrhetinic acid | NF-κB, H-ras, Bax, cytochrome C, Bcl-2, Bcl-xL, Bak, caspase-3, PPARγ | [84,85,86] |
| Gypenoside | NF-κB, PPAR , VCAM-1, TF, iNOS, Ras | [87,88,89,90,91,92] |
| Lupeol | NF-κB, cFLIP, survivin, Bax, caspase-3, caspase-9 | [93,94,95,96,97,98,99,100,101,102] |
| Madecassic acid | iNOS, COX-2, TNF-α, IL-1, IL-6 | [103] |
| Momordin | NF-κB, AP-1, Bcl-2, Bax, caspase-3, PARP | [104,105] |
| Oleandrin | NF-κB, AP-1, Fas, ERK, Akt, FGF-1 | [106,107,108,109,110] |
| Oleanolic acid | NF-κB, mTOR, caspases-3, -8, and -9, ICAM-1, VEGF, PARP, Akt | [111,112,113,114] |
| Platycodon D | NF-κB, Egr-1, caspase-3 | [115,116] |
| Pristimerin | NF-κB, PARP-1, JNK, Bax, p27, Bcl-2, Bcl-xL | [117,118,119,120] |
| Saikosaponins | NF-κB, NF-AT, AP-1, IL-6, TNF- , IFN- , PKC , JNK, p53, Fas/FasL | [121,122,123] |
| Ursolic acid | NF-κB, STAT3, Bcl-2, Bax, ICAM-1, p53, PKC | [114,124,125,126,127,128,129,130,131,132] |
| Withanolide | NF-κB, AP-1, IL-6, COX-2, Hsp70, Hsp90, Bax | [133,134,135,136,137,138] |
| Disease | Triterpenoid |
|---|---|
| Diabetes | Astragaloside, Cucurbitacin, Diosgenin, Ginsenoside, Amyrin, Asiatic acid, Avicin, Betulinic acid, Escin, Glycyrrhizin, Oleanolic acid, Platycodon D, Ursolic acid, Withanolide |
| Cardiovascular | Astragaloside, Cucurbitacin, Diosgenin, Ginsenoside, Gypenoside, Oleandrin, Betulinic acid, Escin, Glycyrrhizin, Lupeol, Oleanolic acid, Platycodon D, Saikosaponins, Ursolic acid, Withanolide |
| Arthritis | Cucurbitacin, Diosgenin, Ginsenoside, Amyrin, Boswellic acid, Celastrol, Glycyrrhizin, Lupeol, Oleanolic acid, CDDO-Me, Ursolic acid, Withanolide, |
| Atherosclerosis | Diosgenin, Gypenoside, Betulinic acid, Glycyrrhizin, Oleanolic acid, Ursolic acid |
| Obesity | Diosgenin, Ginsenoside, Betulinic acid, Escin, Glycyrrhizin, Platycodon D, Momordin, Oleanolic acid, Ursolic acid |
| Alzheimer | CDDO-MA, Alpha-onocerin |
| Parkinson | CDDO-MA |
| Multiple sclerosis | Oleanolic acid |
| Depression | Asiatic acid |
| Osteoporosis | Ursolic acid |
| Cerebral ischemia | Escin, Asiatic acid |
| Memory loss | CDDO-MA |
2. Source and Structure of Triterpenoids

3. Molecular Targets of Triterpenoids
3.1. NF-κB
3.2. STAT3
3.3. Other Pathways
4. Role of Triterpenoids in Cancer Prevention
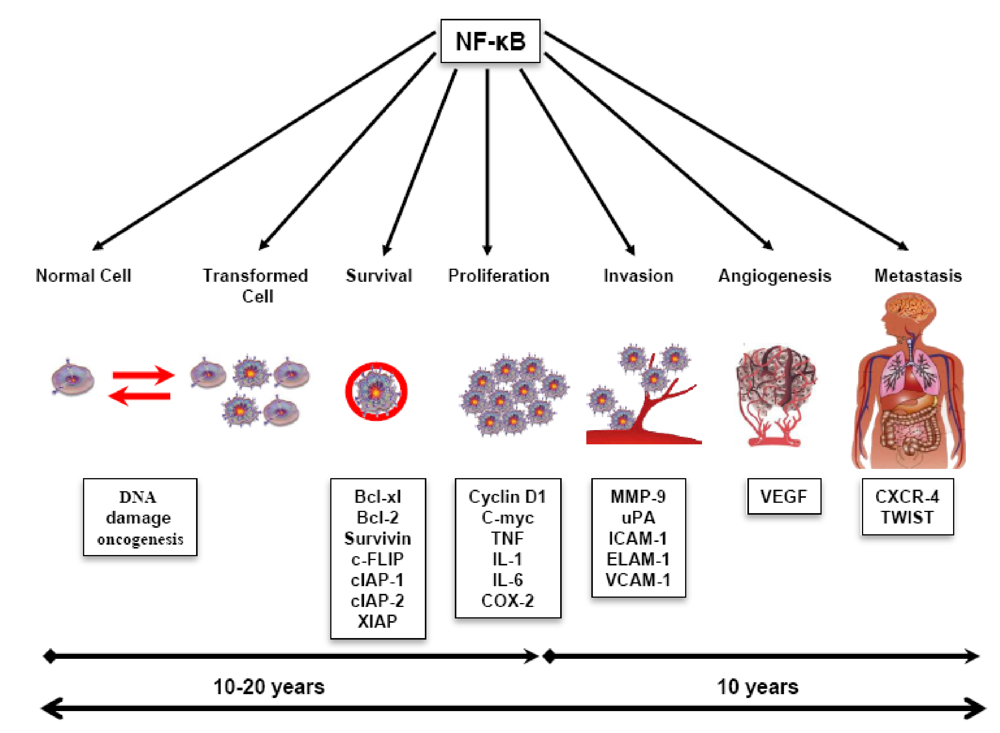
4.1. Role of Triterpenoids in Inflammation
4.2. Role of Triterpenoids in Tumor Cell Survival, Apoptosis, and Proliferation
4.3. Role of Triterpenoids in Invasion, Metastasis, and Angiogenesis
5. Role of Triterpenoids in Cancer Treatment
| Triterpenoids | Cancer | Phase | Status | Sponsors |
|---|---|---|---|---|
| CDDO-Me | Solid tumors or | I | Terminated | MDACC |
| Lymphoid malignancies | ||||
| CDDO | Solid Tumors | I | Completed | NCI |
| or Lymphoma | ||||
| CDDO-Me | Liver disease | I/II | Terminated | RPI |
| Ginsenoside | Breast cancer | II | Ongoing | SIU |
| Ginsenoside | Hypertension | II | Completed | SMH |
| Ginsenoside | Ischemic Stroke | II/III | Completed | XH |
| Betulinic acid | Dysplastic nervus syndrome | I/II | Ongoing | UI |
| Escin | Arm lymphedema | II | Completed | UW |
| Glycyrrhizin | Hepatitis C | III | Ongoing | SP |
| Glycyrrhetinic acid | End stage renal disease | II | Ongoing | UHI |
| Glycyrrhetinic acid | AME | II/III | Completed | BWH |
6. Conclusions
References
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar]
- Sporn, M.B.; Newton, D.L. Chemoprevention of cancer with retinoids. Fed. Proc. 1979, 38, 2528–2534. [Google Scholar]
- Aggarwal, B.B.; Vijayalekshmi, R.V.; Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin. Cancer Res. 2009, 15, 425–430. [Google Scholar]
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 2007, 117, 1175–1183. [Google Scholar]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: how hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Suh, N. Chemoprevention of cancer. Carcinogenesis 2000, 21, 525–530. [Google Scholar]
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P. Biosynthetic diversity in plant triterpene cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar]
- Kinghorn, A.D.; Balandrin, M.F. Human Medicinal Agents from Plants (ACS Symposium Series); American Chemical Society: Washington, DC, USA, 1993; Volume 534. [Google Scholar]
- Medeiros, R.; Otuki, M.F.; Avellar, M.C.; Calixto, J.B. Mechanisms underlying the inhibitory actions of the pentacyclic triterpene alpha-amyrin in the mouse skin inflammation induced by phorbol ester 12-O-tetradecanoylphorbol-13-acetate. Eur. J. Pharmacol. 2007, 559, 227–235. [Google Scholar]
- Vitor, C.E.; Figueiredo, C.P.; Hara, D.B.; Bento, A.F.; Mazzuco, T.L.; Calixto, J.B. Therapeutic action and underlying mechanisms of a combination of two pentacyclic triterpenes, alpha- and beta-amyrin, in a mouse model of colitis. Br. J. Pharmacol. 2009, 157, 1034–1044. [Google Scholar]
- Haridas, V.; Nishimura, G.; Xu, Z.X.; Connolly, F.; Hanausek, M.; Walaszek, Z.; Zoltaszek, R.; Gutterman, J.U. Avicin D: a protein reactive plant isoprenoid dephosphorylates Stat 3 by regulating both kinase and phosphatase activities. PLoS One 2009, 4, e5578. [Google Scholar]
- Xu, Z.X.; Ding, T.; Haridas, V.; Connolly, F.; Gutterman, J.U. Avicin D, a plant triterpenoid, induces cell apoptosis by recruitment of Fas and downstream signaling molecules into lipid rafts. PLoS One 2009, 4, e8532. [Google Scholar]
- Zhang, C.; Li, B.; Gaikwad, A.S.; Haridas, V.; Xu, Z.; Gutterman, J.U.; Duvic, M. Avicin D selectively induces apoptosis and downregulates p-STAT-3, bcl-2, and survivin in cutaneous T-cell lymphoma cells. J. Invest. Dermatol. 2008, 128, 2728–2735. [Google Scholar]
- Xu, Z.X.; Liang, J.; Haridas, V.; Gaikwad, A.; Connolly, F.P.; Mills, G.B.; Gutterman, J.U. A plant triterpenoid, avicin D, induces autophagy by activation of AMP-activated protein kinase. Cell Death Differ. 2007, 14, 1948–1957. [Google Scholar]
- Gaikwad, A.; Poblenz, A.; Haridas, V.; Zhang, C.; Duvic, M.; Gutterman, J. Triterpenoid electrophiles (avicins) suppress heat shock protein-70 and x-linked inhibitor of apoptosis proteins in malignant cells by activation of ubiquitin machinery: implications for proapoptotic activity. Clin. Cancer Res. 2005, 11, 1953–1962. [Google Scholar]
- Haridas, V.; Arntzen, C.J.; Gutterman, J.U. Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), inhibit activation of nuclear factor-kappaB by inhibiting both its nuclear localization and ability to bind DNA. Proc. Natl. Acad. Sci. USA 2001, 98, 11557–11562. [Google Scholar]
- Tang, X.L.; Yang, X.Y.; Jung, H.J.; Kim, S.Y.; Jung, S.Y.; Choi, D.Y.; Park, W.C.; Park, H. Asiatic acid induces colon cancer cell growth inhibition and apoptosis through mitochondrial death cascade. Biol. Pharm. Bull. 2009, 32, 1399–1405. [Google Scholar]
