Intradermal Application of Crotamine Induces Inflammatory and Immunological Changes In Vivo
Abstract
:1. Introduction
2. Results
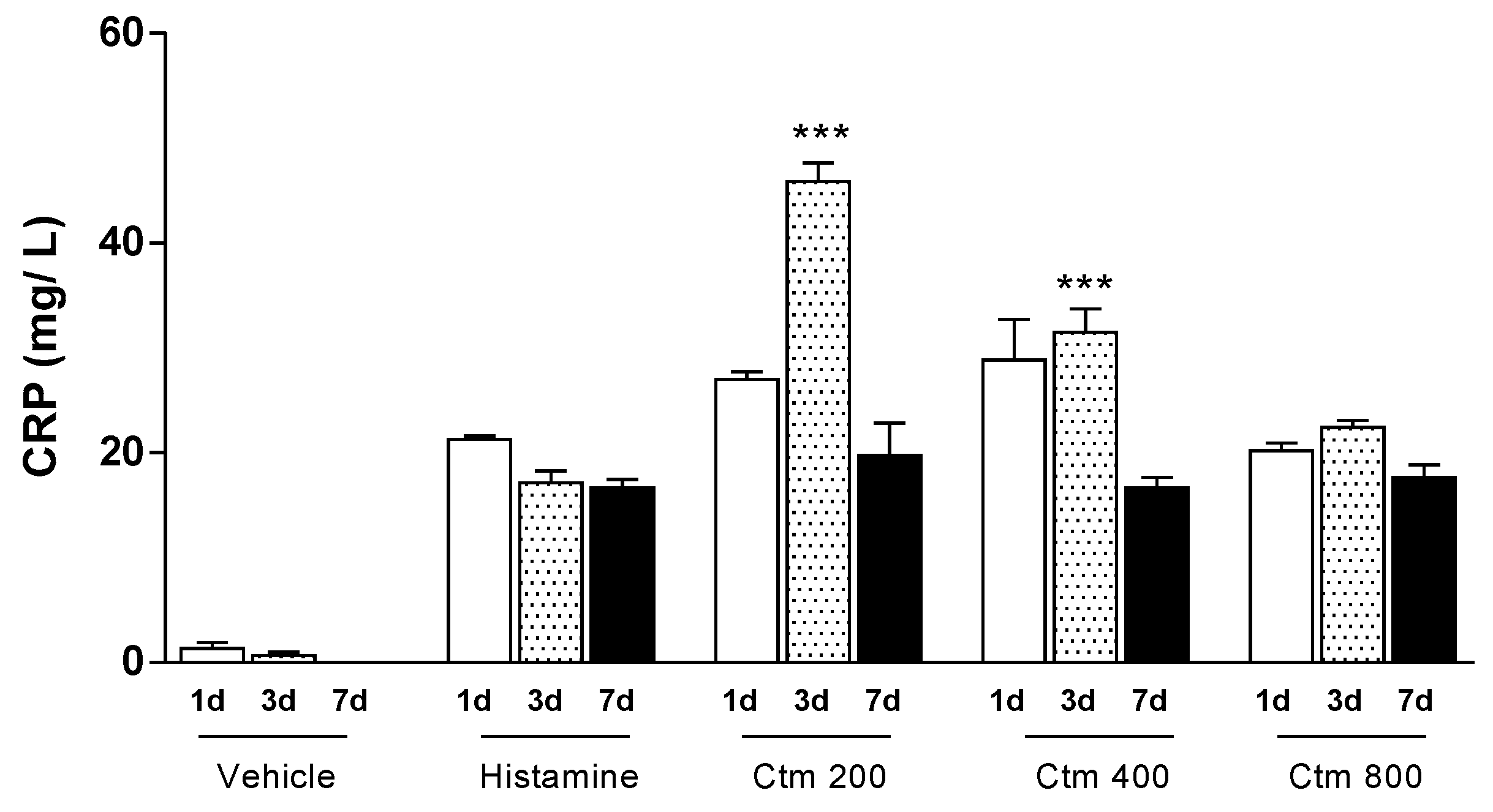
2.1. Crotamine Induced C-Reactive Protein Production
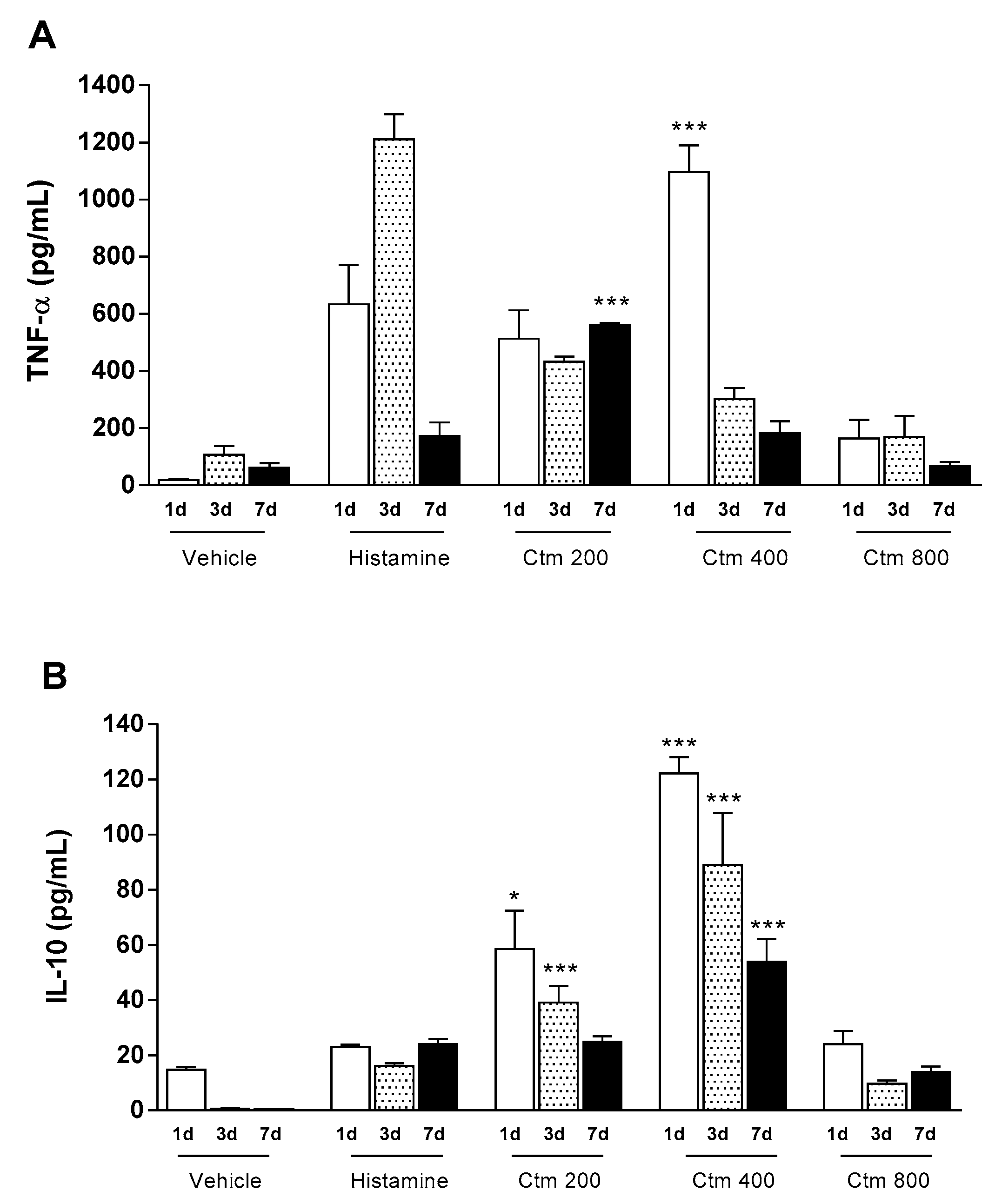
2.2. Crotamine Affected Pro- and Anti-Inflammatory Systemic Cytokines
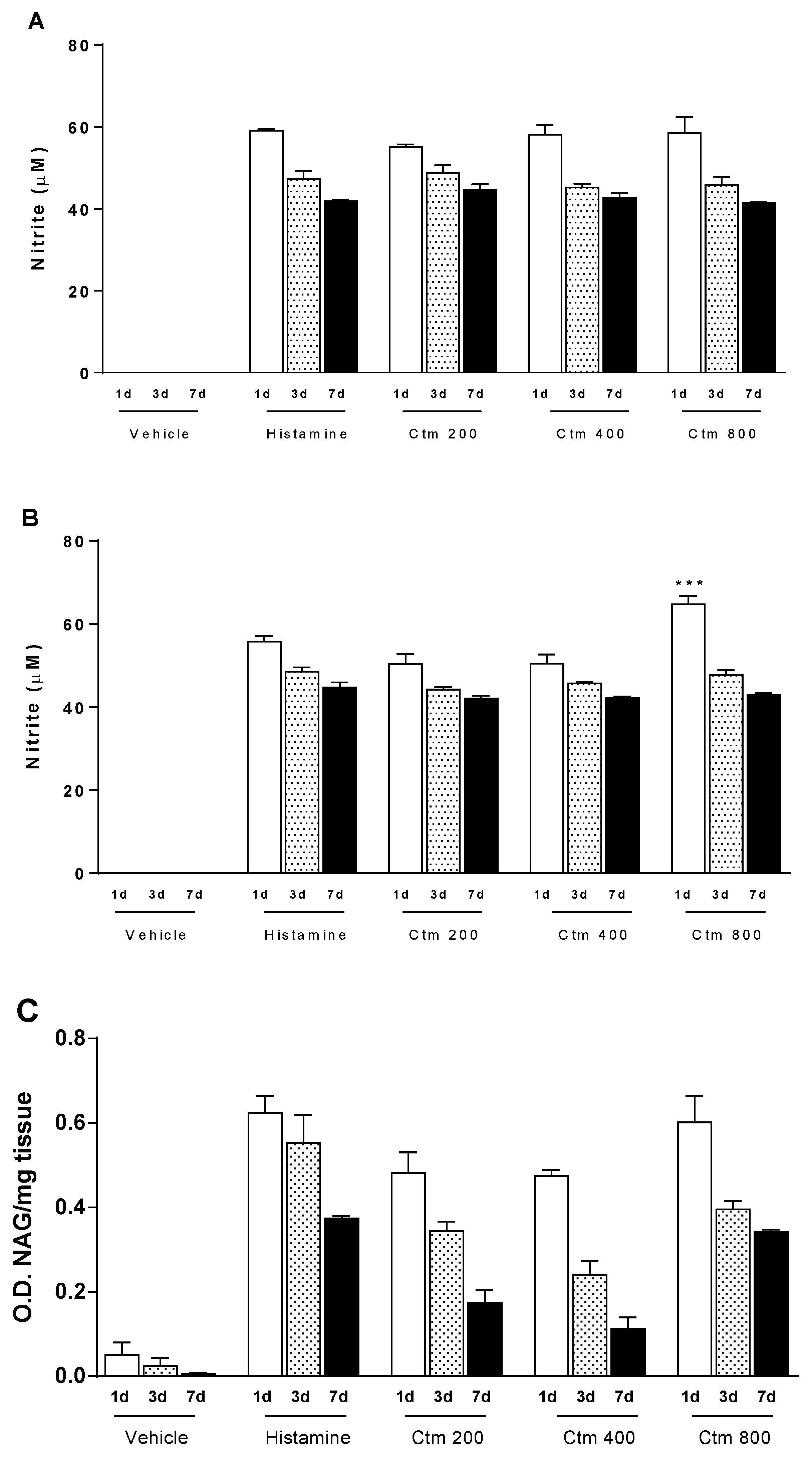
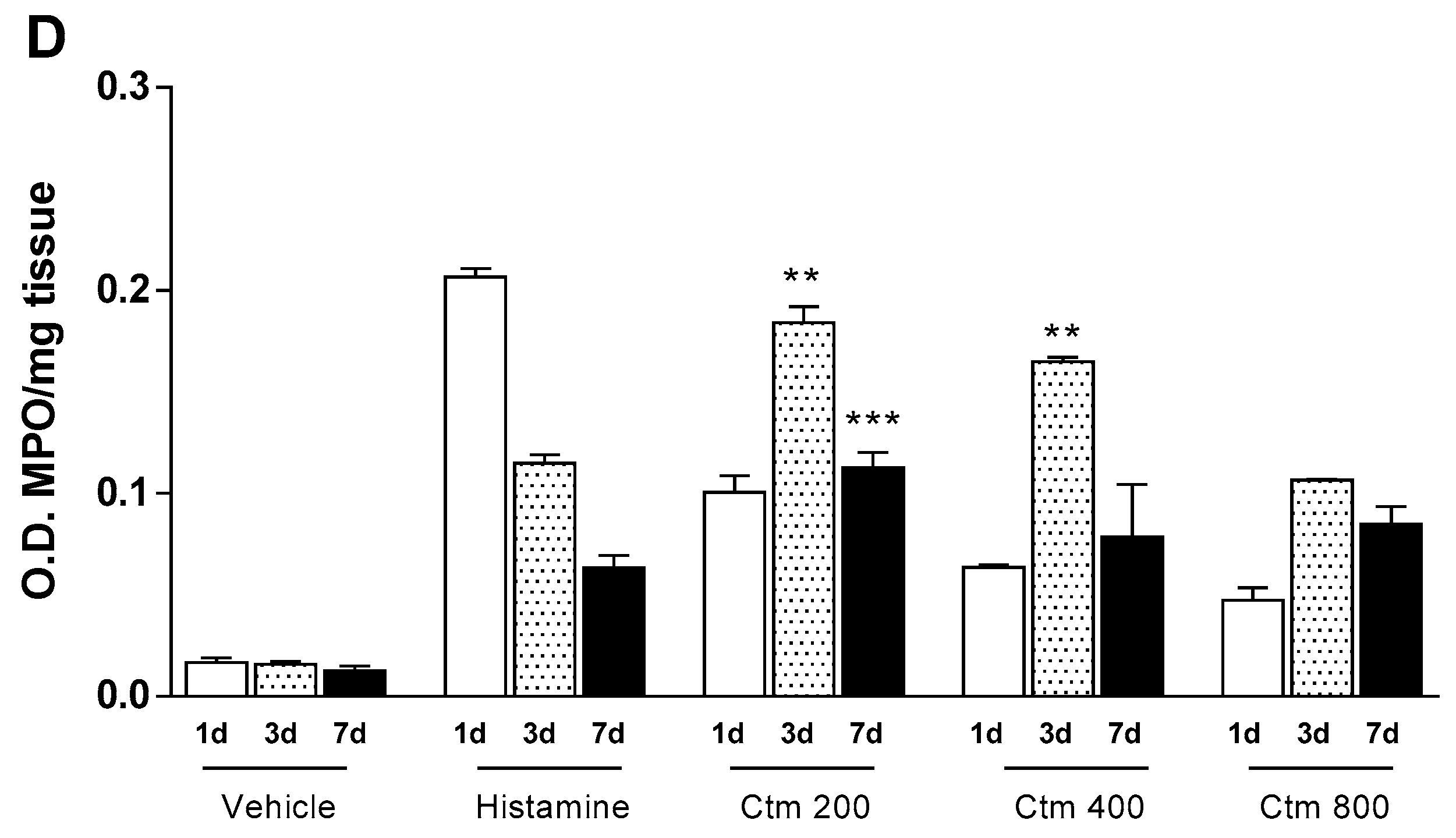
2.3. Crotamine Induces Increased Levels of Nitric Oxide and Cellular Markers
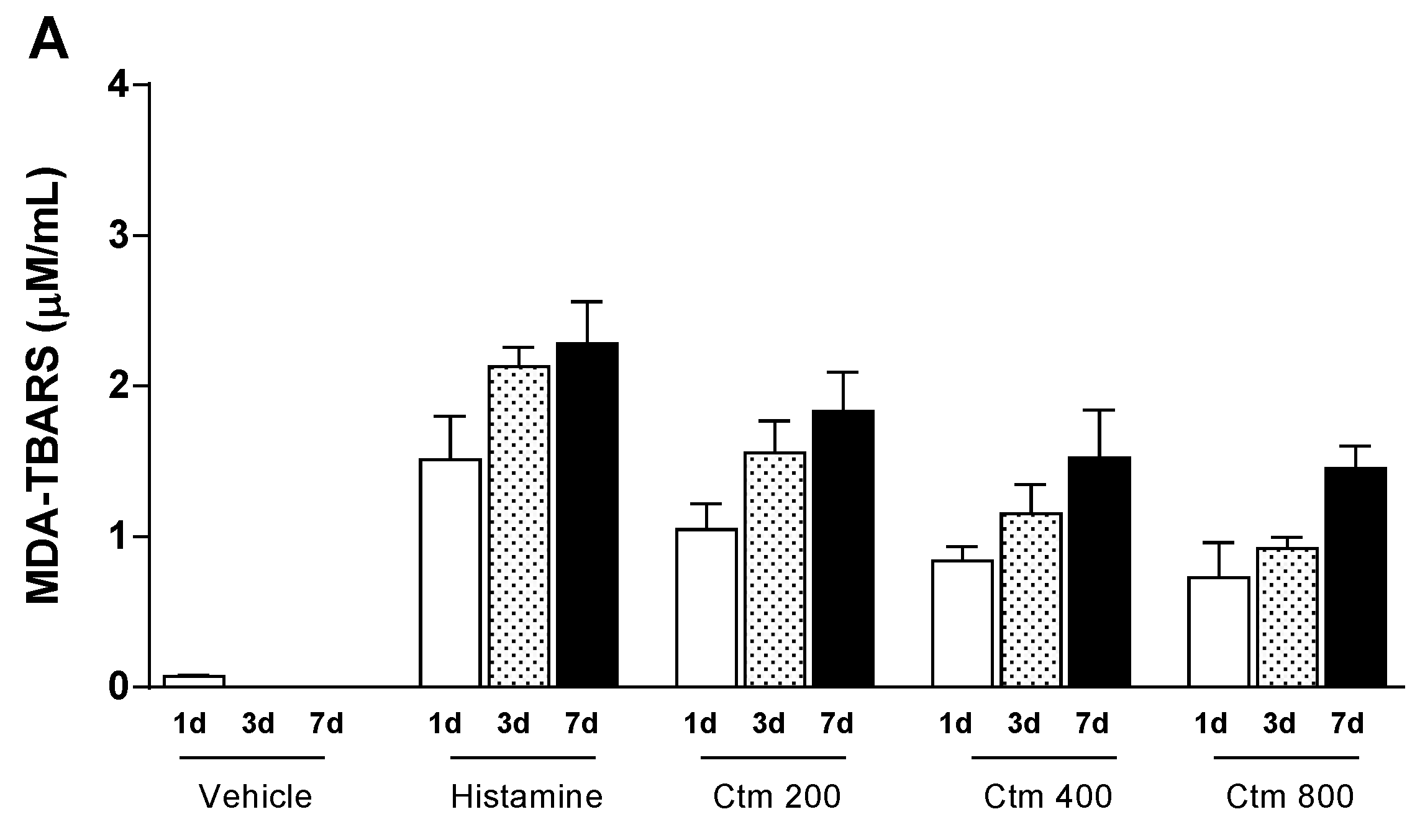
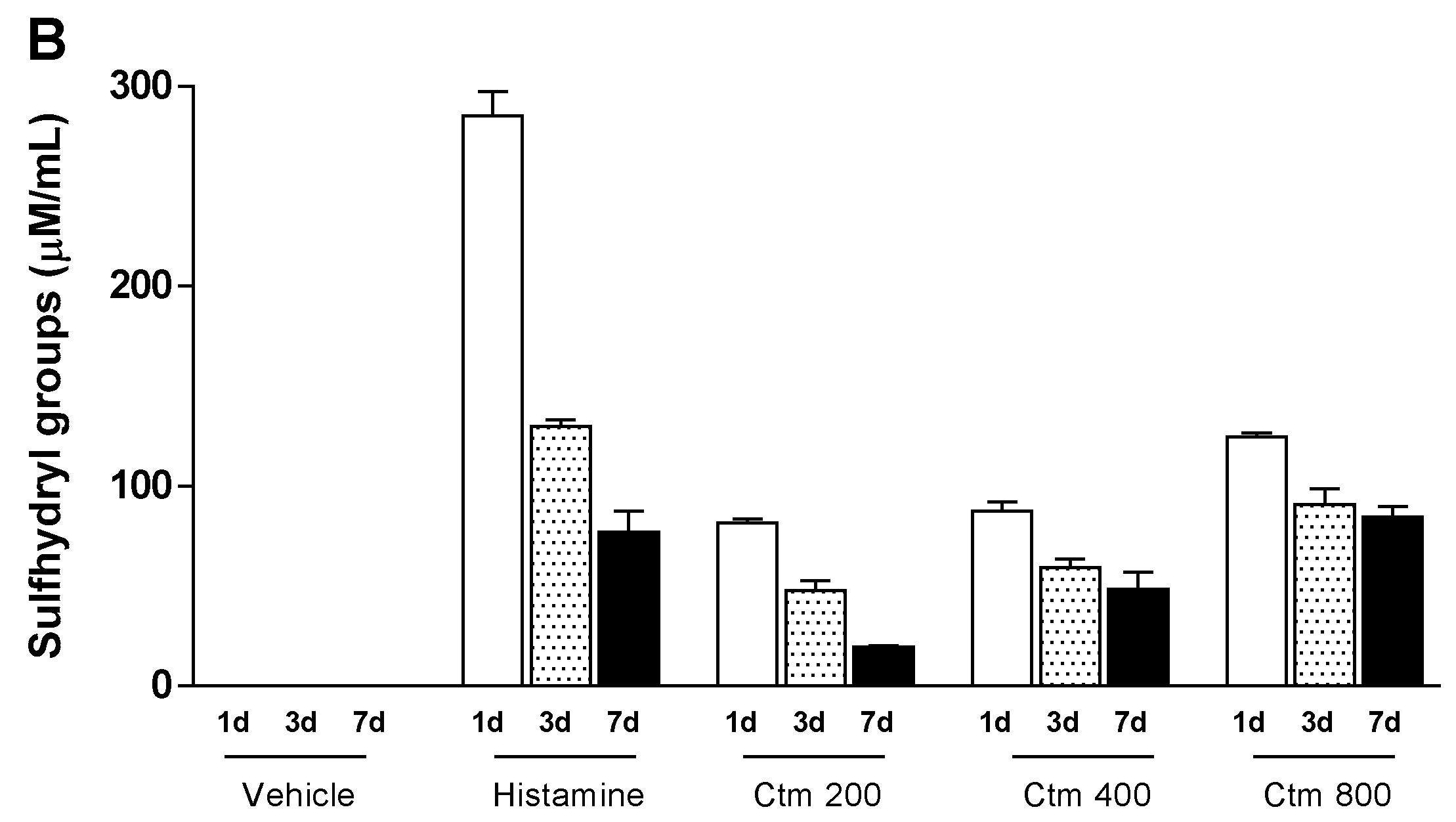
2.4. Crotamine Changed the Systemic Redox State
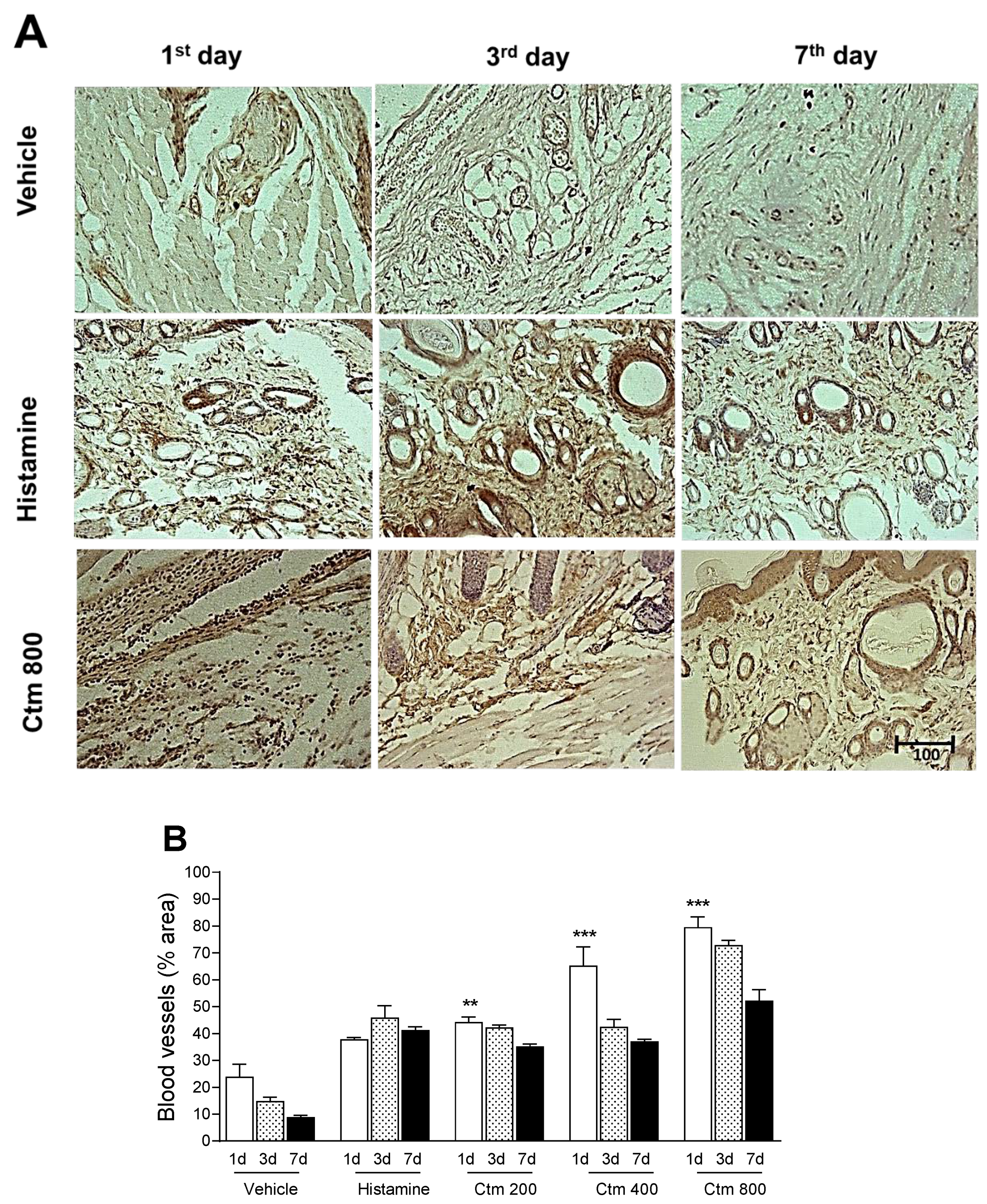
2.5. Crotamine Stimulated Angiogenesis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials and Venom
5.2. Purification of Crotamine
5.3. Animals
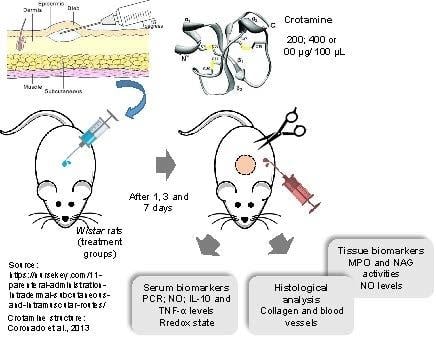
5.4. Experimental Design and Sample Collection
5.5. Clinical Parameters for Immunological and Inflammatory Evaluation
5.5.1. C-Reactive protein (CRP) and Cytokines Measurements
5.5.2. Nitric Oxide (NO) Assay
5.5.3. Systemic Redox State
5.5.4. Myeloperoxidase and N-Acethyglycosaminidase Assays
5.5.5. Histological Analysis
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicastro, G.; Franzoni, L.; De Chiara, C.; Mancin, A.C.; Giglio, J.R.; Spisni, A. Solution structure of crotamine, a Na+ channel affecting toxin from Crotalus durissus terrificus venom. Eur. J. Biochem. 2003, 270, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, A.; Kerkis, I.; Radis-Baptista, G.; Oliveira, E.B.; Vianna-Morgante, A.M.; Pereira, L.V.; Yamane, T. Crotamine is a novel cell-penetrating protein from the venom of rattlesnake Crotalus durissus terrificus. FASEB J. 2004, 18, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Silva, F.D.S.; Pereira, A.; Kerkis, A.; Radis-Baptista, G. Biological versatility of crotamine—A cationic peptide from the venom of a South American rattlesnake. Expert Opin. Investig. Drugs 2010, 19, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.A.; Gabdulkhakov, A.; Georgieva, D.; Sankaran, B.; Murakami, M.T.; Arni, R.K.; Betzel, C. Structure of the polypeptide crotamine from the Brazilian rattlesnake Crotalus durissus terrificus. Acta Cryst. D 2013, 69, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.; Bosch, B.; Hanke, W.; de Lima, V.M.F. Membrane-modifying properties of crotamine, a small peptide-toxin from Crotalus durissus terifficus venom. Biochim. Biophys. Acta 2014, 1840, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.L.; Tu, A.T. Chemical and functional homology of myotoxin a from prairie rattlesnake venom and crotamine from South American rattlesnake venom. Biochim. Biophys. Acta Protein Struct. 1978, 532, 147–154. [Google Scholar] [CrossRef]
