Rice Phyllosphere Bacillus Species and Their Secreted Metabolites Suppress Aspergillus flavus Growth and Aflatoxin Production In Vitro and in Maize Seeds
Abstract
:1. Introduction
2. Results
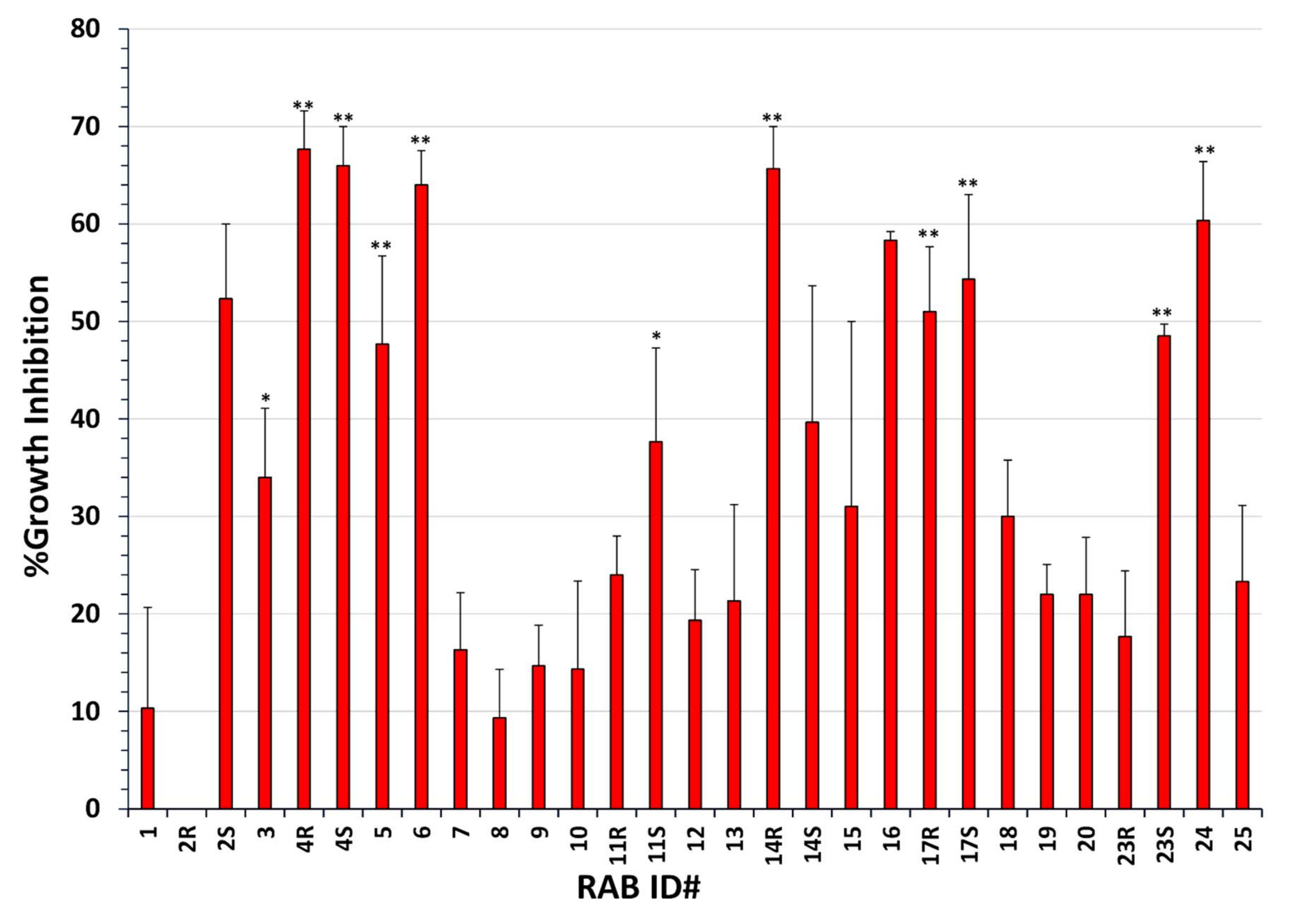
2.1. Many RABs Showed Significant Fungistatic Activity on A. flavus Strains
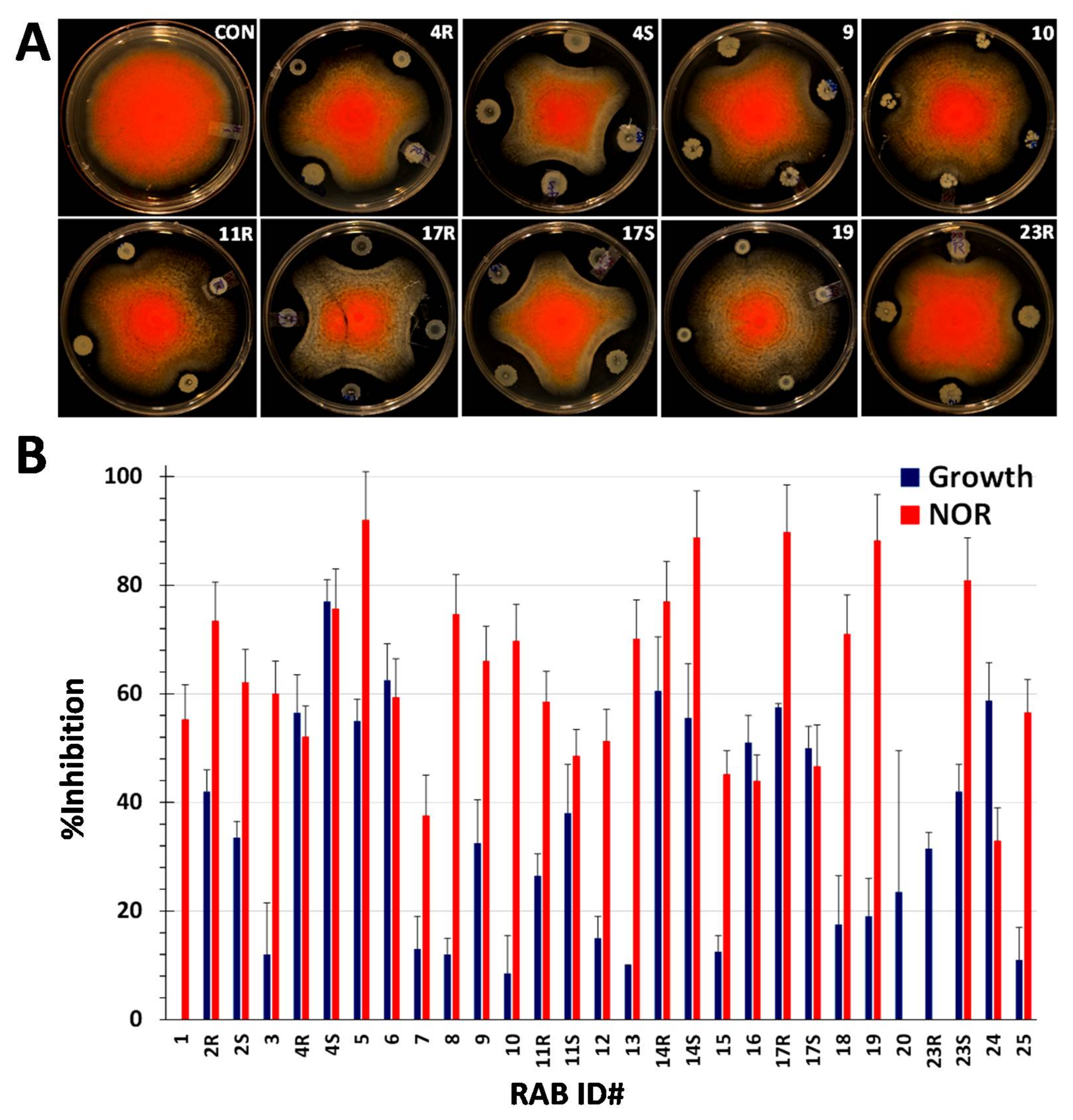
