Reciprocal Interactions between Lactoferrin and Bacterial Endotoxins and Their Role in the Regulation of the Immune Response
Abstract
:1. Introduction
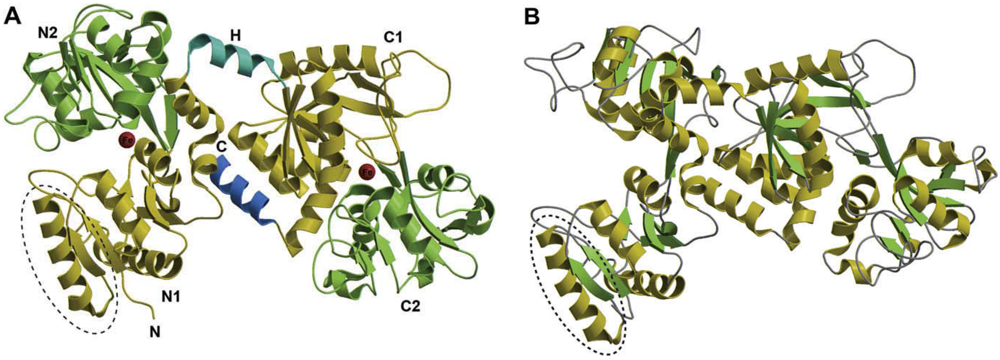
2. Structure of Lf and Molecular Basis of Lf-LPS Interaction
3. Lf Interference with LPS Inflammatory Activity
| Cell | Lf type | LPS-induced functions | Suggested mechanism | References |
|---|---|---|---|---|
| Mouse RAW 264.7 | huLf, bLf | Cytokine production (TNF-α IL-1β, IL-6, IL-8) | Inhibition of NF-kB activation | [42,45-47,49] |
| Human THP-1 | ||||
| Human Mono Mac 6 | ||||
| HUVEC | huLf | Cytokine production (IL-8) | Interaction with sCD14/LPS complex | [49] |
| Human PBMC | Lfcin-derived peptides | Cytokine production (TNF-α) | LPS inactivation via structural changes | [48] |
| Human THP-1 | huLf, bLf, Lfcin B | Cytokine production (IL-6, IL-1, TNF-α) | Not determined | [42,47] |
| Primary human monocytes | ||||
| Human PBMC | ||||
| HUVEC | huLf | Endothelial adhesion molecule expression | Interaction with sCD14/LPS complex | [17] |
| Human neutrophils | huLf | Hydrogen peroxide production | Inhibition of LPS binding to L-selectin | [44] |
| Human neutrophils | Neutrophil released Lf | Priming for enhanced superoxide production | LPS sequestration | [52,53] |
| ApoLf* | ||||
| Bovine PBMC | bLf | Proliferation | Not determined | [51] |
| PGE2 production | ||||
| COX-2 and MMP-9 expression | ||||
| Human CaCo2 | huLf | Intestinal mucosa damage | Not determined | [50] |
| Animal | Lf source | Administration | LPS-triggered effects | Lf activity | References |
|---|---|---|---|---|---|
| Mice, Piglets | bLf, huLf, Lfcin-derived peptides | i.v., i.p., p.o. | endotoxin lethal shock | survival | [54,55-57] |
| Mice | bLf, huLf | i.p. | preterm delivery | prevention | [62-64] |
| Mice | huLf | i.v. | hepatitis | protection | [60] |
| Rats, Mice | bLf, huLf | i.v., p.o., i.p. | TNF-α, IL-6, IL-10, NO production | decreased | [61,65-67] |
| Rats | bLf | p.o. | arthritis and hyperalgesia | prevention | [61] |
| Mice | bLf | i.p. | diarrhea | prevention | [58] |
| Mice | huLf | i.p. | liver mitochondrial dysfunction | protection | [68] |
| Rats | bLf, huLf | i.p. | albumin extravasation, neutrophilia | prevention | [59] |
4. Biological Activity of Lf-Bound LPS: TLR4 -Dependent and -Independent Effects
5. Concluding Remarks

Acknowledgements
References
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [PubMed]
- Westphal, O.; Luderitz, O.; Rietschel, E.T.; Galanos, C. Bacterial lipopolysaccharide and its lipid A component: Some historical and some current aspects. Biochem. Soc. Trans. 1981, 9, 191–195. [Google Scholar] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; Freudenberg, M.; Ricciardi-Castagnoli, P.; Layton, B.; Beutler, B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [PubMed]
- Schumann, R.R.; Leong, S.R.; Flaggs, G.W.; Gray, P.W.; Wright, S.D.; Mathison, J.C.; Tobias, P.S.; Ulevitch, R.J. Structure and function of lipopolysaccharide binding protein. Science 1990, 249, 1429–1431. [Google Scholar] [PubMed]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [PubMed]
- Pugin, J.; Ulevitch, R.J.; Tobias, P.S. A critical role for monocytes and CD14 in endotoxin-induced endothelial cell activation. J. Exp. Med. 1993, 178, 2193–2200. [Google Scholar] [PubMed]
- Wright, S.D. CD14 and innate recognition of bacteria. J. Immunol. 1995, 155, 6–8. [Google Scholar] [PubMed]
- Pugin, J.; Schurer-Maly, C.C.; Leturcq, D.; Moriarty, A.; Ulevitch, R.J.; Tobias, P.S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA 1993, 90, 2744–2748. [Google Scholar]
