Integrating Engineering, Manufacturing, and Regulatory Considerations in the Development of Novel Antivenoms
Abstract
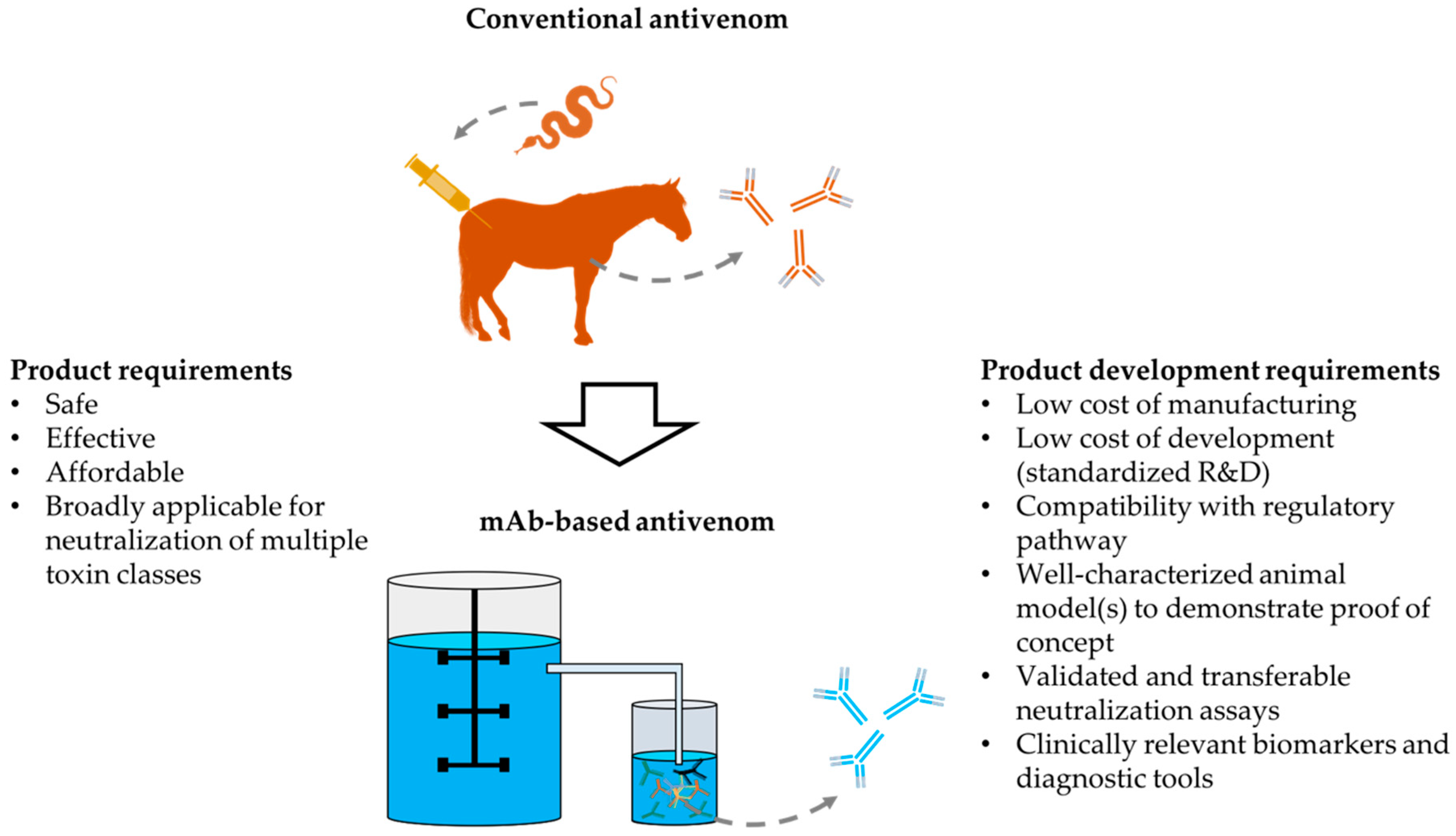
:1. Introduction
2. Considerations for Novel Antivenoms and their Markets
3. Development and Manufacturing Aspects
4. Clinical and Regulatory Aspects
5. Conclusions and Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, J.M.; Williams, D.; Fan, H.W.; Warrell, D.A. Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon 2010, 56, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Calmette, A. L’immunisation artificielle des animaux contre le venin des serpents, et la thérapeutic expérimentale des morsures venimeuses. Comptes Rendus de la Société de Biologie 1894, 46, 120–124. [Google Scholar]
- Laustsen, A.H.; Engmark, M.; Milbo, C.; Johannesen, J.; Lomonte, B.; Gutiérrez, J.M.; Lohse, B. From Fangs to Pharmacology: The Future of Snakebite Envenoming Therapy. Curr. Pharm. Des. 2016, 22, 5270–5293. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Cook, D.A.; Renjifo, C.; Casewell, N.R.; Currier, R.B.; Wagstaff, S.C. Research strategies to improve snakebite treatment: Challenges and progress. J. Proteom. 2011, 74, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Kannt, A.; Wieland, T. Managing risks in drug discovery: Reproducibility of published findings. Naunyn-Schmiedebergs Arch. Pharmacol. 2016, 389, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.I. Consequences of Neglect: Analysis of the Sub-Saharan African Snake Antivenom Market and the Global Context. PLoS Negl. Trop. Dis. 2012, 6, e1670. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Meeting on Monoclonal Antibodies against Rabies and Evaluation of Mechanisms to Improve Access to Other Blood-Derived Immunoglobulins; World Health Organization: Silver Spring, MD, USA, 2017. [Google Scholar]
- Laustsen, A.H.; Johansen, K.H.; Engmark, M.; Andersen, M.R. Recombinant snakebite antivenoms: A cost-competitive solution to a neglected tropical disease? PLoS Negl. Trop. Dis. 2017, 11, e0005361. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Johansen, K.H.; Engmark, M.; Andersen, M.R. Snakebites: Costing recombinant antivenoms. Nature 2016, 538, 41. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Meyers, A.J.; McLean, M.D.; Arbabi-Ghahroudi, M.; MacKenzie, R.; Hall, J.C. In Vivo Neutralization of α-Cobratoxin with High-Affinity Llama Single-Domain Antibodies (VHHs) and a VHH-Fc Antibody. PLoS ONE 2013, 8, e69495. [Google Scholar] [CrossRef] [PubMed]