- Ownby, C.L.; Aird, S.D.; Kaiser, I.I. Physiological and immunological properties of small myotoxins from the venom of the midget faded rattlesnake (Crotalus viridis concolor). Toxicon 1988, 26, 319–323. [Google Scholar] [CrossRef]
- Peigneur, S.; Orts, D.J.; Prieto da Silva, A.R.; Oguiura, N.; Boni-Mitake, M.; de Oliveira, E.B.; Zaharenko, A.J.; de Freitas, J.C.; Tytgat, J. Crotamine pharmacology revisited: Novel insights based on the inhibition of KV channels. Mol. Pharmacol. 2012, 82, 90–96. [Google Scholar] [CrossRef]
- Batista da Cunha, D.; Silvestrini, A.V.; Gomes da Silva, A.C.; Estevam, D.M.P.; Pollettini, F.L.; de Oliveira Navarro, J.; Alves, A.A.; Beretta, A.L.R.Z.; Bizzacchi, J.M.A.; Pereira, L.C.; et al. Mechanistic insights into functional characteristics of native crotamine. Toxicon 2018, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.A.; Nascimento, F.D.; Kerkis, A.; Oliveira, V.; Oliveira, E.B.; Pereira, A.; Rádis-Baptista, G.; Nader, H.B.; Yamane, T.; Kerkis, I.; et al. Cytotoxic effects of crotamine are mediated through lysosomal membrane permeabilization. Toxicon 2008, 52, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Toyama, M.H.; Carneiro, E.M.; Marangoni, S.; Barbosa, R.L.; Corso, G.; Boschero, A.C. Biochemical characterization of two crotamine isoforms isolated by a single step RP-HPLC from Crotalus durissus terrificus (South American rattlesnake) venom and their action on insulin secretion by pancreatic islets. Biochim. Biophys. Acta 2000, 1474, 56–60. [Google Scholar] [CrossRef]
- Oguiura, N.; Boni-Mitake, M.; Affonso, R.; Zhang, G. In vitro antibacterial and hemolytic activities of crotamine, a small basic myotoxin from rattlesnake Crotalus durissus. J. Antibiot. 2011, 64, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Yamane, E.S.; Bizerra, F.C.; Oliveira, E.B.; Moreira, J.T.; Rajabi, M.; Nunes, G.L.; de Souza, A.O.; da Silva, I.D.; Yamane, T.; Karpel, R.L.; et al. Unraveling the antifungal activity of a South American rattlesnake toxin crotamine. Biochimie 2013, 95, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, S.R.A.; de Barros, N.B.; Ferreira, A.S.; Moreira-Dill, L.S.; Calderon, L.A.; Soares, A.M.; Nicolete, R. Biodegradable microparticles containing crotamine isolated from Crotalus durissus terrificus display antileishmanial activity in vitro. Pharmacology 2015, 95, 78–86. [Google Scholar] [CrossRef] [PubMed]
