Efficacy of Potentially Probiotic Fruit-Derived Lactobacillus fermentum, L. paracasei and L. plantarum to Remove Aflatoxin M1 In Vitro
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Chemicals, Bacterial Isolates, and Inoculum Preparation
4.2. Evaluation of AFM1 Removal and Recovery of AFM1 from Bacterial Cells
4.3. Quantification of AFM1
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Saf. 2010, 59, 57–81. [Google Scholar] [CrossRef]
- Elsanhoty, R.M.; Salam, S.A.; Ramadan, M.F.; Badr, F.H. Detoxification of aflatoxin M1 in yoghurt using probiotics and lactic acid bacteria. Food Cont. 2014, 43, 129–134. [Google Scholar] [CrossRef]
- Koppen, R.; Koch, M.; Slegel, D. Determination of mycotoxins in foods: Current state of analytical methods and limitations. Appl. Microbiol. Biotechnol. 2010, 86, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Some traditional herbal medicine, some mycotoxins and styrene. In Monographs on the Evaluation of Carginogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2002. [Google Scholar]
- Ayar, A.; Sert, D.; Lon, A.H. A study on the occurrence of aflatoxin in raw milk due to feeds. J. Food Saf. 2007, 27, 199–207. [Google Scholar] [CrossRef]
- Ministry of Health; National Agency for Health Surveillance; Brazilian Legislation. On the Maximum Tolerated Limit (MTL) for Mycotoxins in Foods; Resolution No. 7 of 18 February 2011; National Agency for Health Surveillance: Brasilia, Brazil, 2011. [Google Scholar]
- Corassin, C.H.; Bovo, F.; Rossim, R.E.; Oliveira, C.A.F. Efficiency of Saccharomyces cerevisiae and lactic acid bacteria strains to bind aflatoxin M1 in UHT skim milk. Food Cont. 2013, 31, 80–83. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 12–34. [Google Scholar]
- Wang, B.; Mahoney, N.E.; Pan, Z.; Khir, R.; Wu, B.; Ma, H.; Zhao, L. Effectiveness of pulsed light treatment for degradation and detoxification of aflatoxin B1 and B2 in rough rice and rice bran. Food Cont. 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, A.; Garcia, H.S.; Steele, J.L. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food Chem. Toxicol. 2009, 47, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Niño, J.C.; Cavazos-Garduño, A.; Hernandez-Mendoza, A.; Applegate, B.; Ferruzzi, M.C.; Martin-González, M.F.S.; García, H.S. Assessment of probiotic strains ability to reduce the bioaccessibility of aflatoxin M1 in artificially contaminated milk using an in vitro digestive model. Food Cont. 2013, 31, 202–207. [Google Scholar] [CrossRef]