- Park, B.C.; Paek, S.H.; Lee, Y.S.; Kim, S.J.; Lee, E.S.; Choi, H.G.; Yong, C.S.; Kim, J.A. Inhibitory effects of asiatic acid on 7,12-dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice. Biol. Pharm. Bull. 2007, 30, 176–179. [Google Scholar]
- Bunpo, P.; Kataoka, K.; Arimochi, H.; Nakayama, H.; Kuwahara, T.; Vinitketkumnuen, U.; Ohnishi, Y. Inhibitory effects of asiatic acid and CPT-11 on growth of HT-29 cells. J. Med. Invest. 2005, 52, 65–73. [Google Scholar]
- Gurfinkel, D.M.; Chow, S.; Hurren, R.; Gronda, M.; Henderson, C.; Berube, C.; Hedley, D.W.; Schimmer, A.D. Disruption of the endoplasmic reticulum and increases in cytoplasmic calcium are early events in cell death induced by the natural triterpenoid Asiatic acid. Apoptosis 2006, 11, 1463–1471. [Google Scholar]
- Hsu, Y.L.; Kuo, P.L.; Lin, L.T.; Lin, C.C. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J. Pharmacol. Exp. Ther. 2005, 313, 333–344. [Google Scholar]
- Lee, Y.S.; Jin, D.Q.; Kwon, E.J.; Park, S.H.; Lee, E.S.; Jeong, T.C.; Nam, D.H.; Huh, K.; Kim, J.A. Asiatic acid, a triterpene, induces apoptosis through intracellular Ca2+ release and enhanced expression of p53 in HepG2 human hepatoma cells. Cancer Lett. 2002, 186, 83–91. [Google Scholar]
- Park, B.C.; Bosire, K.O.; Lee, E.S.; Lee, Y.S.; Kim, J.A. Asiatic acid induces apoptosis in SK-MEL-2 human melanoma cells. Cancer Lett. 2005, 218, 81–90. [Google Scholar]
- Yun, K.J.; Kim, J.Y.; Kim, J.B.; Lee, K.W.; Jeong, S.Y.; Park, H.J.; Jung, H.J.; Cho, Y.W.; Yun, K.; Lee, K.T. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B inactivation in RAW 264.7 macrophages: possible involvement of the IKK and MAPK pathways. Int. Immunopharmacol. 2008, 8, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Hufnagl, P.; Binder, B.R.; Wojta, J. Antiinflammatory activity of astragaloside IV is mediated by inhibition of NF-kappaB activation and adhesion molecule expression. Thromb. Haemost. 2003, 90, 904–914. [Google Scholar]
- Chen, Z.; Wu, Q.; Chen, Y.; He, J. Effects of betulinic acid on proliferation and apoptosis in Jurkat cells and its in vitro mechanism. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 634–638. [Google Scholar]
- Chintharlapalli, S.; Papineni, S.; Ramaiah, S.K.; Safe, S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007, 67, 2816–2823. [Google Scholar]
- Ganguly, A.; Das, B.; Roy, A.; Sen, N.; Dasgupta, S.B.; Mukhopadhayay, S.; Majumder, H.K. Betulinic acid, a catalytic inhibitor of topoisomerase I, inhibits reactive oxygen species-mediated apoptotic topoisomerase I-DNA cleavable complex formation in prostate cancer cells but does not affect the process of cell death. Cancer Res. 2007, 67, 11848–11858. [Google Scholar]
- Karna, E.; Palka, J.A. Mechanism of betulinic acid inhibition of collagen biosynthesis in human endometrial adenocarcinoma cells. Neoplasma 2009, 56, 361–366. [Google Scholar]
- Kasperczyk, H.; La Ferla-Bruhl, K.; Westhoff, M.A.; Behrend, L.; Zwacka, R.M.; Debatin, K.M.; Fulda, S. Betulinic acid as new activator of NF-kappaB: molecular mechanisms and implications for cancer therapy. Oncogene 2005, 24, 6945–6956. [Google Scholar]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulinic acid induces cytochrome c release and apoptosis in a Bax/Bak-independent, permeability transition pore dependent fashion. Apoptosis 2009, 14, 191–202. [Google Scholar]
- Kunnumakkara, A.B.; Nair, A.S.; Sung, B.; Pandey, M.K.; Aggarwal, B.B. Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Mol. Cancer Res. 2009, 7, 118–128. [Google Scholar]
- Rabi, T.; Shukla, S.; Gupta, S. Betulinic acid suppresses constitutive and TNFalpha-induced NF-kappaB activation and induces apoptosis in human prostate carcinoma PC-3 cells. Mol. Carcinog. 2008, 47, 964–973. [Google Scholar]
- Rzeski, W.; Stepulak, A.; Szymanski, M.; Sifringer, M.; Kaczor, J.; Wejksza, K.; Zdzisinska, B.; Kandefer-Szerszen, M. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 374, 11–20. [Google Scholar]
- Takada, Y.; Aggarwal, B.B. Betulinic acid suppresses carcinogen-induced NF-kappa B activation through inhibition of I kappa B alpha kinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J. Immunol. 2003, 171, 3278–3286. [Google Scholar]
- Thurnher, D.; Turhani, D.; Pelzmann, M.; Wannemacher, B.; Knerer, B.; Formanek, M.; Wacheck, V.; Selzer, E. Betulinic acid: a new cytotoxic compound against malignant head and neck cancer cells. Head Neck 2003, 25, 732–740. [Google Scholar]
- Yoon, J.J.; Lee, Y.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Protective role of betulinic acid on TNF-alpha-induced cell adhesion molecules in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010, 391, 96–101. [Google Scholar]
- Yun, Y.; Han, S.; Park, E.; Yim, D.; Lee, S.; Lee, C.K.; Cho, K.; Kim, K. Immunomodulatory activity of betulinic acid by producing pro-inflammatory cytokines and activation of macrophages. Arch. Pharm. Res. 2003, 26, 1087–1095. [Google Scholar]
- Cuaz-Perolin, C.; Billiet, L.; Bauge, E.; Copin, C.; Scott-Algara, D.; Genze, F.; Buchele, B.; Syrovets, T.; Simmet, T.; Rouis, M. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE-/- mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 272–277. [Google Scholar]
- Kiela, P.R.; Midura, A.J.; Kuscuoglu, N.; Jolad, S.D.; Solyom, A.M.; Besselsen, D.G.; Timmermann, B.N.; Ghishan, F.K. Effects of Boswellia serrata in mouse models of chemically induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G798–G808. [Google Scholar]
- Lu, M.; Xia, L.; Hua, H.; Jing, Y. Acetyl-keto-beta-boswellic acid induces apoptosis through a death receptor 5-mediated pathway in prostate cancer cells. Cancer Res. 2008, 68, 1180–1186. [Google Scholar]
- Moussaieff, A.; Shohami, E.; Kashman, Y.; Fride, E.; Schmitz, M.L.; Renner, F.; Fiebich, B.L.; Munoz, E.; Ben-Neriah, Y.; Mechoulam, R. Incensole acetate, a novel anti-inflammatory compound isolated from Boswellia resin, inhibits nuclear factor-kappa B activation. Mol. Pharmacol. 2007, 72, 1657–1664. [Google Scholar]
- Popovic, M.; Laumonnier, Y.; Burysek, L.; Syrovets, T.; Simmet, T. Thrombin-induced expression of endothelial CX3CL1 potentiates monocyte CCL2 production and transendothelial migration. J. Leukoc. Biol. 2008, 84, 215–223. [Google Scholar]
- Syrovets, T.; Buchele, B.; Krauss, C.; Laumonnier, Y.; Simmet, T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. J. Immunol. 2005, 174, 498–506. [Google Scholar]
- Syrovets, T.; Gschwend, J.E.; Buchele, B.; Laumonnier, Y.; Zugmaier, W.; Genze, F.; Simmet, T. Inhibition of IkappaB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. J. Biol. Chem. 2005, 280, 6170–6180. [Google Scholar]
- Takada, Y.; Ichikawa, H.; Badmaev, V.; Aggarwal, B.B. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B-regulated gene expression. J. Immunol. 2006, 176, 3127–3140. [Google Scholar]