- El-Chamy-Maluf, S.; Dal Mas, C.; Oliveira, E.B.; Melo, P.M.; Carmona, A.K.; Gazarini, M.L.; Hayashi, M.A. Inhibition of malaria parasite Plasmodium falciparum development by crotamine, a cell penetrating peptide from the snake venom. Peptides 2016, 78, 11–16. [Google Scholar] [CrossRef]
- Mancin, A.C.; Soares, A.M.; Andriao-Escarso, S.H.; Faça, V.M.; Greene, L.J.; Zuccolotto, S.; Pela, I.R.; Giglio, J.R. The analgesic activity of crotamine, a neurotoxin from Crotalus durissus terrificus (South American rattlesnake) venom: A biochemical and pharmacological study. Toxicon 1998, 36, 1927–1937. [Google Scholar] [CrossRef]
- Rodrigues, M.; de la Torre, B.G.; Andreu, D.; Santos, N.C. Kinetic uptake profiles of cell penetrating peptides in lymphocytes and monocytes. Biochim. Biophys. Acta 2013, 1830, 4554–4563. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, Y.K.; Krupa, M.; Nguyen, A.N.; Do, B.H.; Chung, B.; Vu, T.T.T.; Kim, S.C.; Choe, H. Crotamine stimulates phagocytic activity by inducing nitric oxide and TNF-α via p38 and NFκ-B signaling in RAW 264.7 macrophages. BMB Rep. 2016, 49, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, F.D.; Hayashi, M.A.; Kerkis, A.; Oliveira, V.; Oliveira, E.B.; Rádis-Baptista, G.; Nader, H.B.; Yamane, T.; Tersariol, I.L.S.; Kerkis, I. Crotamine mediates gene delivery into cells through the binding to heparin sulfate proteoglycans. J. Biol. Chem. 2007, 282, 21349–21360. [Google Scholar] [CrossRef]
- Radis-Baptista, G.; Kerkis, I. Crotamine, a Small Basic Polypeptide Myotoxin from Rattlesnake Venom with Cell-Penetrating Properties. Curr. Pharm. Des. 2011, 17, 4351–4361. [Google Scholar] [CrossRef]
- Chen, P.-C.; Hayashi, M.A.F.; Oliveira, E.B.; Karpel, R.L. DNA-Interactive Properties of Crotamine, a Cell-Penetrating Polypeptide and a Potential Drug Carrier. PLoS ONE 2012, 7, e48913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.; Kerkis, A.; Hayashi, M.A.; Pereira, A.S.; Silva, F.S.; Oliveira, E.B.; Prieto da Silva, A.R.; Yamane, T.; Rádis-Baptista, G.; Kerkis, I. Crotamine toxicity