- Onilude, A.A.; Fagade, O.E.; Bello, M.M.; Fadahunsi, I.F. Inhibition of aflatoxin-producing aspergilli by lactic acid bacteria isolates from indigenously fermented cereal gruels. Afr. J. Biotechnol. 2005, 4, 1404–1408. [Google Scholar]
- Azeem, N.; Nawaz, M.; Anjum, A.A.; Saeed, S.; Sana, S.; Mustafa, A.; Yousuf, M.R. Activity and anti-aflatoxigenic effect of indigenously characterized probiotic lactobacilli against Aspergillus flavus—A common poultry feed contaminant. Animals 2019, 15, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsanhoty, R.M.; Ramadan, M.F.; El-Gohery, S.S.; Abol-Ela, M.A.A. Ability of selected microorganisms for removing aflatoxins in vitro and fate of aflatoxins in contaminated wheat during baladi bread baking. Food Cont. 2013, 33, 287–292. [Google Scholar] [CrossRef]
- Naeem, M.; Ilyas, M.; Haider, S.; Baig, S.; Saleem, M. Isolation characterization and identification of lactic acid bacteria from fruit juices and their efficacy against antibiotics. Pak. J. Bot. 2012, 44, 323–328. [Google Scholar]
- Ilha, E.C.; Silva, T.; Lorenz, J.G.; De Oliveira Rocha, G.; Sant’Anna, E.S. Lactobacillus paracasei isolated from grape sourdough: Acid, bile, salt, and heat tolerance after spray drying with skim milk and cheese whey. Eur. Food Res. Technol. 2015, 240, 977–984. [Google Scholar] [CrossRef]
- Garcia, E.F.; Luciano, W.A.; Xavier, D.E.; Da Costa, W.C.; De Sousa Oliveira, K.; Franco, O.L.; De Morais, M.A.J.; Lucena, B.T.; Picão, R.C.; Magnani, M.; et al. Identification of lactic acid bacteria in fruit pulp processing byproducts and potential probiotic properties of selected Lactobacillus strains. Front. Microbiol. 2016, 7, 1371. [Google Scholar] [CrossRef] [Green Version]
- Ahlberg, S.H.; Joutsjoki, V.; Korhonen, H.J. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food Microbiol. 2015, 207, 87–102. [Google Scholar] [CrossRef]
- Khanian, M.; Karimi-Torshizi, M.A.; Allameh, A. Alleviation of aflatoxin-related oxidative damage to liver and improvement of growth performance in broiler chickens consumed Lactobacillus plantarum 299v for entire growth period. Toxicon 2019, 158, 57–62. [Google Scholar] [CrossRef]