- Heumann, D.; Roger, T. Initial responses to endotoxins and Gram-negative bacteria. Clin. Chim. Acta 2002, 323, 59–72. [Google Scholar] [PubMed]
- Shimazu, R.; Akashi, S.; Ogata, H.; Nagai, Y.; Fukudome, K.; Miyake, K.; Kimoto, M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 1999, 189, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Munford, R.S. Detoxifying endotoxin: Time, place and person. J. Endotoxin. Res. 2005, 11, 69–84. [Google Scholar] [PubMed]
- Legrand, D.; Pierce, A.; Elass, E.; Carpentier, M.; Mariller, C.; Mazurier, J. Lactoferrin structure and functions. Adv. Exp. Med. Biol. 2008, 606, 163–194. [Google Scholar] [PubMed]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [PubMed]
- Appelmelk, B.J.; An, Y.Q.; Geerts, M.; Thijs, B.G.; de Boer, H.A.; MacLaren, D. M.; de Graaff, J.; Nuijens, J.H. Lactoferrin is a lipid A-binding protein. Infect. Immun. 1994, 62, 2628–2632. [Google Scholar] [PubMed]
- Baveye, S.; Elass, E.; Fernig, D.G.; Blanquart, C.; Mazurier, J.; Legrand, D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 2000, 68, 6519–6525. [Google Scholar] [CrossRef] [PubMed]
- Britigan, B.E.; Lewis, T.S.; Waldschmidt, M.; McCormick, M.L.; Krieg, A.M. Lactoferrin binds CpG-containing oligonucleotides and inhibits their immunostimulatory effects on human B cells. J. Immunol. 2001, 167, 2921–2928. [Google Scholar] [PubMed]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Interactions of lactoferrin with cells involved in immune function. Biochem. Cell Biol. 2006, 84, 282–290. [Google Scholar] [PubMed]
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie 2009, 91, 11–18. [Google Scholar] [PubMed]
- Baker, E.N.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Baker, H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [PubMed]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136. [Google Scholar] [PubMed]
- Senkovich, O.; Cook, W.J.; Mirza, S.; Hollingshead, S.K.; Protasevich, II; Briles, D.E.; Chattopadhyay, D. Structure of a complex of human lactoferrin N-lobe with pneumococcal surface protein a provides insight into microbial defense mechanism. J. Mol. Biol. 2007, 370, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Sabatucci, A.; Vachette, P.; Vasilyev, V.B.; Beltramini, M.; Sokolov, A.; Pulina, M.; Salvato, B.; Angelucci, C.B.; Maccarrone, M.; Cozzani, I.; Dainese, E. Structural characterization of the ceruloplasmin: Lactoferrin complex in solution. J. Mol. Biol. 2007, 371, 1038–1046. [Google Scholar] [PubMed]
- Mann, D.M.; Romm, E.; Migliorini, M. Delineation of the glycosaminoglycan-binding site in the human inflammatory response protein lactoferrin. J. Biol. Chem. 1994, 269, 23661–23667. [Google Scholar] [PubMed]
- van Berkel, P.H.; Geerts, M.E.; van Veen, H.A.; Mericskay, M.; de Boer, H.A.; Nuijens, J.H. N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem. J. 1997, 328, 145–151. [Google Scholar] [PubMed]
- Elass-Rochard, E.; Roseanu, A.; Legrand, D.; Trif, M.; Salmon, V.; Motas, C.; Montreuil, J.; Spik, G. Lactoferrin-lipopolysaccharide interaction: Involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 1995, 312, 839–845. [Google Scholar] [PubMed]
- Legrand, D.; van Berkel, P.H.; Salmon, V.; van Veen, H.A.; Slomianny, M.C.; Nuijens, J.H.; Spik, G. The N-terminal Arg2, Arg3 and Arg4 of human lactoferrin interact with sulphated molecules but not with the receptor present on Jurkat human lymphoblastic T-cells. Biochem. J. 1997, 327, 841–846. [Google Scholar] [PubMed]