and efficacy in mouse models of melanoma. Expert Opin. Investig. Drugs 2011, 20, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Hayashi, M.A.; Prieto da Silva, A.R.; Pereira, A.; De Sá Júnior, P.L.; Zaharenko, A.J.; Rádis-Baptista, G.; Kerkis, A.; Yamane, T. State of the art in the studies on crotamine, a cell penetrating peptide from South American rattlesnake. Biomed. Res. Int. 2014, 2014, 67598. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Oliveira e silva, S.; Rostelato-Ferreira, S.; Rocha-e-Silva, T.A.A.; Randazzo-Moura, P.; Dal-Belo, C.A.; Sanchez, E.F.; Borja-Oliveira, C.R.; Rodrigues-Simioni, L. Beneficial effect of crotamine in the treatment of myasthenic rats. Muscle Nerve 2013, 47, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Vargas, L.; Lara, M.; Güllich, A.; Mandredini, V.; Ponce-Soto, L.; Mello-Carpes, P. Intrahippocampal infusion of crotamine isolated from Crotalus durissus terrificus alters plasma and brain biochemical parameters. Int. J. Environ. Res. Public Health 2014, 11, 11438–11449. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake venom toxins: Toxicity and medicinal applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Mordoh, J.; Medrano, E.E.; Bonaparte, Y.P.; Lustig, E.S.; Rumi, L. Special report: Studies to determine the possible antitumoral properties of cobra venom and crotoxin complex A and B. Medicina 1988, 48, 337–344. [Google Scholar]
- Iglesias, C.V.; Aparicio, R.; Rodrigues-Simioni, L.; Camargo, E.A.; Antunes, E.; Marangoni, S.; Toyama, M.H. Effects of morin on snake venom phospholipase A2 (PLA2). Toxicon 2005, 46, 751–758. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M. Venoms, venomics, antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef] [Green Version]
- Lomonte, B.; Calvete, J.J. Strategies in “snake venomics” aiming at an integrative view of compositional, functional and immunological characteristics of venoms. J. Venom. Anim. Toxins 2017, 23, 26. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G.A. Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.Y.; Kini, R.M. From snake venom toxins to therapeutics—Cardiovascular examples. Toxicon 2012, 59, 497–506. [Google Scholar] [CrossRef] [PubMed]
