Serum Phenylacetylglutamine among Potential Risk Factors for Arterial Stiffness Measuring by Carotid–Femoral Pulse Wave Velocity in Patients with Kidney Transplantation
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
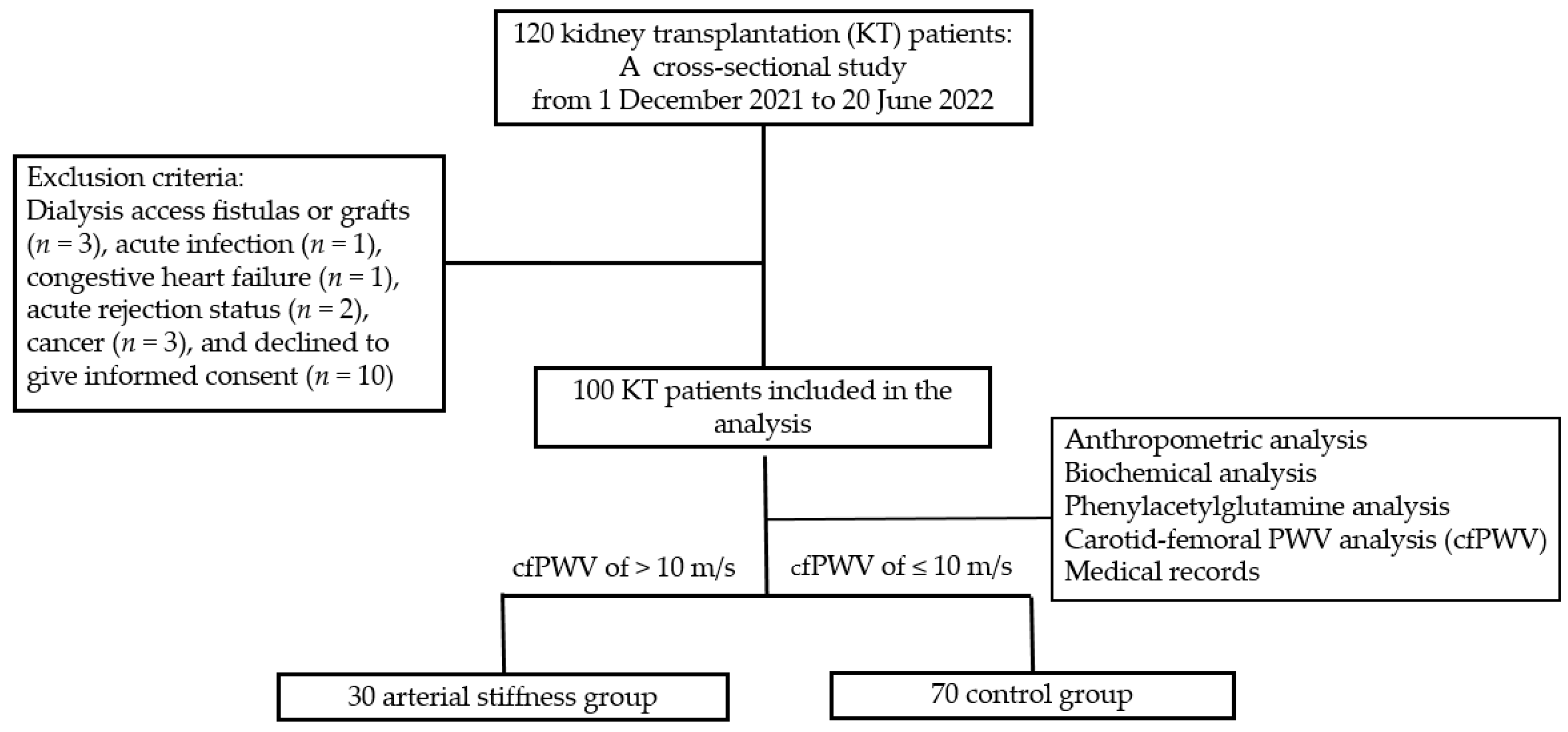
5.1. Patients
5.2. Anthropometric and Biochemical Investigations
5.3. High-Performance Liquid Chromatography–Mass Spectrometry for Determining Serum PAG Concentrations
5.4. Blood Pressure and Arterial Stiffness Measurements
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández, D.; Sánchez-Fructuoso, A.; González-Posada, J.M.; Arias, M.; Campistol, J.M.; Rufino, M.; Morales, J.M.; Moreso, F.; Pérez, G.; Torres, A.; et al. A novel risk score for mortality in renal transplant recipients beyond the first posttransplant year. Transplantation 2009, 88, 803–809. [Google Scholar] [CrossRef]
- Lentine, K.L.; Rocca Rey, L.A.; Kolli, S.; Bacchi, G.; Schnitzler, M.A.; Abbott, K.C.; Xiao, H.; Brennan, D.C. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clin. J. Am. Soc. Nephrol. 2008, 3, 1090–1101. [Google Scholar] [CrossRef]
- Seoane-Pillado, M.T.; Pita-Fernández, S.; Valdés-Cañedo, F.; Seijo-Bestilleiro, R.; Pértega-Díaz, S.; Fernández-Rivera, C.; Alonso-Hernández, Á.; González-Martín, C.; Balboa-Barreiro, V. Incidence of cardiovascular events and associated risk factors in kidney transplant patients: A competing risks survival analysis. BMC Cardiovasc. Disord. 2017, 17, 72. [Google Scholar] [CrossRef]
- Townsend, R.R. Arterial stiffness in CKD: A review. Am. J. Kidney Dis. 2019, 73, 240–247. [Google Scholar] [CrossRef]
- Tsai, J.P.; Hsu, B.G. Arterial stiffness: A brief review. Tzu Chi Med. J. 2020, 33, 115–121. [Google Scholar]
- Castelli, R.; Gidaro, A.; Casu, G.; Merella, P.; Profili, N.I.; Donadoni, M.; Maioli, M.; Delitala, A.P. Aging of the arterial system. Int. J. Mol. Sci. 2023, 24, 6910. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [PubMed]