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A.; El Khoury, A. Assorted methods for decontamination of aflatoxin m1 in milk using microbial adsorbents. Toxins 2019, 11, 304. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, H.; Lee, S.; Lee, J.; Ha, J.; Choi, Y.; Yoon, Y.; Choi, K.H. Microbe-mediated aflatoxin decontamination of dairy products and feeds. J. Dairy Sci. 2017, 100, 871–880. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 1998, 61, 466–468. [Google Scholar] [CrossRef]
- Pierides, H.; El-Nezami, K.; Peltonem, S.; Salminem, J.; Ahokas, J.T. Ability of dairy strains of lactic acid bacteria to bind aflatoxin M1 in a food model. J. Food Prot. 2000, 63, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Jebali, R.; Abbes, S.; Salah-Abbès, J.B.; Younes, R.B.; Haous, Z.; Oueslati, R. Ability of Lactobacillus plantarum MON03 to mitigate aflatoxins (B1 and M1) immunotoxicities in mice. J. Immunotoxicol. 2014, 12, 290–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panwar, R.; Kumar, N.; Kashyap, V.; Ram, C.; Kapila, R. Aflatoxin M1 detoxification ability of probiotic lactobacilli of Indian origin in in vitro digestion model. Prob. Antimicrob. Proteins 2019, 11, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Risa, A.; Divinyi, D.M.; Baka, E.; Krifaton, C. Aflatoxin B1 detoxification by cell-free extracts of Rhodococcus strains. Acta Microbiol. et Immunol. Hung. 2017, 64, 423–438. [Google Scholar] [CrossRef] [Green Version]
- Abbès, S.; Salah-Abbès, J.B.; Sharafi, H.; Jebali, R.; Noghabi, K.A.; Oueslati, R. Ability of Lactobacillus rhamnosus GAF01 to remove AFM1 in vitro and to counteract AFM1 immunotoxicity in vivo. J. Immunotoxicol. 2013, 10, 279–286. [Google Scholar] [CrossRef]
- Bovo, F.; Corassin, C.H.; Rosim, R.E.; De Oliveira, C.A. Efficiency of lactic acid bacteria strains for decontamination of aflatoxin M1 in phosphate buffer saline solution and in skim milk. Food Bioproc. Technol. 2012, 5, 1–5. [Google Scholar]
- Brazilian Legislation, Ministry of Health, National Agency for Health Surveillance. Guide for Validation of Analytical and Bioanalytical Methods. National Agency for Health Surveillance. Resolution nº899; National Agency for Health Surveillance: Brasilia, Brazil, 2003. [Google Scholar]