- Wang, H.; Syrovets, T.; Kess, D.; Buchele, B.; Hainzl, H.; Lunov, O.; Weiss, J.M.; Scharffetter-Kochanek, K.; Simmet, T. Targeting NF-kappa B with a natural triterpenoid alleviates skin inflammation in a mouse model of psoriasis. J. Immunol. 2009, 183, 4755–4763. [Google Scholar]
- Yuan, H.Q.; Kong, F.; Wang, X.L.; Young, C.Y.; Hu, X.Y.; Lou, H.X. Inhibitory effect of acetyl-11-keto-beta-boswellic acid on androgen receptor by interference of Sp1 binding activity in prostate cancer cells. Biochem. Pharmacol. 2008, 75, 2112–2121. [Google Scholar]
- Jung, H.W.; Chung, Y.S.; Kim, Y.S.; Park, Y.K. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp. Mol. Med. 2007, 39, 715–721. [Google Scholar]
- Kim, D.Y.; Park, J.W.; Jeoung, D.; Ro, J.Y. Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur. J. Pharmacol. 2009, 612, 98–105. [Google Scholar]
- Lee, J.H.; Koo, T.H.; Yoon, H.; Jung, H.S.; Jin, H.Z.; Lee, K.; Hong, Y.S.; Lee, J.J. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem. Pharmacol. 2006, 72, 1311–1321. [Google Scholar]
- Sethi, G.; Ahn, K.S.; Pandey, M.K.; Aggarwal, B.B. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood 2007, 109, 2727–2735. [Google Scholar]
- Zhang, T.; Li, Y.; Yu, Y.; Zou, P.; Jiang, Y.; Sun, D. Characterization of celastrol to inhibit hsp90 and cdc37 interaction. J. Biol. Chem. 2009, 284, 35381–35389. [Google Scholar]
- Chan, K.T.; Li, K.; Liu, S.L.; Chu, K.H.; Toh, M.; Xie, W.D. Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562. Cancer Lett. 2010, 289, 46–52. [Google Scholar]
- Lui, V.W.; Yau, D.M.; Wong, E.Y.; Ng, Y.K.; Lau, C.P.; Ho, Y.; Chan, J.P.; Hong, B.; Ho, K.; Cheung, C.S.; Tsang, C.M.; Tsao, S.W.; Chan, A.T. Cucurbitacin I elicits anoikis sensitization, inhibits cellular invasion and in vivo tumor formation ability of nasopharyngeal carcinoma cells. Carcinogenesis 2009, 30, 2085–2094. [Google Scholar]
- Sun, C.; Zhang, M.; Shan, X.; Zhou, X.; Yang, J.; Wang, Y.; Li-Ling, J.; Deng, Y. Inhibitory effect of cucurbitacin E on pancreatic cancer cells growth via STAT3 signaling. J. Cancer Res. Clin. Oncol. 2009, 136, 603–610. [Google Scholar]
- Sun, J.; Blaskovich, M.A.; Jove, R.; Livingston, S.K.; Coppola, D.; Sebti, S.M. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene 2005, 24, 3236–3245. [Google Scholar]
- Thoennissen, N.H.; Iwanski, G.B.; Doan, N.B.; Okamoto, R.; Lin, P.; Abbassi, S.; Song, J.H.; Yin, D.; Toh, M.; Xie, W.D.; Said, J.W.; Koeffler, H.P. Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res. 2009, 69, 5876–5884. [Google Scholar]
- Yasuda, S.; Yogosawa, S.; Izutani, Y.; Nakamura, Y.; Watanabe, H.; Sakai, T. Cucurbitacin B induces G(2) arrest and apoptosis via a reactive oxygen species-dependent mechanism in human colon adenocarcinoma SW480 cells. Mol. Nutr. Food Res. 2009, 54, 559–565. [Google Scholar]
- Chiang, C.T.; Way, T.D.; Tsai, S.J.; Lin, J.K. Diosgenin, a naturally occurring steroid, suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett. 2007, 581, 5735–5742. [Google Scholar]
- Corbiere, C.; Liagre, B.; Terro, F.; Beneytout, J.L. Induction of antiproliferative effect by diosgenin through activation of p53, release of apoptosis-inducing factor (AIF) and modulation of caspase-3 activity in different human cancer cells. Cell Res. 2004, 14, 188–196. [Google Scholar]
- Leger, D.Y.; Liagre, B.; Beneytout, J.L. Role of MAPKs and NF-kappaB in diosgenin-induced megakaryocytic differentiation and subsequent apoptosis in HEL cells. Int. J. Oncol. 2006, 28, 201–207. [Google Scholar]
- Raju, J.; Bird, R.P. Diosgenin, a naturally occurring steroid [corrected] saponin suppresses 3-hydroxy-3-methylglutaryl CoA reductase expression and induces apoptosis in HCT-116 human colon carcinoma cells. Cancer Lett. 2007, 255, 194–204. [Google Scholar]
- Shishodia, S.; Aggarwal, B.B. Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, I kappa B kinase activation and NF-kappa B-regulated gene expression. Oncogene 2006, 25, 1463–1473. [Google Scholar]
- Srinivasan, S.; Koduru, S.; Kumar, R.; Venguswamy, G.; Kyprianou, N.; Damodaran, C. Diosgenin targets Akt-mediated prosurvival signaling in human breast cancer cells. Int. J. Cancer 2009, 125, 961–967. [Google Scholar]
- Harikumar, K.B.; Sung, B.; Pandey, M.K.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Escin, a Pentacyclic Triterpene, Chemosensitizes Human Tumor Cells through Inhibition of NF-{kappa}B Signaling Pathway. Mol. Pharmacol. 2010, 77, 818–827. [Google Scholar]
- Tan, S.M.; Li, F.; Rajendran, P.; Prem Kumar, A.; Hui, K.M.; Sethi, G. Identification of {beta}-escin as a novel inhibitor of STAT3/JAK2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2010. [Google Scholar]
- Chen, N.H.; Liu, J.W.; Zhong, J.J. Ganoderic acid Me inhibits tumor invasion through down-regulating matrix metalloproteinases 2/9 gene expression. J. Pharmacol. Sci. 2008, 108, 212–216. [Google Scholar]
- Jiang, J.; Grieb, B.; Thyagarajan, A.; Sliva, D. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling. Int. J. Mol. Med. 2008, 21, 577–584. [Google Scholar]
- Li, C.H.; Chen, P.Y.; Chang, U.M.; Kan, L.S.; Fang, W.H.; Tsai, K.S.; Lin, S.B. Ganoderic acid X, a lanostanoid triterpene, inhibits topoisomerases and induces apoptosis of cancer cells. Life Sci. 2005, 77, 252–265. [Google Scholar]
- Miyamoto, I.; Liu, J.; Shimizu, K.; Sato, M.; Kukita, A.; Kukita, T.; Kondo, R. Regulation of osteoclastogenesis by ganoderic acid DM isolated from Ganoderma lucidum. Eur. J. Pharmacol. 2009, 602, 1–7. [Google Scholar]
- Tang, W.; Liu, J.W.; Zhao, W.M.; Wei, D.Z.; Zhong, J.J. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006, 80, 205–211. [Google Scholar]
- Kim, S.M.; Lee, S.Y.; Cho, J.S.; Son, S.M.; Choi, S.S.; Yun, Y.P.; Yoo, H.S.; Yoon do, Y.; Oh, K.W.; Han, S.B.; Hong, J.T. Combination of ginsenoside Rg3 with docetaxel enhances the susceptibility of prostate cancer cells via inhibition of NF-kappaB. Eur. J. Pharmacol. 2010, 631, 1–9. [Google Scholar]
- Liu, T.G.; Huang, Y.; Cui, D.D.; Huang, X.B.; Mao, S.H.; Ji, L.L.; Song, H.B.; Yi, C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer 2009, 9, 250. [Google Scholar]
- Wang, J.; Qiao, L.; Li, Y.; Yang, G. Ginsenoside Rb1 attenuates intestinal ischemia-reperfusion-induced liver injury by inhibiting NF-kappaB activation. Exp. Mol. Med. 2008, 40, 686–698. [Google Scholar]
- Zhang, Z.; Li, X.; Lv, W.; Yang, Y.; Gao, H.; Yang, J.; Shen, Y.; Ning, G. Ginsenoside Re reduces insulin resistance through inhibition of c-Jun NH2-terminal kinase and nuclear factor-kappaB. Mol. Endocrinol. 2008, 22, 186–195. [Google Scholar]