- Julve Parreño, J.M.; Huet, E.; Fernández-Del-Carmen, A.; Segura, A.; Venturi, M.; Gandía, A.; Pan, W.-S.; Albaladejo, I.; Forment, J.; Pla, D.; et al. A synthetic biology approach for consistent production of plant-made recombinant polyclonal antibodies against snake venom toxins. Plant. Biotechnol. J. 2017, 16, 727–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, J.M.A.; Oliveira, T.S.; Silveira, C.R.F.; Caporrino, M.C.; Rodriguez, D.; Moura-da-Silva, A.M.; Ramos, O.H.P.; Rucavado, A.; Gutiérrez, J.M.; Magalhães, G.S.; et al. A neutralizing recombinant single chain antibody, scFv, against BaP1, A P-I hemorrhagic metalloproteinase from Bothrops asper snake venom. Toxicon 2014, 87, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; John, T.R.; Kaiser, I.I. Specificity and binding affinity of an anti-crotoxin combinatorial antibody selected from a phage-displayed library. Biochem. Pharmacol. 1995, 50, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Gutiérrez, J.; Ramírez, M.; Díaz, C. Neutralization of myotoxic phospholipases A2 from the venom of the snake Bothrops asper by monoclonal antibodies. Toxicon 1992, 30, 239–245. [Google Scholar] [CrossRef]
- Boulain, J.C.; Ménez, A.; Couderc, J.; Faure, G.; Liacopoulos, P.; Fromageot, P. Neutralizing monoclonal antibody specific for Naja nigricollis toxin alpha: Preparation, characterization, and localization of the antigenic binding site. Biochemistry 1982, 21, 2910–2915. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Kahan, L. Production and partial characterization of monoclonal antibodies to Bothrops asper (terciopelo) myotoxin. Toxicon 1988, 26, 675–689. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Gutiérrez, J.M.; Knudsen, C.; Johansen, K.H.; Bermúdez-Méndez, E.; Cerni, F.A.; Jürgensen, J.A.; Ledsgaard, L.; Martos-Esteban, A.; Øhlenschlæger, M.; et al. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon 2018, 146, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Laustsen, A.H. Recent Advances in Next Generation Snakebite Antivenoms. Trop. Med. Infect. Dis. 2018, 3, 42. [Google Scholar] [CrossRef]
- Laustsen, A.H. Guiding recombinant antivenom development by omics technologies. New Biotechnol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C. Landmark Green Light for Mosquirix Malaria Vaccine. Nat. Biotechnol. 2015, 33, 1015–1016. [Google Scholar] [CrossRef] [PubMed]
- Antivenom Market By Type [Vaccines And Hyperimmune Sera (Homologous & Heterologous)], By Animal (Snakes, Scorpions, Spiders, And Others), And By Region—Global Industry Analysis, Size, Share, Growth, Trends, And Forecasts (2018–2023) Market Data Forecast. Available online: https://www.marketdataforecast.com/market-reports/global-antivenom-market-1580/ (accessed on 18 May 2018).