- Haskard, C.A.; El-Nezami, H.S.; Kankaanpää, P.E.; Salminen, S.; Ahokas, J.T. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 2001, 67, 3086–3091. [Google Scholar] [CrossRef] [Green Version]
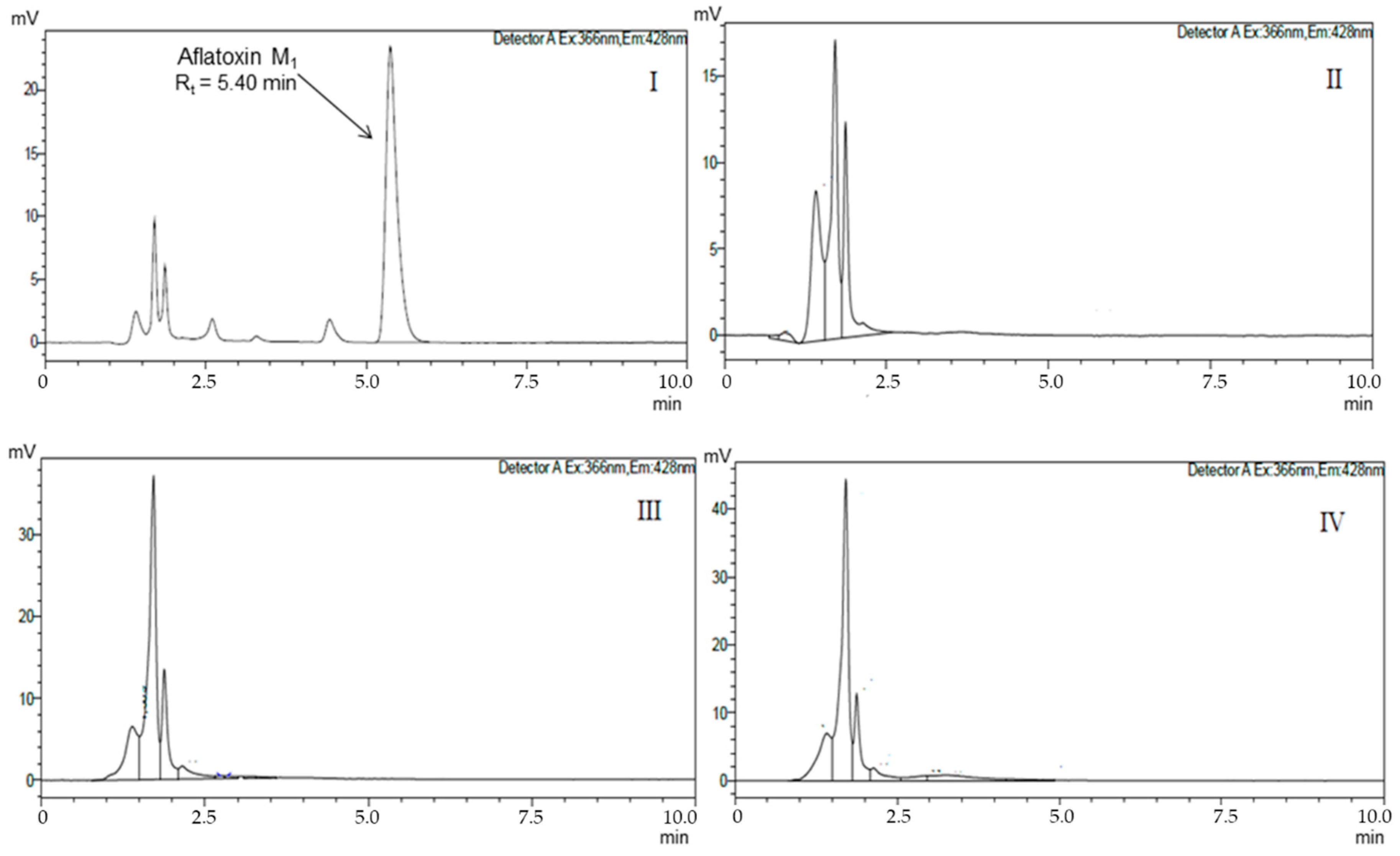
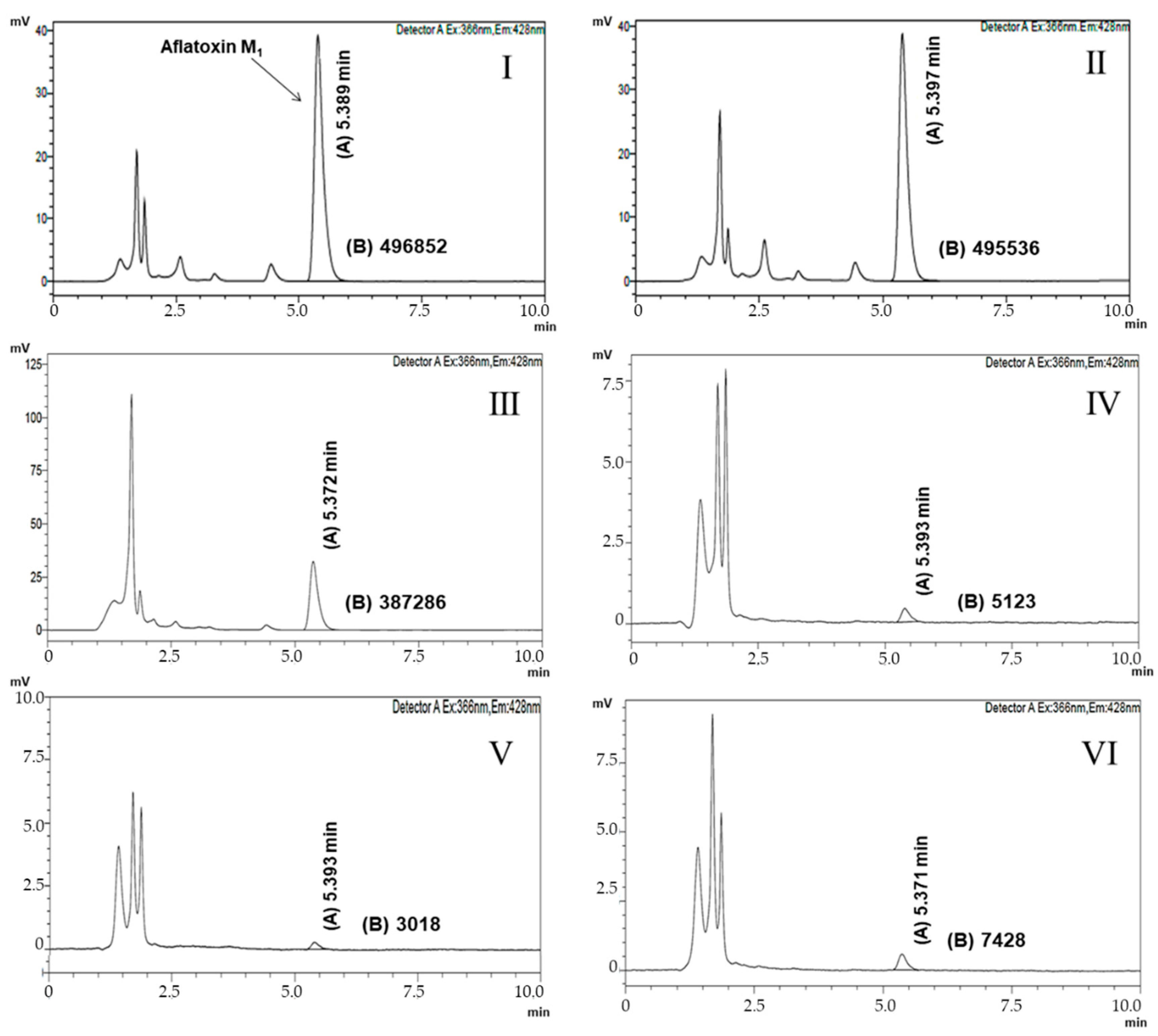
| Isolates | AFM1 Removal (%) | |||
|---|---|---|---|---|
| 1 h-Incubation | 24 h-Incubation | |||
| Viable Cells | Heat-Killed Cells | Viable Cells | Heat-Killed Cells | |
| L. paracasei 108 | 73.0 ± 1.2 b,B | 72.9 ± 1.1 b,B | 78.9 ± 0.5 a,A | 78.7 ± 1.2 a,A |
| L. plantarum 49 | 78.1 ± 1.6 a,A | 75.8 ± 1.0 a,A,B | 77.0 ± 2.7 a,A | 76.6 ± 1.5 a,A |
| L. fermentum 111 | 78.6 ± 2.1 a,A | 78.4 ± 0.65 a,A | 80.0 ± 1.7 a,A | 78.3 ± 2.5 a,A |
| Isolates | AFM1 Recovery, % | |||
|---|---|---|---|---|
| 1 h-Incubation | 24 h-Incubation | |||
| Viable Cells | Heat-Killed Cells | Viable Cells | Heat-Killed Cells | |
| L. paracasei 108 | 34.6 ± 1.1 b,B | 28.5 ± 1.7 d,C | 31.7 ± 1.2 c,A | 40.3 ± 1.6 a,A |
| L. plantarum 49 | 13.4 ± 1.5 c,C | 43.8 ± 1.5 a,B | 18.8 ± 1.0 b,B | 10.9 ± 1.2 d,C |
| L. fermentum 111 | 60.6 ± 1.6 a,A | 47.9 ± 1.5 b,A | 14.1 ± 1.4 c,C | 14.9 ± 1.6 c,B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, P.O.d.; Matos, C.J.d.; Nascimento, Y.M.; Tavares, J.F.; Souza, E.L.d.; Magalhães, H.I.F. Efficacy of Potentially Probiotic Fruit-Derived Lactobacillus fermentum, L. paracasei and L. plantarum to Remove Aflatoxin M1 In Vitro. Toxins 2021, 13, 4. https://doi.org/10.3390/toxins13010004
Cruz POd, Matos CJd, Nascimento YM, Tavares JF, Souza ELd, Magalhães HIF. Efficacy of Potentially Probiotic Fruit-Derived Lactobacillus fermentum, L. paracasei and L. plantarum to Remove Aflatoxin M1 In Vitro. Toxins. 2021; 13(1):4. https://doi.org/10.3390/toxins13010004
Chicago/Turabian StyleCruz, Paloma Oliveira da, Clarisse Jales de Matos, Yuri Mangueira Nascimento, Josean Fechine Tavares, Evandro Leite de Souza, and Hemerson Iury Ferreira Magalhães. 2021. "Efficacy of Potentially Probiotic Fruit-Derived Lactobacillus fermentum, L. paracasei and L. plantarum to Remove Aflatoxin M1 In Vitro" Toxins 13, no. 1: 4. https://doi.org/10.3390/toxins13010004