- De Lima, D.C.; Alvarez Abreu, P.; de Freitas, C.C.; Santos, D.O.; Borges, R.O.; dos Santos, T.C.; Castro, H.C. Snake Venom: Any Clue for Antibiotics and CAM? Evid. Based Complement. Altern. Med. 2005, 2, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, C.; Larios, S.; Angulo, Y.; Pizarro-Cerda, J.; Gorvel, J.-P.; Moreno, E.; Lomonte, B. Antimicrobial activity of myotoxic phospholipases A2 from crotalid snake venoms and synthetic peptide variants derived from their C-terminal region. Toxicon 2005, 45, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Perumal Samy, R.; Gopalakrishnakone, P.; Thwin, M.M.; Chow, T.K.V.; Bow, H.; Yap, E.H.; Thong, T.W.J. Antibacterial activity of snake, scorpion and bee venoms: A comparison with purified venom phospholipase A2 enzymes. J. Appl. Microbiol. 2007, 102, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Muller, V.D.; Soares, R.O.; dos Santos-Junior, N.N.; Trabuco, A.C.; Cintra, A.C.; Figueiredo, L.T.; Aquino, V.H. Phospholipase A2 isolated from the venom of Crotalus durissus terrificus inactivates dengue virus and other enveloped viruses by disrupting the viral envelope. PLoS ONE 2014, 9, e112351. [Google Scholar] [CrossRef] [PubMed]
- Deolindo, P.; Teixeira-Ferreira, A.S.; Melo, E.J.; Arnholdt, A.C.V.; de Souza, W.; Alves, E.W.; DaMatta, R.A. Programmed cell death in Trypanosoma cruzi induced by Bothrops jararaca venom. Memórias do Instituto Oswaldo Cruz 2005, 100, 33–38. [Google Scholar] [CrossRef] [PubMed]
- De Melo Alves Paiva, R.; de Freitas Figueiredo, R.; Antonucci, G.A.; Paiva, H.H.; de Lourdes Pires Bianchi, M.; Rodrigues, K.C.; Sampaio, S.V. Cell cycle arrest evidence, parasiticidal and bactericidal properties induced by l-amino acid oxidase from Bothrops atrox snake venom. Biochimie 2011, 93, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Corin, R.E.; Viskatis, L.J.; Vidal, J.C.; Etcheverry, M.A. Cytotoxicity of crotoxin on murine erythroleukemia cells in vitro. Investig. New Drugs 1993, 11, 11–15. [Google Scholar] [CrossRef]
- Donato, N.J.; Martin, C.A.; Perez, M.; Newman, R.A.; Vidal, J.C.; Etcheverry, M.E. Regulation of epidermal growth factor receptor activity by crotoxin, a snake venom phospholipase A2 toxin. A novel growth inhibitory mechanism. Biochem. Pharmacol. 1996, 11, 1535–1543. [Google Scholar] [CrossRef]
- Costa, L.A.; Miles, H.A.; Diez, R.A.; Araujo, C.E.; Coni, M.C.M.; Cervellino, J.C. Phase I study of VRCTC-310, a purified phospholipase A2 purified from snake venom, in patients with refractory cancer: Safety and pharmacokinetic data. Anticancer Drugs 1997, 9, 829–834. [Google Scholar] [CrossRef]