- Chaves, L.F.; Chuang, T.-W.; Sasa, M.; Gutiérrez, J.M. Snakebites are associated with poverty, weather fluctuations, and El Niño. Sci. Adv. 2015, 1, e1500249. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake Envenoming: A. Disease of Poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef] [PubMed]
- Bawaskar, H.S.; Bawaskar, P.H.; Bawaskar, P.H. Snake bite in India: A neglected disease of poverty. Lancet 2017, 390, 1947–1948. [Google Scholar] [CrossRef]
- Williams, D.J.; Gutiérrez, J.M.; Calvete, J.J.; Wüster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteom. 2011, 74, 1735–1767. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H. Toxin-centric development approach for next-generation antivenoms. Toxicon 2018, 150, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A.H. Basics of Antibody Phage Display Technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Rodríguez, Y.; Quesada-Bernat, S.; Pla, D. Toxin-resolved antivenomics-guided assessment of the immunorecognition landscape of antivenoms. Toxicon 2018, 148, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Lohse, B.; Lomonte, B.; Engmark, M.; Gutiérrez, J.M. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a Toxicity Score. Toxicon 2015, 104, 43–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvete, J.J.; Lomonte, B. A bright future for integrative venomics. Toxicon 2015, 107, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Gutiérrez, J.M. Priority actions and progress to substantially and sustainably reduce the mortality, morbidity and socioeconomic burden of tropical snakebite. Toxins 2016, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.K.; Næsted, H.; Müller, C.; Tolstrup, A.B.; Frandsen, T.P. Recombinant antibody mixtures: Production strategies and cost considerations. Arch. Biochem. Biophys. 2012, 526, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Windyga, J.; Trelinski, J.; von Depka Prondzinski, M.; Giagounidis, A.; Doyen, C.; Janssens, A.; Alvarez-Román, M.T.; Jarque, I.; Loscertales, J.; et al. Rozrolimupab, a mixture of 25 recombinant human monoclonal RhD antibodies, in the treatment of primary immune thrombocytopenia. Blood 2012, 120, 3670–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, H.J.; Poulsen, T.T.; Dahlman, A.; Kjær, I.; Koefoed, K.; Sen, J.W.; Weilguny, D.; Bjerregaard, B.; Andersen, C.R.; Horak, I.D.; et al. Pan-HER, an Antibody Mixture Simultaneously Targeting EGFR, HER2, and HER3, Effectively Overcomes Tumor Heterogeneity and Plasticity. Clin. Cancer Res. 2015, 21, 4110–4122. [Google Scholar] [CrossRef] [PubMed]
- Montagut, C.; Argilés, G.; Ciardiello, F.; Poulsen, T.T.; Dienstmann, R.; Kragh, M.; Kopetz, S.; Lindsted, T.; Ding, C.; Vidal, J.; et al. Efficacy of Sym004 in Patients with Metastatic Colorectal Cancer with Acquired Resistance to Anti-EGFR Therapy and Molecularly Selected by Circulating Tumor DNA Analyses: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e175245. [Google Scholar] [CrossRef] [PubMed]
- Klutz, S.; Holtmann, L.; Lobedann, M.; Schembecker, G. Cost evaluation of antibody production processes in different operation modes. Chem. Eng. Sci. 2016, 141, 63–74. [Google Scholar] [CrossRef]
- Hammerschmidt, N.; Tscheliessnig, A.; Sommer, R.; Helk, B.; Jungbauer, A. Economics of recombinant antibody production processes at various scales: Industry-standard compared to continuous precipitation. Biotechnol. J. 2014, 9, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Gronemeyer, P.; Ditz, R.; Strube, J. Trends in Upstream and Downstream Process Development for Antibody Manufacturing. Bioengineering 2014, 1, 188–212. [Google Scholar] [CrossRef] [PubMed]