- Curtin, J.F.; Liu, N.; Candolfi, M.; Xiong, W.; Assi, H.; Yagiz, K.; Edwards, M.R.; Michelsen, K.S.; Kroeger, K.M.; Liu, C.; Muhammad, A.K.; Clark, M.C.; Arditi, M.; Comin-Anduix, B.; Ribas, A.; Lowenstein, P.R.; Castro, M.G. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009, 6, e10. [Google Scholar]
- Hsiang, C.Y.; Lai, I.L.; Chao, D.C.; Ho, T.Y. Differential regulation of activator protein 1 activity by glycyrrhizin. Life Sci. 2002, 70, 1643–1656. [Google Scholar]
- Matsui, S.; Sonoda, Y.; Sekiya, T.; Aizu-Yokota, E.; Kasahara, T. Glycyrrhizin derivative inhibits eotaxin 1 production via STAT6 in human lung fibroblasts. Int. Immunopharmacol. 2006, 6, 369–375. [Google Scholar]
- Menegazzi, M.; Di Paola, R.; Mazzon, E.; Genovese, T.; Crisafulli, C.; Dal Bosco, M.; Zou, Z.; Suzuki, H.; Cuzzocrea, S. Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol. Res. 2008, 58, 22–31. [Google Scholar]
- Niwa, K.; Lian, Z.; Onogi, K.; Yun, W.; Tang, L.; Mori, H.; Tamaya, T. Preventive effects of glycyrrhizin on estrogen-related endometrial carcinogenesis in mice. Oncol. Rep. 2007, 17, 617–622. [Google Scholar]
- Takei, H.; Baba, Y.; Hisatsune, A.; Katsuki, H.; Miyata, T.; Yokomizo, K.; Isohama, Y. Glycyrrhizin inhibits interleukin-8 production and nuclear factor-kappaB activity in lung epithelial cells, but not through glucocorticoid receptors. J. Pharmacol. Sci. 2008, 106, 460–468. [Google Scholar]
- Wang, J.Y.; Guo, J.S.; Li, H.; Liu, S.L.; Zern, M.A. Inhibitory effect of glycyrrhizin on NF-kappaB binding activity in CCl4- plus ethanol-induced liver cirrhosis in rats. Liver 1998, 18, 180–185. [Google Scholar]
- Chintharlapalli, S.; Papineni, S.; Jutooru, I.; McAlees, A.; Safe, S. Structure-dependent activity of glycyrrhetinic acid derivatives as peroxisome proliferator-activated receptor {gamma} agonists in colon cancer cells. Mol. Cancer Ther. 2007, 6, 1588–1598. [Google Scholar]
- Lee, C.S.; Kim, Y.J.; Lee, M.S.; Han, E.S.; Lee, S.J. 18beta-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci. 2008, 83, 481–489. [Google Scholar]
- Satomi, Y.; Nishino, H.; Shibata, S. Glycyrrhetinic acid and related compounds induce G1 arrest and apoptosis in human hepatocellular carcinoma HepG2. Anticancer Res. 2005, 25, 4043–4047. [Google Scholar]
- Aktan, F.; Henness, S.; Roufogalis, B.D.; Ammit, A.J. Gypenosides derived from Gynostemma pentaphyllum suppress NO synthesis in murine macrophages by inhibiting iNOS enzymatic activity and attenuating NF-kappaB-mediated iNOS protein expression. Nitric Oxide 2003, 8, 235–242. [Google Scholar]
- Chen, J.C.; Lu, K.W.; Lee, J.H.; Yeh, C.C.; Chung, J.G. Gypenosides induced apoptosis in human colon cancer cells through the mitochondria-dependent pathways and activation of caspase-3. Anticancer Res. 2006, 26, 4313–4326. [Google Scholar]
- Huang, T.H.; Li, Y.; Razmovski-Naumovski, V.; Tran, V.H.; Li, G.Q.; Duke, C.C.; Roufogalis, B.D. Gypenoside XLIX isolated from Gynostemma pentaphyllum inhibits nuclear factor-kappaB activation via a PPAR-alpha-dependent pathway. J. Biomed. Sci. 2006, 13, 535–548. [Google Scholar]
- Huang, T.H.; Tran, V.H.; Roufogalis, B.D.; Li, Y. Gypenoside XLIX, a naturally occurring PPAR-alpha activator, inhibits cytokine-induced vascular cell adhesion molecule-1 expression and activity in human endothelial cells. Eur. J. Pharmacol. 2007, 565, 158–165. [Google Scholar]
- Huang, T.H.; Tran, V.H.; Roufogalis, B.D.; Li, Y. Gypenoside XLIX, a naturally occurring gynosaponin, PPAR-alpha dependently inhibits LPS-induced tissue factor expression and activity in human THP-1 monocytic cells. Toxicol. Appl. Pharmacol. 2007, 218, 30–36. [Google Scholar]
- Lu, H.F.; Chen, Y.S.; Yang, J.S.; Chen, J.C.; Lu, K.W.; Chiu, T.H.; Liu, K.C.; Yeh, C.C.; Chen, G.W.; Lin, H.J.; Chung, J.G. Gypenosides induced G0/G1 arrest via inhibition of cyclin E and induction of apoptosis via activation of caspases-3 and -9 in human lung cancer A-549 cells. In Vivo 2008, 22, 215–221. [Google Scholar]
- Lee, T.K.; Poon, R.T.; Wo, J.Y.; Ma, S.; Guan, X.Y.; Myers, J.N.; Altevogt, P.; Yuen, A.P. Lupeol suppresses cisplatin-induced nuclear factor-kappaB activation in head and neck squamous cell carcinoma and inhibits local invasion and nodal metastasis in an orthotopic nude mouse model. Cancer Res. 2007, 67, 8800–8809. [Google Scholar]
- Murtaza, I.; Saleem, M.; Adhami, V.M.; Hafeez, B.B.; Mukhtar, H. Suppression of cFLIP by lupeol, a dietary triterpene, is sufficient to overcome resistance to TRAIL-mediated apoptosis in chemoresistant human pancreatic cancer cells. Cancer Res. 2009, 69, 1156–1165. [Google Scholar]
- Nigam, N.; Prasad, S.; George, J.; Shukla, Y. Lupeol induces p53 and cyclin-B-mediated G2/M arrest and targets apoptosis through activation of caspase in mouse skin. Biochem. Biophys. Res. Commun. 2009, 381, 253–258. [Google Scholar]
- Prasad, S.; Kalra, N.; Shukla, Y. Induction of apoptosis by lupeol and mango extract in mouse prostate and LNCaP cells. Nutr. Cancer 2008, 60, 120–130. [Google Scholar]
- Prasad, S.; Madan, E.; Nigam, N.; Roy, P.; George, J.; Shukla, Y. Induction of apoptosis by lupeol in human epidermoid carcinoma A431 cells through regulation of mitochondrial, Akt/PKB and NFkappaB signaling pathways. Cancer Biol. Ther. 2009, 8, 1632–1639. [Google Scholar]
- Saleem, M.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene 2004, 23, 5203–5214. [Google Scholar]
- Saleem, M.; Kaur, S.; Kweon, M.H.; Adhami, V.M.; Afaq, F.; Mukhtar, H. Lupeol, a fruit and vegetable based triterpene, induces apoptotic death of human pancreatic adenocarcinoma cells via inhibition of Ras signaling pathway. Carcinogenesis 2005, 26, 1956–1964. [Google Scholar]
- Saleem, M.; Murtaza, I.; Tarapore, R.S.; Suh, Y.; Adhami, V.M.; Johnson, J.J.; Siddiqui, I.A.; Khan, N.; Asim, M.; Hafeez, B.B.; Shekhani, M.T.; Li, B.; Mukhtar, H. Lupeol inhibits proliferation of human prostate cancer cells by targeting beta-catenin signaling. Carcinogenesis 2009, 30, 808–817. [Google Scholar]
- Saleem, M.; Murtaza, I.; Witkowsky, O.; Kohl, A.M.; Maddodi, N. Lupeol triterpene, a novel diet-based microtubule targeting agent: disrupts survivin/cFLIP activation in prostate cancer cells. Biochem. Biophys. Res. Commun. 2009, 388, 576–582. [Google Scholar]
- Zhang, L.; Zhang, Y.; Zhang, L.; Yang, X.; Lv, Z. Lupeol, a dietary triterpene, inhibited growth, and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Invest 2009, 27, 163–170. [Google Scholar]
- Won, J.H.; Shin, J.S.; Park, H.J.; Jung, H.J.; Koh, D.J.; Jo, B.G.; Lee, J.Y.; Yun, K.; Lee, K.T. Anti-inflammatory Effects of Madecassic Acid via the Suppression of NF-kappaB Pathway in LPS-Induced RAW 264.7 Macrophage Cells. Planta Med. 2010, 76, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Lee, J.W.; Hahm, E.R.; Jung, K.C.; Lee, J.H.; Park, C.H.; Rhee, H.S.; Ryu, J.M.; Kim, H.K.; Yang, C.H. Momordin I, an inhibitor of AP-1, suppressed osteoclastogenesis through inhibition of NF-kappaB and AP-1 and also reduced osteoclast activity and survival. Biochem. Biophys. Res. Commun. 2005, 337, 815–823. [Google Scholar]