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Matey-Hernandez, M.L.; Keehn, L.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Kuo, C.F.; et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Heart J. 2018, 39, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut microbiota and cardiovascular disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Zhao, Z.; Song, X.; Qu, H.; Liu, H. Phenylacetylglutamine is associated with the degree of coronary atherosclerotic severity assessed by coronary computed tomographic angiography in patients with suspected coronary artery disease. Atherosclerosis 2021, 333, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.; et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell 2020, 180, 862–877.e22. [Google Scholar] [CrossRef]
- Lehmann, E.D.; Gosling, R.G.; Sönksen, P.H. Arterial wall compliance in diabetes. Diabet. Med. 1992, 9, 114–119. [Google Scholar] [CrossRef]
- Zhan, B.; Huang, X.; Wang, J.; Qin, X.; Zhang, J.; Cao, J.; Song, Y.; Liu, L.; Li, P.; Yang, R.; et al. Association between lipid profiles and arterial stiffness in Chinese patients with hypertension: Insights from the CSPPT. Angiology 2019, 70, 515–522. [Google Scholar] [CrossRef]
- Pavlovska, I.; Kunzova, S.; Jakubik, J.; Hruskova, J.; Skladana, M.; Rivas-Serna, I.M.; Medina-Inojosa, J.R.; Lopez-Jimenez, F.; Vysoky, R.; Geda, Y.E.; et al. Associations between high triglycerides and arterial stiffness in a population-based sample: Kardiovize Brno 2030 study. Lipids Health Dis. 2020, 19, 170. [Google Scholar] [CrossRef]
- Yue, M.; Liu, H.; He, M.; Wu, F.; Li, X.; Pang, Y.; Yang, X.; Zhou, G.; Ma, J.; Liu, M.; et al. Gender-specific association of metabolic syndrome and its components with arterial stiffness in the general Chinese population. PLoS ONE 2017, 12, e0186863. [Google Scholar] [CrossRef] [PubMed]
- Topouchian, J.; Labat, C.; Gautier, S.; Bäck, M.; Achimastos, A.; Blacher, J.; Cwynar, M.; de la Sierra, A.; Pall, D.; Fantin, F.; et al. Effects of metabolic syndrome on arterial function in different age groups: The advanced approach to arterial stiffness study. J. Hypertens. 2018, 36, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, N.; Mahmoudi, M.; Halperin, F.; Wu, J.C.; Pakpour, S. Gut microbiota and cardiovascular disease: Opportunities and challenges. Microbiome 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Agnoletti, D.; Piani, F.; Cicero, A.F.G.; Borghi, C. The gut microbiota and vascular aging: A state-of-the-art and systematic review of the literature. J. Clin. Med. 2022, 11, 3557. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Povia, J.P.; Lwiindi, P.C.; Kirabo, A. Recent advances in microbiota-associated metabolites in heart failure. Biomedicines 2023, 11, 2313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, B.; Sun, Y.; Deng, H.; Wang, H.; Qiao, Z. Alteration of the gut microbiota and metabolite phenylacetylglutamine in patients with severe chronic heart failure. Front. Cardiovasc. Med. 2023, 9, 1076806. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Nemet, I.; Prasad Saha, P.; Haghikia, A.; Li, X.S.; Mohan, M.L.; Lovano, B.; Castel, L.; Witkowski, M.; Buffa, J.A.; et al. Gut microbiota-generated phenylacetylglutamine and heart failure. Circ. Heart Fail. 2023, 16, e009972. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, X.; Feng, X.; Wei, M.; Luo, Y.; Zhao, T.; Xiao, B.; Xia, J. Phenylacetylglutamine, a novel biomarker in acute ischemic stroke. Front. Cardiovasc. Med. 2021, 8, 798765. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zuo, K.; Fu, Y.; Li, J.; Wang, H.; Xu, L.; Yang, X. Dysbiosis of gut microbiota and metabolite phenylacetylglutamine in coronary artery disease patients with stent stenosis. Front. Cardiovasc. Med. 2022, 9, 832092. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, F.; Brunkwall, L.; Smith, E.; Orho-Melander, M.; Nilsson, P.M.; Fernandez, C.; Melander, O. The gut microbiota-related metabolite phenylacetylglutamine associates with increased risk of incident coronary artery disease. J. Hypertens. 2020, 38, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; Beaumont, M.; Pallister, T.; Villar, J.; Goodrich, J.K.; Clark, A.; Pascual, J.; Ley, R.E.; Spector, T.D.; Bell, J.T.; et al. Gut-microbiota metabolite axis in early renal function decline. PLoS ONE 2015, 10, e0134311. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xin, W.; Xiong, J.; Yao, M.; Zhang, B.; Zhao, J. The intestinal microbiota and metabolites in the gut-kidney-heart axis of chronic kidney disease. Front. Pharmacol. 2022, 13, 837500. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Wang, C.H.; Kuo, C.H.; Lin, Y.L.; Tsai, J.P.; Hsu, B.G. Serum p-cresyl sulfate is a predictor of central arterial stiffness in patients on maintenance hemodialysis. Toxins 2019, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Lai, Y.H.; Liu, C.H.; Wang, C.H.; Hsu, B.G.; Tsai, J.P. Association between serum indoxyl sulfate levels with carotid-femoral pulse wave velocity in patients with chronic kidney disease. Ren. Fail. 2021, 43, 796–802. [Google Scholar] [CrossRef]
- Huang, P.Y.; Hsu, B.G.; Lai, Y.H.; Wang, C.H.; Tsai, J.P. Serum trimethylamine N-oxide level is positively associated with aortic stiffness measuring by carotid-femoral pulse wave velocity in patients undergoing maintenance hemodialysis. Toxins 2023, 15, 572. [Google Scholar] [CrossRef]
- Poesen, R.; Claes, K.; Evenepoel, P.; de Loor, H.; Augustijns, P.; Kuypers, D.; Meijers, B. Microbiota-derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J. Am. Soc. Nephrol. 2016, 27, 3479–3487. [Google Scholar] [CrossRef]
- Sirich, T.L.; Funk, B.A.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W.; Sirich, T.L.; Funk, B.A.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J. Am. Soc. Nephrol. 2014, 25, 615–622. [Google Scholar] [CrossRef]
- Melilli, E.; Manonelles, A.; Montero, N.; Grinyo, J.; Martinez-Castelao, A.; Bestard, O.; Cruzado, J. Impact of immunosuppressive therapy on arterial stiffness in kidney transplantation: Are all treatments the same? Clin. Kidney J. 2018, 11, 413–421. [Google Scholar] [CrossRef]
- Elezaby, A.; Dexheimer, R.; Sallam, K. Cardiovascular effects of immunosuppression agents. Front. Cardiovasc. Med. 2022, 21, 981838. [Google Scholar] [CrossRef]
- Strózecki, P.; Adamowicz, A.; Włodarczyk, Z.; Manitius, J. The influence of calcineurin inhibitors on pulse wave velocity in renal transplant recipients. Ren. Fail. 2007, 29, 679–684. [Google Scholar] [CrossRef]
- Strózecki, P.; Adamowicz, A.; Włodarczyk, Z.; Manitius, J. Factors associated with increased arterial stiffness in renal transplant recipients. Med. Sci. Monit. 2010, 16, CR301–CR306. [Google Scholar] [PubMed]