- Novais, J.L.; Titchener-Hooker, N.J.; Hoare, M. Economic comparison between conventional and disposables-based technology for the production of biopharmaceuticals. Biotechnol. Bioeng. 2001, 75, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Klutz, S.; Magnus, J.; Lobedann, M.; Schwan, P.; Maiser, B.; Niklas, J.; Temming, M.; Schembecker, G. Developing the biofacility of the future based on continuous processing and single-use technology. J. Biotechnol. 2015, 213, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.A.; Gottschalk, U. Single-use disposable technologies for biopharmaceutical manufacturing. Trends Biotechnol. 2013, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.S.; Washbrook, J.; Titchener-Hooker, N.J. Decision-Support Tool for Assessing Biomanufacturing Strategies under Uncertainty: Stainless Steel versus Disposable Equipment for Clinical Trial Material Preparation. Biotechnol. Prog. 2005, 21, 486–497. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. RSV Vaccine Research and Development Technology Roadmap: Priority Activities for Development, Testing, Licensure and Global Use of RSV Vaccines, with a Specific Focus on the Medical Need for Young Children in Low-and Middle-income Countries; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. WHO Preferred Product Characteristics for Respiratory Syncytial Virus (RSV) Vaccines; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Pathmeswaran, A.; Kasturiratne, A.; Fonseka, M.; Nandasena, S.; Lalloo, D.G.; de Silva, H.J. Identifying the biting species in snakebite by clinical features: An epidemiological tool for community surveys. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Solano, G.; Pla, D.; Herrera, M.; Segura, Á.; Vargas, M.; Villalta, M.; Sánchez, A.; Sanz, L.; Lomonte, B.; et al. Preclinical Evaluation of the Efficacy of Antivenoms for Snakebite Envenoming: State-of-the-Art and Challenges Ahead. Toxins 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Sells, P.G. Animal experimentation in snake venom research and in vitro alternatives. Toxicon 2003, 42, 115–133. [Google Scholar] [CrossRef]
- Liberti, L.; Breckenridge, A.; Hoekman, J.; Leufkens, H.; Lumpkin, M.; McAuslane, N.; Stolk, P.; Zhi, K.; Rägo, L. Accelerating access to new medicines: Current status of facilitated regulatory pathways used by emerging regulatory authorities. J. Public Health Policy 2016, 37, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Doua, J.Y.; Van Geertruyden, J.-P. Registering medicines for low-income countries: How suitable are the stringent review procedures of the World Health Organization, the US Food and Drug Administration and the European Medicines Agency? Trop. Med. Int. Health 2014, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.F. Developing drug prototypes: Pharmacology replaces safety and tolerability? Nat. Rev. Drug Discov. 2010, 9, 865. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laustsen, A.H.; Dorrestijn, N. Integrating Engineering, Manufacturing, and Regulatory Considerations in the Development of Novel Antivenoms. Toxins 2018, 10, 309. https://doi.org/10.3390/toxins10080309
Laustsen AH, Dorrestijn N. Integrating Engineering, Manufacturing, and Regulatory Considerations in the Development of Novel Antivenoms. Toxins. 2018; 10(8):309. https://doi.org/10.3390/toxins10080309
Chicago/Turabian StyleLaustsen, Andreas Hougaard, and Netty Dorrestijn. 2018. "Integrating Engineering, Manufacturing, and Regulatory Considerations in the Development of Novel Antivenoms" Toxins 10, no. 8: 309. https://doi.org/10.3390/toxins10080309