- Wu, H.F.; Monroe, D.M.; Church, F.C. Characterization of the glycosaminoglycan-binding region of lactoferrin. Arch. Biochem. Biophys. 1995, 317, 85–92. [Google Scholar] [PubMed]
- Ellison, R.T., 3rd; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Invest. 1991, 88, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Giansanti, F.; Boffi, A.; Ajello, M.; Valenti, P.; Chiancone, E.; Antonini, G. Ca2+ binding to bovine lactoferrin enhances protein stability and influences the release of bacterial lipopolysaccharide. Biochem. Cell Biol. 2002, 80, 41–48. [Google Scholar] [PubMed]
- Haridas, M.; Anderson, B.F.; Baker, E.N. Structure of human diferric lactoferrin refined at 2.2 Ǻ resolution. Acta. Crystallogr. D. Biol. Crystallogr. 1995, 51, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-dimensional structure of diferric bovine lactoferrin at 2.8 Ǻ resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.C.; Wines, B.D.; Baker, H.; Langley, R.J.; Baker, E.N.; Fraser, J.D. The crystal structure of staphylococcal superantigen-like protein 11 in complex with sialyl Lewis X reveals the mechanism for cell binding and immune inhibition. Mol. Microbiol. 2007, 66, 1342–1355. [Google Scholar] [PubMed]
- Choi, B.K.; Actor, J.K.; Rios, S.; d'Anjou, M.; Stadheim, T.A.; Warburton, S.; Giaccone, E.; Cukan, M.; Li, H.; Kull, A.; Sharkey, N.; Gollnick, P.; Kocieba, M.; Artym, J.; Zimecki, M.; Kruzel, M.L.; Wildt, S. Recombinant human lactoferrin expressed in glycoengineered Pichia pastoris: Effect of terminal N-acetylneuraminic acid on in vitro secondary humoral immune response. Glycoconj. J. 2008, 25, 581–593. [Google Scholar] [PubMed]
- Zimecki, M.; Kocieba, M.; Kruzel, M. Immunoregulatory activities of lactoferrin in the delayed type hypersensitivity in mice are mediated by a receptor with affinity to mannose. Immunobiology 2002, 205, 120–131. [Google Scholar] [PubMed]
- Groot, F.; Geijtenbeek, T.B.; Sanders, R.W.; Baldwin, C.E.; Sanchez-Hernandez, M.; Floris, R.; van Kooyk, Y.; de Jong, E.C.; Berkhout, B. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN--gp120 interaction. J. Virol. 2005, 79, 3009–3015. [Google Scholar] [PubMed]
- Brandenburg, K.; Jurgens, G.; Muller, M.; Fukuoka, S.; Koch, M.H. Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol. Chem. 2001, 382, 1215–1225. [Google Scholar] [PubMed]
- Brandenburg, K.; Kusumoto, S.; Seydel, U. Conformational studies of synthetic lipid A analogues and partial structures by infrared spectroscopy. Biochim. Biophys. Acta 1997, 1329, 183–201. [Google Scholar] [PubMed]
- Schromm, A.B.; Brandenburg, K.; Loppnow, H.; Moran, A.P.; Koch, M.H.; Rietschel, E.T.; Seydel, U. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur. J. Biochem. 2000, 267, 2008–2013. [Google Scholar] [PubMed]
- Mattsby-Baltzer, I.; Roseanu, A.; Motas, C.; Elverfors, J.; Engberg, I.; Hanson, L.A. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr. Res. 1996, 40, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Elass-Rochard, E.; Legrand, D.; Salmon, V.; Roseanu, A.; Trif, M.; Tobias, P.S.; Mazurier, J.; Spik, G. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect. Immun. 1998, 66, 486–491. [Google Scholar] [PubMed]
- Baveye, S.; Elass, E.; Mazurier, J.; Legrand, D. Lactoferrin inhibits the binding of lipopolysaccharides to L-selectin and subsequent production of reactive oxygen species by neutrophils. FEBS Lett. 2000, 469, 5–8. [Google Scholar] [PubMed]
- Choe, Y.H.; Lee, S.W. Effect of lactoferrin on the production of tumor necrosis factor-alpha and nitric oxide. J. Cell Biochem. 1999, 76, 30–36. [Google Scholar] [PubMed]