- Gungor, O.; Kircelli, F.; Carrero, J.J.; Hur, E.; Demirci, M.S.; Asci, G.; Toz, H. The effect of immunosuppressive treatment on arterial stiffness and matrix Gla protein levels in renal transplant recipients. Clin. Nephrol. 2011, 75, 491–496. [Google Scholar] [CrossRef]
- Melilli, E.; Bestard-Matamoros, O.; Manonelles-Montero, A.; Sala-Bassa, N.; Mast, R.; Grinyó-Boira, J.M.; Cruzado, J.M. Arterial stiffness in kidney transplantation: A single center case-control study comparing belatacept versus calcineurin inhibitor immunosuppressive based regimen. Nefrologia 2015, 35, 58–65. [Google Scholar]
- Vinh, A.; Chen, W.; Blinder, Y.; Weiss, D.; Taylor, W.R.; Goronzy, J.J.; Weyand, C.M.; Harrison, D.G.; Guzik, T.J. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 2010, 122, 2529–2537. [Google Scholar] [CrossRef]
- Khoo, S.B.; Lin, Y.L.; Ho, G.J.; Lee, M.C.; Hsu, B.G. Association of endothelial dysfunction with sarcopenia and muscle function in a relatively young cohort of kidney transplant recipients. Peer J. 2021, 9, e12521. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
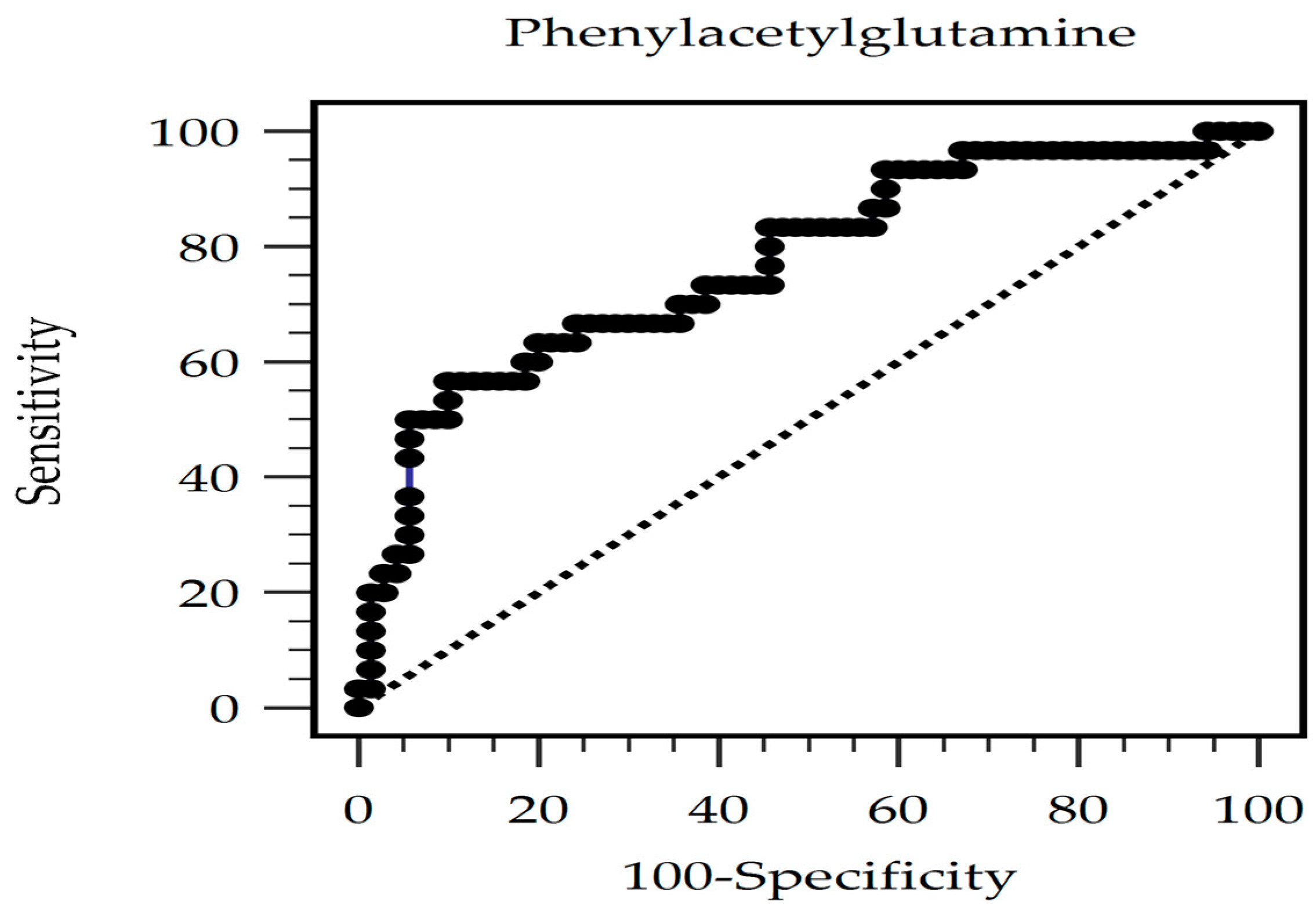

| Characteristic | All Participants (n = 100) | Control Group (n = 70) | Arterial Stiffness Group (n = 30) | p Value |
|---|---|---|---|---|
| Age (years) | 54.29 ± 11.54 | 52.57 ± 11.58 | 58.30 ± 10.56 | 0.022 * |
| KT vintage (months) | 93.42 ± 56.31 | 91.43 ± 54.99 | 98.07 ± 59.97 | 0.592 |
| Height (cm) | 160.61 ± 10.02 | 159.52 ± 10.17 | 163.15 ± 9.32 | 0.097 |
| Body weight (kg) | 64.59 ± 14.43 | 64.54 ± 14.03 | 64.70 ± 15.57 | 0.958 |
| Body mass index (kg/m2) | 24.61 ± 4.56 | 24.84 ± 4.65 | 24.07 ± 4.37 | 0.440 |
| Carotid–femoral PWV (m/s) | 9.16 ± 2.05 | 8.10 ± 1.22 | 11.62 ± 1.36 | <0.001 * |
| Systolic blood pressure (mmHg) | 140.47 ± 17.46 | 136.89 ± 16.41 | 148.83 ± 17.22 | 0.001 * |
| Diastolic blood pressure (mmHg) | 83.33 ± 11.28 | 82.26 ± 10.80 | 85.83 ± 12.15 | 0.147 |
| Total cholesterol (mg/dL) | 188.16 ± 46.88 | 186.29 ± 46.00 | 192.53 ± 49.38 | 0.544 |
| Triglyceride (mg/dL) | 123.50 (86.25–164.50) | 115.50 (82.75–150.50) | 138.50 (97.25–181.50) | 0.132 |
| HDL-C (mg/dL) | 53.02 ± 17.31 | 53.47 ± 15.74 | 51.97 ± 20.79 | 0.693 |
| LDL-C (mg/dL) | 105.49 ± 36.63 | 101.75 ± 30.52 | 114.20 ± 47.45 | 0.120 |