- Kim, J.H.; Ju, E.M.; Lee, D.K.; Hwang, H.J. Induction of apoptosis by momordin I in promyelocytic leukemia (HL-60) cells. Anticancer Res. 2002, 22, 1885–1889. [Google Scholar]
- Afaq, F.; Saleem, M.; Aziz, M.H.; Mukhtar, H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion markers in CD-1 mouse skin by oleandrin. Toxicol. Appl. Pharmacol. 2004, 195, 361–369. [Google Scholar]
- Manna, S.K.; Sah, N.K.; Newman, R.A.; Cisneros, A.; Aggarwal, B.B. Oleandrin suppresses activation of nuclear transcription factor-kappaB, activator protein-1, and c-Jun NH2-terminal kinase. Cancer Res. 2000, 60, 3838–3847. [Google Scholar]
- Newman, R.A.; Kondo, Y.; Yokoyama, T.; Dixon, S.; Cartwright, C.; Chan, D.; Johansen, M.; Yang, P. Autophagic cell death of human pancreatic tumor cells mediated by oleandrin, a lipid-soluble cardiac glycoside. Integr. Cancer Ther. 2007, 6, 354–364. [Google Scholar]
- Smith, J.A.; Madden, T.; Vijjeswarapu, M.; Newman, R.A. Inhibition of export of fibroblast growth factor-2 (FGF-2) from the prostate cancer cell lines PC3 and DU145 by Anvirzel and its cardiac glycoside component, oleandrin. Biochem. Pharmacol. 2001, 62, 469–472. [Google Scholar]
- Sreenivasan, Y.; Raghavendra, P.B.; Manna, S.K. Oleandrin-mediated expression of Fas potentiates apoptosis in tumor cells. J. Clin. Immunol. 2006, 26, 308–322. [Google Scholar]
- Chu, R.; Zhao, X.; Griffin, C.; Staub, R.E.; Shoemaker, M.; Climent, J.; Leitman, D.; Cohen, I.; Shtivelman, E.; Fong, S. Selective concomitant inhibition of mTORC1 and mTORC2 activity in estrogen receptor negative breast cancer cells by BN107 and oleanolic acid. Int. J. Cancer 2009. [Google Scholar]
- Deeb, D.; Gao, X.; Dulchavsky, S.A.; Gautam, S.C. CDDO-Me inhibits proliferation, induces apoptosis, down-regulates Akt, mTOR, NF-kappaB and NF-kappaB-regulated antiapoptotic and proangiogenic proteins in TRAMP prostate cancer cells. J. Exp. Ther. Oncol. 2008, 7, 31–39. [Google Scholar]
- Deeb, D.; Gao, X.; Jiang, H.; Janic, B.; Arbab, A.S.; Rojanasakul, Y.; Dulchavsky, S.A.; Gautam, S.C. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem. Pharmacol. 2010, 79, 350–360. [Google Scholar]
- Yan, S.L.; Huang, C.Y.; Wu, S.T.; Yin, M.C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol in Vitro 24, 842–848. [PubMed]
- Ahn, K.S.; Hahn, B.S.; Kwack, K.; Lee, E.B.; Kim, Y.S. Platycodin D-induced apoptosis through nuclear factor-kappaB activation in immortalized keratinocytes. Eur. J. Pharmacol. 2006, 537, 1–11. [Google Scholar]
- Shin, D.Y.; Kim, G.Y.; Li, W.; Choi, B.T.; Kim, N.D.; Kang, H.S.; Choi, Y.H. Implication of intracellular ROS formation, caspase-3 activation and Egr-1 induction in platycodon D-induced apoptosis of U937 human leukemia cells. Biomed. Pharmacother. 2009, 63, 86–94. [Google Scholar]
- Byun, J.Y.; Kim, M.J.; Eum, D.Y.; Yoon, C.H.; Seo, W.D.; Park, K.H.; Hyun, J.W.; Lee, Y.S.; Lee, J.S.; Yoon, M.Y.; Lee, S.J. Reactive oxygen species-dependent activation of Bax and poly(ADP-ribose) polymerase-1 is required for mitochondrial cell death induced by triterpenoid pristimerin in human cervical cancer cells. Mol. Pharmacol. 2009, 76, 734–744. [Google Scholar]
- Tiedemann, R.E.; Schmidt, J.; Keats, J.J.; Shi, C.X.; Zhu, Y.X.; Palmer, S.E.; Mao, X.; Schimmer, A.D.; Stewart, A.K. Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF-kappaB with antimyeloma activity in vitro and in vivo. Blood 2009, 113, 4027–4037. [Google Scholar]
- Wu, C.C.; Chan, M.L.; Chen, W.Y.; Tsai, C.Y.; Chang, F.R.; Wu, Y.C. Pristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondria. Mol. Cancer Ther. 2005, 4, 1277–1285. [Google Scholar]
- Yang, H.; Landis-Piwowar, K.R.; Lu, D.; Yuan, P.; Li, L.; Reddy, G.P.; Yuan, X.; Dou, Q.P. Pristimerin induces apoptosis by targeting the proteasome in prostate cancer cells. J. Cell Biochem. 2008, 103, 234–244. [Google Scholar]
- Hsu, Y.L.; Kuo, P.L.; Chiang, L.C.; Lin, C.C. Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in induction of apoptosis and cell cycle arrest by saikosaponin d in human hepatoma cell lines. Cancer Lett. 2004, 213, 213–221. [Google Scholar]
- Leung, C.Y.; Liu, L.; Wong, R.N.; Zeng, Y.Y.; Li, M.; Zhou, H. Saikosaponin-d inhibits T cell activation through the modulation of PKCtheta, JNK, and NF-kappaB transcription factor. Biochem. Biophys. Res. Commun. 2005, 338, 1920–1927. [Google Scholar]
- Wong, V.K.; Zhou, H.; Cheung, S.S.; Li, T.; Liu, L. Mechanistic study of saikosaponin-d (Ssd) on suppression of murine T lymphocyte activation. J. Cell Biochem. 2009, 107, 303–315. [Google Scholar]
- Manu, K.A.; Kuttan, G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-kappaB mediated activation of bcl-2 in B16F-10 melanoma cells. Int. Immunopharmacol. 2008, 8, 974–981. [Google Scholar]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375–4383. [Google Scholar]
- Achiwa, Y.; Hasegawa, K.; Udagawa, Y. Regulation of the phosphatidylinositol 3-kinase-Akt and the mitogen-activated protein kinase pathways by ursolic acid in human endometrial cancer cells. Biosci. Biotechnol. Biochem. 2007, 71, 31–37. [Google Scholar]
- Huang, H.C.; Huang, C.Y.; Lin-Shiau, S.Y.; Lin, J.K. Ursolic acid inhibits IL-1beta or TNF-alpha-induced C6 glioma invasion through suppressing the association ZIP/p62 with PKC-zeta and downregulating the MMP-9 expression. Mol. Carcinog. 2009, 48, 517–531. [Google Scholar]
- Li, Y.; Xing, D.; Chen, Q.; Chen, W.R. Enhancement of chemotherapeutic agent-induced apoptosis by inhibition of NF-kappaB using ursolic acid. Int. J. Cancer 2010, 127, 462–473. [Google Scholar]
- Pathak, A.K.; Bhutani, M.; Nair, A.S.; Ahn, K.S.; Chakraborty, A.; Kadara, H.; Guha, S.; Sethi, G.; Aggarwal, B.B. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol. Cancer Res. 2007, 5, 943–955. [Google Scholar]
- Shan, J.Z.; Xuan, Y.Y.; Zheng, S.; Dong, Q.; Zhang, S.Z. Ursolic acid inhibits proliferation and induces apoptosis of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J. Zhejiang Univ. Sci. B 2009, 10, 668–674. [Google Scholar]
- Wang, X.; Li, L.; Wang, B.; Xiang, J. Effects of ursolic acid on the proliferation and apoptosis of human ovarian cancer cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 761–764. [Google Scholar]
- Zhang, Y.X.; Kong, C.Z.; Wang, L.H.; Li, J.Y.; Liu, X.K.; Xu, B.; Xu, C.L.; Sun, Y.H. Ursolic acid overcomes Bcl-2-mediated resistance to apoptosis in prostate cancer cells involving activation of JNK-induced Bcl-2 phosphorylation and degradation. J. Cell Biochem. 2010, 109, 764–773. [Google Scholar]