- Haversen, L.; Ohlsson, B.G.; Hahn-Zoric, M.; Hanson, L.A.; Mattsby-Baltzer, I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell Immunol. 2002, 220, 83–95. [Google Scholar] [PubMed]
- Crouch, S.P.; Slater, K.J.; Fletcher, J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood 1992, 80, 235–240. [Google Scholar] [PubMed]
- Andra, J.; Lohner, K.; Blondelle, S.E.; Jerala, R.; Moriyon, I.; Koch, M.H.; Garidel, P.; Brandenburg, K. Enhancement of endotoxin neutralization by coupling of a C12-alkyl chain to a lactoferricin-derived peptide. Biochem. J. 2005, 385, 135–143. [Google Scholar] [PubMed]
- Elass, E.; Masson, M.; Mazurier, J.; Legrand, D. Lactoferrin inhibits the lipopolysaccharide-induced expression and proteoglycan-binding ability of interleukin-8 in human endothelial cells. Infect. Immun. 2002, 70, 1860–1866. [Google Scholar] [PubMed]
- Hirotani, Y.; Ikeda, K.; Kato, R.; Myotoku, M.; Umeda, T.; Ijiri, Y.; Tanaka, K. Protective effects of lactoferrin against intestinal mucosal damage induced by lipopolysaccharide in human intestinal Caco-2 cells. Yakugaku Zasshi 2008, 128, 1363–1368. [Google Scholar] [PubMed]
- Dawes, M.E.; Tyler, J.W.; Marsh, A.E.; Larson, R.L.; Steevens, B.J.; Lakritz, J. In Vitro effects of lactoferrin on lipopolysaccharide-induced proliferation, gene expression, and prostanoid production by bovine peripheral blood mononuclear cells. Am. J. Vet. Res. 2008, 69, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Mao, J.; Rasmussen, G.T.; Serody, J.S.; Britigan, B.E. Interaction of lactoferrin and lipopolysaccharide (LPS): Effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J. Infect. Dis. 1992, 166, 1375–1378. [Google Scholar] [PubMed]
- Wang, D.; Pabst, K.M.; Aida, Y.; Pabst, M.J. Lipopolysaccharide-inactivating activity of neutrophils is due to lactoferrin. J. Leukoc. Biol. 1995, 57, 865–874. [Google Scholar] [PubMed]
- Zagulski, T.; Lipinski, P.; Zagulska, A.; Broniek, S.; Jarzabek, Z. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. Br. J. Exp. Pathol. 1989, 70, 697–704. [Google Scholar] [PubMed]
- Zhang, G.H.; Mann, D.M.; Tsai, C.M. Neutralization of endotoxin in vitro and in vivo by a human lactoferrin-derived peptide. Infect. Immun. 1999, 67, 1353–1358. [Google Scholar] [PubMed]
- Kruzel, M.L.; Harari, Y.; Chen, C.Y.; Castro, G.A. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation 2000, 24, 33–44. [Google Scholar] [PubMed]
- Lee, W.J.; Farmer, J.L.; Hilty, M.; Kim, Y.B. The protective effects of lactoferrin feeding against endotoxin lethal shock in germfree piglets. Infect. Immun. 1998, 66, 1421–1426. [Google Scholar] [PubMed]
- Talukder, M.J.; Harada, E. Bovine lactoferrin protects lipopolysaccharide-induced diarrhea modulating nitric oxide and prostaglandin E2 in mice. Can. J. Physiol. Pharmacol. 2007, 85, 200–208. [Google Scholar] [PubMed]
- Yajima, M.; Yajima, T.; Kuwata, T. Intraperitoneal injection of lactoferrin ameliorates severe albumin extravasation and neutrophilia in LPS-induced inflammation in neonatal rats. Biomed. Res. 2005, 26, 249–255. [Google Scholar] [PubMed]
- Yamaguchi, M.; Matsuura, M.; Kobayashi, K.; Sasaki, H.; Yajima, T.; Kuwata, T. Lactoferrin protects against development of hepatitis caused by sensitization of Kupffer cells by lipopolysaccharide. Clin. Diagn. Lab. Immunol. 2001, 8, 1234–1239. [Google Scholar] [PubMed]
- Hayashida, K.; Kaneko, T.; Takeuchi, T.; Shimizu, H.; Ando, K.; Harada, E. Oral administration of lactoferrin inhibits inflammation and nociception in rat adjuvant-induced arthritis. J. Vet. Med. Sci. 2004, 66, 149–154. [Google Scholar] [PubMed]