| Fasting glucose (mg/dL) | 94.00 (88.00–109.75) | 92.00 (87.00–99.00) | 109.50 (93.25–148.25) | 0.001 * |
| Blood urea nitrogen (mg/dL) | 25.00 (17.25–35.00) | 24.50 (16.75–33.25) | 26.00 (19.75–41.00) | 0.167 |
| Creatinine (mg/dL) | 1.50 (1.19–2.00) | 1.37 (1.16–1.90) | 1.81 (1.30–2.18) | 0.063 |
| eGFR (mL/min/1.73 m2) | 48.43 ± 23.70 | 49.86 ± 22.11 | 45.11 ± 27.16 | 0.361 |
| Total calcium (mg/dL) | 9.35 ± 0.81 | 9.31 ± 0.72 | 9.42 ± 1.00 | 0.566 |
| Phosphorus (mg/dL) | 3.32 ± 0.76 | 3.29 ± 0.77 | 3.38 ± 0.75 | 0.584 |
| Intact parathyroid hormone (pg/mL) | 85.75 (52.76–153.53) | 85.75 (57.45–153.75) | 88.45 (50.08–153.85) | 0.593 |
| Phenylacetylglutamine (ng/mL) | 395.61 (353.41–578.86) | 353.61 (285.57–478.39) | 648.40 (370.90–803.30) | <0.001 * |
| Female, n (%) | 54 (54.0) | 40 (57.1) | 14 (46.7) | 0.335 |
| Diabetes, n (%) | 34 (34.0) | 18 (25.7) | 16 (53.3) | 0.008 * |
| Hypertension, n (%) | 41 (41.0) | 25 (35.7) | 16 (53.3) | 0.101 |
| Living donor, n (%) | 20 (20.0) | 14 (20.0) | 6 (20.0) | 1.000 |
| Steroid use, n (%) | 88 (88.0) | 63 (90.0) | 25 (83.3) | 0.347 |
| Cyclosporine use, n (%) | 17 (17.0) | 14 (20.0) | 3 (10.0) | 0.222 |
| Tacrolimus use, n (%) | 80 (80.0) | 53 (75.7) | 27 (90.0) | 0.102 |
| Mycophenolate mofetil use, n (%) | 79 (79.0) | 57 (81.4) | 22 (73.3) | 0.362 |
| Statin use, n (%) | 41 (41.0) | 31 (44.3) | 10 (33.3) | 0.308 |
| Fibrate use, n (%) | 18 (18.0) | 12 (17.1) | 6 (20.0) | 0.733 |
| Causes of KT | ||||
| Diabetes, n (%) | 32 (32.0) | 19 (27.1) | 13 (43.3) | 0.326 |
| Glomerulonephritis, n (%) | 38 (38.0) | 30 (42.9) | 8 (26.7) | |
| Hypertension, n (%) | 8 (8.0) | 5 (7.1) | 3 (10.0) | |
| Other, n (%) | 22 (22.0) | 16 (22.9) | 6 (20.0) |
| Variables | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Phenylacetylglutamine, 1 ng/mL | 1.004 | 1.002–1.007 | 0.001 * |
| Age, 1 year | 1.074 | 1.011–1.140 | 0.021 * |
| Glucose, 1 mg/dL | 1.015 | 1.001–1.025 | 0.033 * |
| Systolic blood pressure, 1 mmHg | 1.034 | 1.002–1.067 | 0.037 * |
| Diabetes mellitus (present) | 0.997 | 0.255–3.611 | 0.996 |
| Variables | Carotid–Femoral Pulse Wave Velocity (m/s) | ||||
|---|---|---|---|---|---|