- Sampaio, S.; Brigatte, P.; Sousa-e-Silva, M.C.; dos-Santos, E.; Rangel-Santos, A.; Curi, R.; Cury, Y. Contribution of crotoxin for the inhibitory effect of Crotalus durissus terrificus snake venom on macrophage function. Toxicon 2003, 41, 899–907. [Google Scholar] [CrossRef]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; de Moura, A.A.; Grabner, A.N.; Mazzi, M.V.; Soares, A.M. Antitumoral Activity of Snake Venom Proteins: New Trends in Cancer Therapy. BioMed Res. Int. 2014, 2014, 203639. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.S.; Lara, M.V.S.; Gonçalves, R.; Mandredini, V.; Ponce-Soto, L.A.; Marangoni, S.; Mello-Carpes, P.B. The intrahippocampal infusion of crotamine from Crotalus durissus terrificus venom enhances memory persistence in rats. Toxicon 2014, 85, 52–58. [Google Scholar] [CrossRef]
- Skobe, M.; Detmar, M. Structure, function and molecular control of the skin lymphatic system. J. Investig. Dermatol. Symp. Proc. 2000, 5, 14–19. [Google Scholar] [CrossRef]
- Parslow, T.G. Immunogens, antigens and vaccines. In Medical Immunology, 10th ed.; Parslow, T.G., Stites, D.P., Terr, A.I., Imboden, J.B., Eds.; Lange Medical Books/McGraw-Hill Medical Pub. Division: New York, NY, USA, 2001; pp. 72–81. [Google Scholar]
- Mayer, L.E.; De Bona, K.S.; Abdalla, F.H.; de Almeida, F.L.; Pozzobon, R.C.R.; Charão, M.F.; Moretto, M.B.; Moresco, R.N. Perspectives on the laboratory evaluation of the inflammatory response. Rev. Bras. Farm. 2010, 91, 149–161. [Google Scholar]
- Aguiar, F.J.B.; Ferreira-Júnior, M.; Sales, M.M.; Cruz-Neto, L.M.; Fonseca, L.A.M.; Sumita, N.M.; Duarte, A.J.S. C-reactive protein: Clinical applications and proposals for a rational use. Rev. Assoc. Med. Bras. 2013, 59, 85–92. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.M.B.; Sakata, R.K.; Issy, A.M.; Gerola, L.R.; Salomão, R. Citocinas e dor. Rev. Bras. Anestesiol. 2011, 61, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.; Noboru, N.; Young, A.; Thomas, D. Pro and anti-inflammatory cytokine levels (TNF-α, IL-1β, IL-6 and IL-10) in rat model of neuroma. Pathophysiology 2017, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Soto, L.A.; Martins-de-Souza, D.; Marangoni, S. Structural and pharmacological characterization of the crotamine isoforms III-4 (MYX4_CROCu) and III-7 (MYX7_CROCu) isolated from the Crotalus durissus cumanensis venom. Toxicon 