- Mitsuhashi, Y.; Otsuki, K.; Yoda, A.; Shimizu, Y.; Saito, H.; Yanaihara, T. Effect of lactoferrin on lipopolysaccharide (LPS) induced preterm delivery in mice. Acta. Obstet. Gynecol. Scand. 2000, 79, 355–358. [Google Scholar] [PubMed]
- Sasaki, Y.; Otsuki, K.; Hasegawa, A.; Sawada, M.; Chiba, H.; Negishi, M.; Nagatsuka, M.; Okai, T. Preventive effect of recombinant human lactoferrin on lipopolysaccharide-induced preterm delivery in mice. Acta. Obstet. Gynecol. Scand. 2004, 83, 1035–1038. [Google Scholar] [PubMed]
- Otsuki, K.; Yakuwa, K.; Sawada, M.; Hasegawa, A.; Sasaki, Y.; Mitsukawa, K.; Chiba, H.; Nagatsuka, M.; Saito, H.; Okai, T. Recombinant human lactoferrin has preventive effects on lipopolysaccharide-induced preterm delivery and production of inflammatory cytokines in mice. J. Perinat. Med. 2005, 33, 320–323. [Google Scholar] [PubMed]
- Machnicki, M.; Zimecki, M.; Zagulski, T. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. Int. J. Exp. Pathol. 1993, 74, 433–439. [Google Scholar] [PubMed]
- Kruzel, M.L.; Harari, Y.; Mailman, D.; Actor, J.K.; Zimecki, M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin. Exp. Immunol. 2002, 130, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Zimecki, M.; Kruzel, M.L. Effects of lactoferrin on IL-6 production by peritoneal and alveolar cells in cyclophosphamide-treated mice. J. Chemother. 2004, 16, 187–192. [Google Scholar] [PubMed]
- Kruzel, M.L.; Actor, J.K.; Radak, Z.; Bacsi, A.; Saavedra-Molina, A.; Boldogh, I. Lactoferrin decreases LPS-induced mitochondrial dysfunction in cultured cells and in animal endotoxemia model. Innate Immun. 2009. [Google Scholar]
- Na, Y.J.; Han, S.B.; Kang, J.S.; Yoon, Y.D.; Park, S.K.; Kim, H.M.; Yang, K.H.; Joe, C.O. Lactoferrin works as a new LPS-binding protein in inflammatory activation of macrophages. Int. Immunopharmacol. 2004, 4, 1187–1199. [Google Scholar] [PubMed]
- Chodaczek, G.; Zimecki, M.; Lukasiewicz, J.; Lugowski, C. A complex of lactoferrin with monophosphoryl lipid A is an efficient adjuvant of the humoral and cellular immune response in mice. Med. Microbiol. Immunol. 2006, 195, 207–216. [Google Scholar] [PubMed]
- Miyazawa, K.; Mantel, C.; Lu, L.; Morrison, D.C.; Broxmeyer, H.E. Lactoferrin-lipopolysaccharide interactions Effect on lactoferrin binding to monocyte/macrophage-differentiated HL-60 cells. J. Immunol. 1991, 146, 723–729. [Google Scholar] [PubMed]
- Puddu, P.; Carollo, M.G.; Belardelli, F.; Valenti, P.; Gessani, S. Role of endogenous interferon and LPS in the immunomodulatory effects of bovine lactoferrin in murine peritoneal macrophages. J. Leukoc. Biol. 2007, 82, 347–353. [Google Scholar] [PubMed]
- Curran, C.S.; Demick, K.P.; Mansfield, J.M. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell Immunol. 2006, 242, 23–30. [Google Scholar] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Latorre, D.; Puddu, P.; Valenti, P.; Gessani, S. Reciprocal Interactions between Lactoferrin and Bacterial Endotoxins and Their Role in the Regulation of the Immune Response. Toxins 2010, 2, 54-68. https://doi.org/10.3390/toxins2010054
Latorre D, Puddu P, Valenti P, Gessani S. Reciprocal Interactions between Lactoferrin and Bacterial Endotoxins and Their Role in the Regulation of the Immune Response. Toxins. 2010; 2(1):54-68. https://doi.org/10.3390/toxins2010054
Chicago/Turabian StyleLatorre, Daniela, Patrizia Puddu, Piera Valenti, and Sandra Gessani. 2010. "Reciprocal Interactions between Lactoferrin and Bacterial Endotoxins and Their Role in the Regulation of the Immune Response" Toxins 2, no. 1: 54-68. https://doi.org/10.3390/toxins2010054