- Chang, H.C.; Chang, F.R.; Wang, Y.C.; Pan, M.R.; Hung, W.C.; Wu, Y.C. A bioactive withanolide Tubocapsanolide A inhibits proliferation of human lung cancer cells via repressing Skp2 expression. Mol. Cancer Ther. 2007, 6, 1572–1578. [Google Scholar]
- Chen, W.Y.; Chang, F.R.; Huang, Z.Y.; Chen, J.H.; Wu, Y.C.; Wu, C.C. Tubocapsenolide A, a novel withanolide, inhibits proliferation and induces apoptosis in MDA-MB-231 cells by thiol oxidation of heat shock proteins. J. Biol. Chem. 2008, 283, 17184–17193. [Google Scholar]
- Ichikawa, H.; Takada, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol. Cancer Ther. 2006, 5, 1434–1445. [Google Scholar]
- Malik, F.; Kumar, A.; Bhushan, S.; Khan, S.; Bhatia, A.; Suri, K.A.; Qazi, G.N.; Singh, J. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis 2007, 12, 2115–2133. [Google Scholar]
- Mulabagal, V.; Subbaraju, G.V.; Rao, C.V.; Sivaramakrishna, C.; Dewitt, D.L.; Holmes, D.; Sung, B.; Aggarwal, B.B.; Tsay, H.S.; Nair, M.G. Withanolide sulfoxide from Aswagandha roots inhibits nuclear transcription factor-kappa-B, cyclooxygenase and tumor cell proliferation. Phytother. Res. 2009, 23, 987–992. [Google Scholar]
- Ndlovu, N.; van Lint, C.; Van Wesemael, K.; Callebert, P.; Chalbos, D.; Haegeman, G.; Vanden Berghe, W. Hyperactivated NF-{kappa}B and AP-1 transcription factors promote highly accessible chromatin and constitutive transcription across the interleukin-6 gene promoter in metastatic breast cancer cells. Mol. Cell Biol. 2009, 29, 5488–5504. [Google Scholar]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar]
- Bouvier, F.; Rahier, A.; Camara, B. Biogenesis, molecular regulation and function of plant isoprenoids. Prog. Lipid Res. 2005, 44, 357–429. [Google Scholar]
- Keeling, C.I.; Bohlmann, J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006, 170, 657–675. [Google Scholar]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–375. [Google Scholar]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-kappaB: its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar]
- Garcia, R.; Yu, C.L.; Hudnall, A.; Catlett, R.; Nelson, K.L.; Smithgall, T.; Fujita, D.J.; Ethier, S.P.; Jove, R. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 1997, 8, 1267–1276. [Google Scholar]
- Kim, Y.K.; Kim, R.G.; Park, S.J.; Ha, J.H.; Choi, J.W.; Park, H.J.; Lee, K.T. In vitro antiinflammatory activity of kalopanaxsaponin A isolated from Kalopanax pictus in murine macrophage RAW 264.7 cells. Biol. Pharm. Bull. 2002, 25, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yang, M.; Huang, F.; Li, K.; Si, J.; Shi, L.; Chen, S.; Xiao, P. Cytotoxicity of three cycloartane triterpenoids from Cimicifuga dahurica. Cancer Lett. 2005, 226, 65–75. [Google Scholar]
- Migone, T.S.; Lin, J.X.; Cereseto, A.; Mulloy, J.C.; O'Shea, J.J.; Franchini, G.; Leonard, W.J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science 1995, 269, 79–81. [Google Scholar]
- Megeney, L.A.; Perry, R.L.; LeCouter, J.E.; Rudnicki, M.A. bFGF and LIF signaling activates STAT3 in proliferating myoblasts. Dev. Genet. 1996, 19, 139–145. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. NF-kappaB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar]
- Janssens, S.; Tschopp, J. Signals from within: the DNA-damage-induced NF-kappaB response. Cell Death Differ. 2006, 13, 773–784. [Google Scholar]
- Karin, M.; Greten, F.R. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar]
- Bonizzi, G.; Karin, M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar]
- Xie, K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001, 12, 375–391. [Google Scholar]
- Garg, A.; Aggarwal, B.B. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia 2002, 16, 1053–1068. [Google Scholar]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar]
- Ulrich, C.M.; Potter, J.D. Testing for colon neoplasia susceptibility variants at the human Cox2 locus. J. Natl. Cancer Inst. 2001, 93, 1572–1574. [Google Scholar]
- Suh, N.; Wang, Y.; Honda, T.; Gribble, G.W.; Dmitrovsky, E.; Hickey, W.F.; Maue, R.A.; Place, A.E.; Porter, D.M.; Spinella, M.J.; Williams, C.R.; Wu, G.; Dannenberg, A.J.; Flanders, K.C.; Letterio, J.J.; Mangelsdorf, D.J.; Nathan, C.F.; Nguyen, L.; Porter, W.W.; Ren, R.F.; Roberts, A.B.; Roche, N.S.; Subbaramaiah, K.; Sporn, M.B. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res. 1999, 59, 336–341. [Google Scholar]
- Suh, N.; Roberts, A.B.; Birkey Reffey, S.; Miyazono, K.; Itoh, S.; ten Dijke, P.; Heiss, E.H.; Place, A.E.; Risingsong, R.; Williams, C.R.; Honda, T.; Gribble, G.W.; Sporn, M.B. Synthetic triterpenoids enhance transforming growth factor beta/Smad signaling. Cancer Res. 2003, 63, 1371–1376. [Google Scholar]
- Suh, W.S.; Kim, Y.S.; Schimmer, A.D.; Kitada, S.; Minden, M.; Andreeff, M.; Suh, N.; Sporn, M.; Reed, J.C. Synthetic triterpenoids activate a pathway for apoptosis in AML cells involving downregulation of FLIP and sensitization to TRAIL. Leukemia 2003, 17, 2122–2129. [Google Scholar]
- Ahmad, R.; Raina, D.; Meyer, C.; Kharbanda, S.; Kufe, D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J. Biol. Chem. 2006, 281, 35764–35769. [Google Scholar]
- Yore, M.M.; Liby, K.T.; Honda, T.; Gribble, G.W.; Sporn, M.B. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol. Cancer Ther. 2006, 5, 3232–3239. [Google Scholar]
- Couch, R.D.; Browning, R.G.; Honda, T.; Gribble, G.W.; Wright, D.L.; Sporn, M.B.; Anderson, A.C. Studies on the reactivity of CDDO, a promising new chemopreventive and chemotherapeutic agent: implications for a molecular mechanism of action. Bioorg. Med. Chem. Lett 2005, 15, 2215–2219. [Google Scholar]
- Haridas, V.; Kim, S.O.; Nishimura, G.; Hausladen, A.; Stamler, J.S.; Gutterman, J.U. Avicinylation (thioesterification): a protein modification that can regulate the response to oxidative and nitrosative stress. Proc. Natl. Acad. Sci. USA 2005, 102, 10088–10093. [Google Scholar]
- Deeb, D.; Gao, X.; Jiang, H.; Dulchavsky, S.A.; Gautam, S.C. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells by independently targeting pro-survival Akt and mTOR. Prostate 2009, 69, 851–860. [Google Scholar]
- Dirsch, V.M.; Kiemer, A.K.; Wagner, H.; Vollmar, A.M. The triterpenoid quinonemethide pristimerin inhibits induction of inducible nitric oxide synthase in murine macrophages. Eur. J. Pharmacol. 1997, 336, 211–217. [Google Scholar]
- Brunelleschi, S.; Bardelli, C.; Amoruso, A.; Gunella, G.; Ieri, F.; Romani, A.; Malorni, W.; Franconi, F. Minor polar compounds extra-virgin olive oil extract (MPC-OOE) inhibits NF-kappa B translocation in human monocyte/macrophages. Pharmacol. Res. 2007, 56, 542–549. [Google Scholar]