2010, 55, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Kendall, H.; Marshall, R.; Bartold, P. Nitric oxide and tissue destruction. Oral Dis. 2001, 7, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Dusse, L.M.S.; Vieira, L.M.; Carvalho, M.d.G. Revisão sobre óxido nítrico. J. Bras. Patol. Med. Lab. 2003, 39, 343–349. [Google Scholar] [CrossRef]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Picolo, G.; Cury, Y. Peripheral neuronal nitric oxide synthase activity mediates the antinociceptive effect of Crotalus durissus terrificus snake venom, a δ- and κ-opioid receptor agonist. Life Sci. 2004, 75, 559–573. [Google Scholar] [CrossRef]
- Costa, E.S.; Faiad, O.J.; Landgraf, R.G.; Ferreira, A.K.; Brigatte, P.; Curi, R.; Sampaio, S.C. Involvement of formyl peptide receptors in the stimulatory effect of crotoxin on macrophages co-cultivated with tumour cells. Toxicon 2013, 74, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Farias, L.H.S.; Rodrigues, A.P.D.; Coêlho, E.C.; Santos, M.F.; Sampaio, S.C.; Silva, E.O. Crotoxin stimulates an M1 activation profile in murine macrophages during Leishmania amazonensis infection. Parasitology 2017, 144, 1458–1467. [Google Scholar] [CrossRef]
- Miyabara, E.; Tostes, R.; Selistre-de-Araújo, H.; Aoki, M.; Moriscot, A. Role of nitric oxide in myotoxic activity induced by crotoxin in vivo. Toxicon 2004, 43, 425–432. [Google Scholar] [CrossRef]
- Bulet, P.; Stocklin, R.; Menin, L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2004, 198, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S.; Vicent, K.A.; Feron, O.; Kelly, R.A. Innate Immunity and Angiogenesis. Circ. Res. 2004, 96, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Kolla, R.V.; Sidney, J.; Weiskopf, D.; Fleri, W.; Kim, Y.; Peters, B.; Sette, A. Evaluating the immunogenicity of protein drugs by applying in vitro MHC binding data and the immune epitope database and analysis resource. Clin. Dev. Immunol. 2013, 2013, 467852. [Google Scholar] [CrossRef] [PubMed]
- Kropshofer, H.; Singer, T. Overview of Cell-Based Tools for Pre-Clinical Assessment of Immunogenicity of Biotherapeutics. J. Immunotoxicol. 2006, 3, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Wolbink, G.J.; Aarden, L.A.; Dijkmans, B. Dealing with immunogenicity of biologicals: Assessment and clinical relevance. Curr. Opin. Rheumatol. 