| Simple Linear Regression | Multivariate Linear Regression | ||||
| r | p Value | Beta | Adjusted R2 Change | p Value | |
| Female | −0.165 | 0.101 | – | – | – |
| Diabetes | 0.295 | 0.003 * | – | – | – |
| Hypertension | 0.043 | 0.669 | – | – | – |
| Age (years) | 0.284 | 0.004 * | 0.205 | 0.040 | 0.021 * |
| KT vintage (months) | 0.012 | 0.907 | – | – | – |
| Height (cm) | 0.175 | 0.081 | – | – | – |
| Body weight (kg) | 0.107 | 0.288 | – | – | – |
| Body mass index (kg/m2) | 0.019 | 0.849 | – | – | – |
| Systolic blood pressure (mmHg) | 0.389 | <0.001 * | 0.296 | 0.151 | 0.001 * |
| Diastolic blood pressure (mmHg) | 0.079 | 0.433 | – | – | – |
| Total cholesterol (mg/dL) | 0.004 | 0.965 | – | – | – |
| Log-Triglyceride (mg/dL) | 0.213 | 0.033 * | – | – | – |
| HDL-C (mg/dL) | −0.144 | 0.154 | |||
| LDL-C (mg/dL) | 0.146 | 0.146 | – | – | – |
| Log-Glucose (mg/dL) | 0.301 | 0.002 * | 0.244 | 0.080 | 0.006 * |
| Log-BUN (mg/dL) | 0.102 | 0.312 | – | – | – |
| Log-Creatinine (mg/dL) | 0.101 | 0.316 | – | – | – |
| eGFR (mL/min/1.73 m2) | −0.061 | 0.548 | – | – | – |
| Total calcium (mg/dL) | −0.033 | 0.742 | – | – | – |
| Phosphorus (mg/dL) | 0.053 | 0.600 | – | – | – |
| Log-iPTH (pg/mL) | 0.040 | 0.694 | – | – | – |
| Log-PAG (ng/mL) | 0.299 | 0.003 * | 0.215 | 0.040 | 0.016 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-H.; Chen, Y.-C.; Ho, C.-C.; Hsu, B.-G. Serum Phenylacetylglutamine among Potential Risk Factors for Arterial Stiffness Measuring by Carotid–Femoral Pulse Wave Velocity in Patients with Kidney Transplantation. Toxins 2024, 16, 111. https://doi.org/10.3390/toxins16020111
Yang H-H, Chen Y-C, Ho C-C, Hsu B-G. Serum Phenylacetylglutamine among Potential Risk Factors for Arterial Stiffness Measuring by Carotid–Femoral Pulse Wave Velocity in Patients with Kidney Transplantation. Toxins. 2024; 16(2):111. https://doi.org/10.3390/toxins16020111
Chicago/Turabian StyleYang, Hsiao-Hui, Yen-Cheng Chen, Ching-Chun Ho, and Bang-Gee Hsu. 2024. "Serum Phenylacetylglutamine among Potential Risk Factors for Arterial Stiffness Measuring by Carotid–Femoral Pulse Wave Velocity in Patients with Kidney Transplantation" Toxins 16, no. 2: 111. https://doi.org/10.3390/toxins16020111