- Tundis, R.; Bonesi, M.; Deguin, B.; Loizzo, M.R.; Menichini, F.; Conforti, F.; Tillequin, F.; Menichini, F. Cytotoxic activity and inhibitory effect on nitric oxide production of triterpene saponins from the roots of Physospermum verticillatum (Waldst & Kit) (Apiaceae). Bioorg. Med. Chem. 2009, 17, 4542–4547. [Google Scholar]
- Yesilada, E.; Bedir, E.; Calis, I.; Takaishi, Y.; Ohmoto, Y. Effects of triterpene saponins from Astragalus species on in vitro cytokine release. J. Ethnopharmacol. 2005, 96, 71–77. [Google Scholar]
- Chang, U.M.; Li, C.H.; Lin, L.I.; Huang, C.P.; Kan, L.S.; Lin, S.B. Ganoderiol F, a ganoderma triterpene, induces senescence in hepatoma HepG2 cells. Life Sci. 2006, 79, 1129–1139. [Google Scholar]
- Lu, K.W.; Tsai, M.L.; Chen, J.C.; Hsu, S.C.; Hsia, T.C.; Lin, M.W.; Huang, A.C.; Chang, Y.H.; Ip, S.W.; Lu, H.F.; Chung, J.G. Gypenosides inhibited invasion and migration of human tongue cancer SCC4 cells through down-regulation of NFkappaB and matrix metalloproteinase-9. Anticancer Res. 2008, 28, 1093–1099. [Google Scholar]
- Park, Y.S.; Lee, J.H.; Bondar, J.; Harwalkar, J.A.; Safayhi, H.; Golubic, M. Cytotoxic action of acetyl-11-keto-beta-boswellic acid (AKBA) on meningioma cells. Planta Med. 2002, 68, 397–401. [Google Scholar]
- Lee, K.J.; Hwang, S.J.; Choi, J.H.; Jeong, H.G. Saponins derived from the roots of Platycodon grandiflorum inhibit HT-1080 cell invasion and MMPs activities: regulation of NF-kappaB activation via ROS signal pathway. Cancer Lett. 2008, 268, 233–243. [Google Scholar]
- Kim, M.O.; Moon, D.O.; Choi, Y.H.; Shin, D.Y.; Kang, H.S.; Choi, B.T.; Lee, J.D.; Li, W.; Kim, G.Y. Platycodin D induces apoptosis and decreases telomerase activity in human leukemia cells. Cancer Lett. 2008, 261, 98–107. [Google Scholar]
- Xie, Y.; Ye, Y.P.; Sun, H.X.; Li, D. Contribution of the glycidic moieties to the haemolytic and adjuvant activity of platycodigenin-type saponins from the root of Platycodon grandiflorum. Vaccine 2008, 26, 3452–3460. [Google Scholar]
- Fulda, S.; Debatin, K.M. Signaling through death receptors in cancer therapy. Curr. Opin. Pharmacol. 2004, 4, 327–332. [Google Scholar]
- Fesik, S.W.; Shi, Y. Structural biology. Controlling the caspases. Science 2001, 294, 1477–1478. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2002, 2, 420–430. [Google Scholar]
- Debatin, K.M.; Krammer, P.H. Death receptors in chemotherapy and cancer. Oncogene 2004, 23, 2950–2966. [Google Scholar]
- Rieger, L.; Weller, M.; Bornemann, A.; Schabet, M.; Dichgans, J.; Meyermann, R. BCL-2 family protein expression in human malignant glioma: a clinical-pathological correlative study. J. Neurol. Sci. 1998, 155, 68–75. [Google Scholar]
- Gerhauser, C. Cancer chemopreventive potential of apples, apple juice, and apple components. Planta Med. 2008, 74, 1608–1624. [Google Scholar]
- Costa, P.M.; Ferreira, P.M.; Bolzani Vda, S.; Furlan, M.; de Freitas Formenton Macedo Dos Santos, V.A.; Corsino, J.; de Moraes, M.O.; Costa-Lotufo, L.V.; Montenegro, R.C.; Pessoa, C. Antiproliferative activity of pristimerin isolated from Maytenus ilicifolia (Celastraceae) in human HL-60 cells. Toxicol in Vitro 2008, 22, 854–863. [Google Scholar]
- Murayama, T.; Eizuru, Y.; Yamada, R.; Sadanari, H.; Matsubara, K.; Rukung, G.; Tolo, F.M.; Mungai, G.M.; Kofi-Tsekpo, M. Anticytomegalovirus activity of pristimerin, a triterpenoid quinone methide isolated from Maytenus heterophylla (Eckl. & Zeyh.). Antivir. Chem. Chemother. 2007, 18, 133–139. [Google Scholar] [PubMed]
- Liagre, B.; Bertrand, J.; Leger, D.Y.; Beneytout, J.L. Diosgenin, a plant steroid, induces apoptosis in COX-2 deficient K562 cells with activation of the p38 MAP kinase signalling and inhibition of NF-kappaB binding. Int. J. Mol. Med. 2005, 16, 1095–1101. [Google Scholar]
- Haridas, V.; Li, X.; Mizumachi, T.; Higuchi, M.; Lemeshko, V.V.; Colombini, M.; Gutterman, J.U. Avicins, a novel plant-derived metabolite lowers energy metabolism in tumor cells by targeting the outer mitochondrial membrane. Mitochondrion 2007, 7, 234–240. [Google Scholar]
- Pandey, M.K.; Sung, B.; Aggarwal, B.B. Betulinic acid suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase SHP-1 in human multiple myeloma cells. Int. J. Cancer 2010, 127, 282–292. [Google Scholar]
- Martin, R.; Ibeas, E.; Carvalho-Tavares, J.; Hernandez, M.; Ruiz-Gutierrez, V.; Nieto, M.L. Natural triterpenic diols promote apoptosis in astrocytoma cells through ROS-mediated mitochondrial depolarization and JNK activation. PLoS One 2009, 4, e5975. [Google Scholar]
- Martinez-Gonzalez, J.; Rodriguez-Rodriguez, R.; Gonzalez-Diez, M.; Rodriguez, C.; Herrera, M.D.; Ruiz-Gutierrez, V.; Badimon, L. Oleanolic acid induces prostacyclin release in human vascular smooth muscle cells through a cyclooxygenase-2-dependent mechanism. J. Nutr. 2008, 138, 443–448. [Google Scholar]
- Reyes, F.J.; Centelles, J.J.; Lupianez, J.A.; Cascante, M. (2Alpha,3beta)-2,3-dihydroxyolean-12-en-28-oic acid, a new natural triterpene from Olea europea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett. 2006, 580, 6302–6310. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chang, N.W.; Chung, J.G.; Chen, K.C. Saikosaponin-A induces apoptotic mechanism in human breast MDA-MB-231 and MCF-7 cancer cells. Am. J. Chin. Med. 2003, 31, 363–377. [Google Scholar]
- Hsu, M.J.; Cheng, J.S.; Huang, H.C. Effect of saikosaponin, a triterpene saponin, on apoptosis in lymphocytes: association with c-myc, p53, and bcl-2 mRNA. Br. J. Pharmacol. 2000, 131, 1285–1293. [Google Scholar]
- Sun, Y.; Cai, T.T.; Zhou, X.B.; Xu, Q. Saikosaponin a inhibits the proliferation and activation of T cells through cell cycle arrest and induction of apoptosis. Int. Immunopharmacol. 2009, 9, 978–983. [Google Scholar]
- Siu, F.M.; Ma, D.L.; Cheung, Y.W.; Lok, C.N.; Yan, K.; Yang, Z.; Yang, M.; Xu, S.; Ko, B.C.; He, Q.Y.; Che, C.M. Proteomic and transcriptomic study on the action of a cytotoxic saponin (Polyphyllin D): induction of endoplasmic reticulum stress and mitochondria-mediated apoptotic pathways. Proteomics 2008, 8, 3105–3117. [Google Scholar]
- Zou, W.; Liu, X.; Yue, P.; Zhou, Z.; Sporn, M.B.; Lotan, R.; Khuri, F.R.; Sun, S.Y. c-Jun NH2-terminal kinase-mediated up-regulation of death receptor 5 contributes to induction of apoptosis by the novel synthetic triterpenoid methyl-2-cyano-3,12-dioxooleana-1, 9-dien-28-oate in human lung cancer cells. Cancer Res. 2004, 64, 7570–7578. [Google Scholar]
- Pedersen, I.M.; Kitada, S.; Schimmer, A.; Kim, Y.; Zapata, J.M.; Charboneau, L.; Rassenti, L.; Andreeff, M.; Bennett, F.; Sporn, M.B.; Liotta, L.D.; Kipps, T.J.; Reed, J.C. The triterpenoid CDDO induces apoptosis in refractory CLL B cells. Blood 2002, 100, 2965–2972. [Google Scholar]
- Liu, J.J.; Duan, R.D. LY294002 enhances boswellic acid-induced apoptosis in colon cancer cells. Anticancer Res. 2009, 29, 2987–2991. [Google Scholar]