2009, 21, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Brinks, V.; Jiskoot, W.; Schellekens, H. Immunogenicity of therapeutic proteins: The use of animal models. Pharm. Res. 2011, 28, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Parenky, A.; Myler, H.; Amaravadi, L.; Bechtold-Peters, K.; Rosenberg, A.; Kirshner, S.; Quarmby, V. New FDA draft guidance on immunogenicity. AAPS J. 2014, 16, 499–503. [Google Scholar] [CrossRef]
- Diehl, K.-H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vorstenbosch, C.V.D. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Green, L.C.; Wagner, D.A.; Glogowski, K.; Skipper, P.I.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 42, 407–421. [Google Scholar] [CrossRef]
- Faure, P.; Lafond, J.L. Measurement of plasma sulfhydryl and carbonyl groups as a possible indicator of protein oxidation. In Analysis of Free Radicals in Biological Systems; Favier, A., Cadet, J., Kalyanaraman, B., Fontecave, M., Pierre, J.L., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1995; pp. 237–248. [Google Scholar]
- Moscardi, L.C.; Espíndola, T.P.; Ferreira, A.A.; Alves, N.; Amaral, M.E.C.; Aro, A.A.; Dalia, R.A.; Esquisatto, M.A.M.; Mendonça, F.A.S.; Santos, G.M.T.; et al. Lasertherapy as a strategy for treatment Healing under Caloric Restriction–Study in Rats. J. Pharm. Pharmacol. 2018, 6, 647–658. [Google Scholar]
- Souza, D.G.; Cassali, G.D.; Poole, S.; Teixeira, M.M. Effects of inhibition of PDE4 and TNF-α on local and remote injuries following ischaemia and reperfusion injury. Br. J. Pharmacol. 2001, 134, 985–994. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestrini, A.V.P.; de Macedo, L.H.; de Andrade, T.A.M.; Mendes, M.F.; Pigoso, A.A.; Mazzi, M.V. Intradermal Application of Crotamine Induces Inflammatory and Immunological Changes In Vivo. Toxins 2019, 11, 39. https://doi.org/10.3390/toxins11010039
Silvestrini AVP, de Macedo LH, de Andrade TAM, Mendes MF, Pigoso AA, Mazzi MV. Intradermal Application of Crotamine Induces Inflammatory and Immunological Changes In Vivo. Toxins. 2019; 11(1):39. https://doi.org/10.3390/toxins11010039
Chicago/Turabian StyleSilvestrini, Ana Vitória Pupo, Luana Henrique de Macedo, Thiago Antônio Moretti de Andrade, Maíra Felonato Mendes, Acácio Antônio Pigoso, and Maurício Ventura Mazzi. 2019. "Intradermal Application of Crotamine Induces Inflammatory and Immunological Changes In Vivo" Toxins 11, no. 1: 39. https://doi.org/10.3390/toxins11010039