- Liu, J.J.; Huang, B.; Hooi, S.C. Acetyl-keto-beta-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells. Br. J. Pharmacol. 2006, 148, 1099–1107. [Google Scholar]
- Hostanska, K.; Daum, G.; Saller, R. Cytostatic and apoptosis-inducing activity of boswellic acids toward malignant cell lines in vitro. Anticancer Res. 2002, 22, 2853–2862. [Google Scholar]
- Liu, J.J.; Nilsson, A.; Oredsson, S.; Badmaev, V.; Zhao, W.Z.; Duan, R.D. Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis 2002, 23, 2087–2093. [Google Scholar]
- Kim, J.Y.; Park, K.W.; Moon, K.D.; Lee, M.K.; Choi, J.; Yee, S.T.; Shim, K.H.; Seo, K.I. Induction of apoptosis in HT-29 colon cancer cells by crude saponin from Platycodi Radix. Food Chem. Toxicol. 2008, 46, 3753–3758. [Google Scholar]
- Park, D.I.; Lee, J.H.; Moon, S.K.; Kim, C.H.; Lee, Y.T.; Cheong, J.; Choi, B.T.; Choi, Y.H. Induction of apoptosis and inhibition of telomerase activity by aqueous extract from Platycodon grandiflorum in human lung carcinoma cells. Pharmacol. Res. 2005, 51, 437–443. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar]
- He, X.; Liu, R.H. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple's anticancer activity. J. Agric. Food Chem. 2007, 55, 4366–4370. [Google Scholar]
- Juan, M.E.; Planas, J.M.; Ruiz-Gutierrez, V.; Daniel, H.; Wenzel, U. Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells. Br. J. Nutr. 2008, 100, 36–43. [Google Scholar]
- Juan, M.E.; Wenzel, U.; Ruiz-Gutierrez, V.; Daniel, H.; Planas, J.M. Olive fruit extracts inhibit proliferation and induce apoptosis in HT-29 human colon cancer cells. J. Nutr. 2006, 136, 2553–2557. [Google Scholar]
- Marquez Martin, A.; de la Puerta Vazquez, R.; Fernandez-Arche, A.; Ruiz-Gutierrez, V. Supressive effect of maslinic acid from pomace olive oil on oxidative stress and cytokine production in stimulated murine macrophages. Free Radic. Res. 2006, 40, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ren, J.; Tang, J.; Zhang, D.; Li, B.; Li, Y. Estrogen-like activities of saikosaponin-d in vitro: a pilot study. Eur. J. Pharmacol. 626, 159–165. [PubMed]
- Zu, N.; Li, P.; Li, N.; Choy, P.; Gong, Y. Mechanism of saikosaponin-d in the regulation of rat mesangial cell proliferation and synthesis of extracellular matrix proteins. Biochem. Cell Biol. 2007, 85, 169–174. [Google Scholar]
- Jedinak, A.; Muckova, M.; Kost'alova, D.; Maliar, T.; Masterova, I. Antiprotease and antimetastatic activity of ursolic acid isolated from Salvia officinalis. Z Naturforsch C 2006, 61, 777–782. [Google Scholar]
- Suh, N.; Honda, T.; Finlay, H.J.; Barchowsky, A.; Williams, C.; Benoit, N.E.; Xie, Q.W.; Nathan, C.; Gribble, G.W.; Sporn, M.B. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998, 58, 717–723. [Google Scholar]
- Sawada, N.; Kataoka, K.; Kondo, K.; Arimochi, H.; Fujino, H.; Takahashi, Y.; Miyoshi, T.; Kuwahara, T.; Monden, Y.; Ohnishi, Y. Betulinic acid augments the inhibitory effects of vincristine on growth and lung metastasis of B16F10 melanoma cells in mice. Br. J. Cancer 2004, 90, 1672–1678. [Google Scholar]
- Noujaim, D.; van Golen, C.M.; van Golen, K.L.; Grauman, A.; Feldman, E.L. N-Myc and Bcl-2 coexpression induces MMP-2 secretion and activation in human neuroblastoma cells. Oncogene 2002, 21, 4549–4557. [Google Scholar]
- Pan, M.R.; Chang, H.C.; Wu, Y.C.; Huang, C.C.; Hung, W.C. Tubocapsanolide A inhibits transforming growth factor-beta-activating kinase 1 to suppress NF-kappaB-induced CCR7. J. Biol. Chem. 2009, 284, 2746–2754. [Google Scholar]
- Moon, H.I.; Kim, M.R.; Woo, E.R.; Chung, J.H. Triterpenoid from Styrax japonica SIEB. et ZUCC, and its effects on the expression of matrix metalloproteinases-1 and type 1 procollagen caused by ultraviolet irradiated cultured primary human skin fibroblasts. Biol. Pharm. Bull. 2005, 28, 2003–2006. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Chau, C.F.; Chen, K.D.; Chen, D.H.; Yen, G.C. The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain. Mol. Nutr. Food Res. 2007, 51, 1472–1477. [Google Scholar]
- McMahon, G. VEGF receptor signaling in tumor angiogenesis. Oncologist 2000, 5 (Suppl. 1), 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shyu, K.G.; Tsai, S.C.; Wang, B.W.; Liu, Y.C.; Lee, C.C. Saikosaponin C induces endothelial cells growth, migration and capillary tube formation. Life Sci. 2004, 76, 813–826. [Google Scholar]
- Wang, B.F.; Cheng, Y.A.; Dang, S.S. Angiogenesis inhibitory effect of saikosaponin-d on chicken embryo. Zhongguo Zhong Xi Yi Jie He Za Zhi 2009, 29, 425–429. [Google Scholar]
- Sogno, I.; Vannini, N.; Lorusso, G.; Cammarota, R.; Noonan, D.M.; Generoso, L.; Sporn, M.B.; Albini, A. Anti-angiogenic activity of a novel class of chemopreventive compounds: oleanic acid terpenoids. Recent Results Cancer Res. 2009, 181, 209–212. [Google Scholar]
- Shishodia, S.; Sethi, G.; Konopleva, M.; Andreeff, M.; Aggarwal, B.B. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin. Cancer Res. 2006, 12, 1828–1838. [Google Scholar]
- Pang, X.; Yi, Z.; Zhang, X.; Sung, B.; Qu, W.; Lian, X.; Aggarwal, B.B.; Liu, M. Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res. 2009, 69, 5893–5900. [Google Scholar]
- Kwon, H.J.; Shim, J.S.; Kim, J.H.; Cho, H.Y.; Yum, Y.N.; Kim, S.H.; Yu, J. Betulinic acid inhibits growth factor-induced in vitro angiogenesis via the modulation of mitochondrial function in endothelial cells. Jpn. J. Cancer Res. 2002, 93, 417–425. [Google Scholar]
- Zhou, Y.X.; Huang, Y.L. Antiangiogenic effect of celastrol on the growth of human glioma: an in vitro and in vivo study. Chin. Med. J. (Engl.) 2009, 122, 1666–1673. [Google Scholar] [PubMed]
- Kimura, Y.; Taniguchi, M.; Baba, K. Antitumor and antimetastatic effects on liver of triterpenoid fractions of Ganoderma lucidum: mechanism of action and isolation of an active substance. Anticancer Res. 2002, 22, 3309–3318. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting Inflammatory Pathways by Triterpenoids for Prevention and Treatment of Cancer. Toxins 2010, 2, 2428-2466. https://doi.org/10.3390/toxins2102428
Yadav VR, Prasad S, Sung B, Kannappan R, Aggarwal BB. Targeting Inflammatory Pathways by Triterpenoids for Prevention and Treatment of Cancer. Toxins. 2010; 2(10):2428-2466. https://doi.org/10.3390/toxins2102428
Chicago/Turabian StyleYadav, Vivek R., Sahdeo Prasad, Bokyung Sung, Ramaswamy Kannappan, and Bharat B. Aggarwal. 2010. "Targeting Inflammatory Pathways by Triterpenoids for Prevention and Treatment of Cancer" Toxins 2, no. 10: 2428-2466. https://doi.org/10.3390/toxins2102428
APA StyleYadav, V. R., Prasad, S., Sung, B., Kannappan, R., & Aggarwal, B. B. (2010). Targeting Inflammatory Pathways by Triterpenoids for Prevention and Treatment of Cancer. Toxins, 2(10), 2428-2466. https://doi.org/10.3390/toxins2102428