2.2. RABs Differ in Fungistasis and Anti-Mycotoxigenic Activitivities
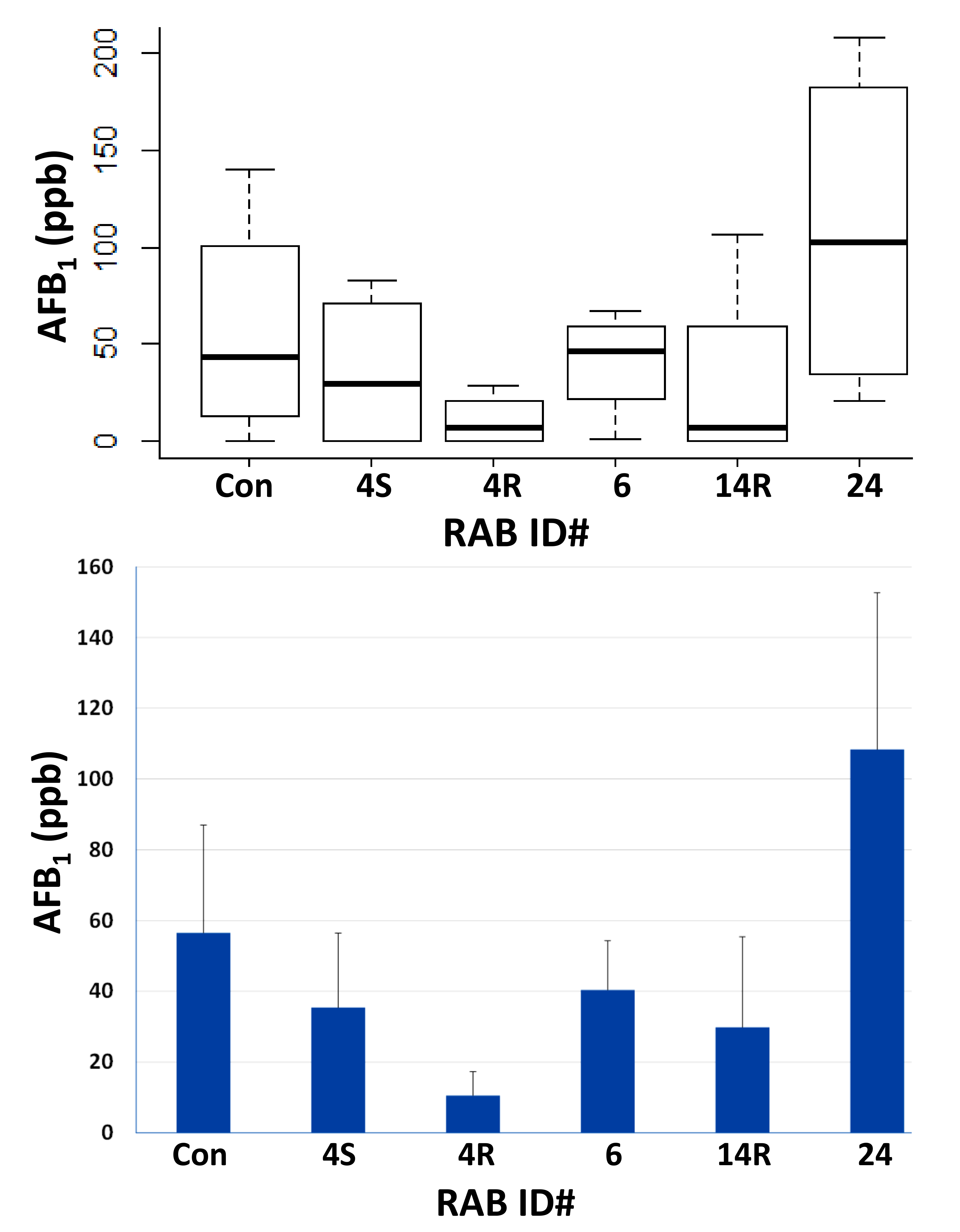
2.3. Field Testing of Top Five RABs on AF Control in Maize
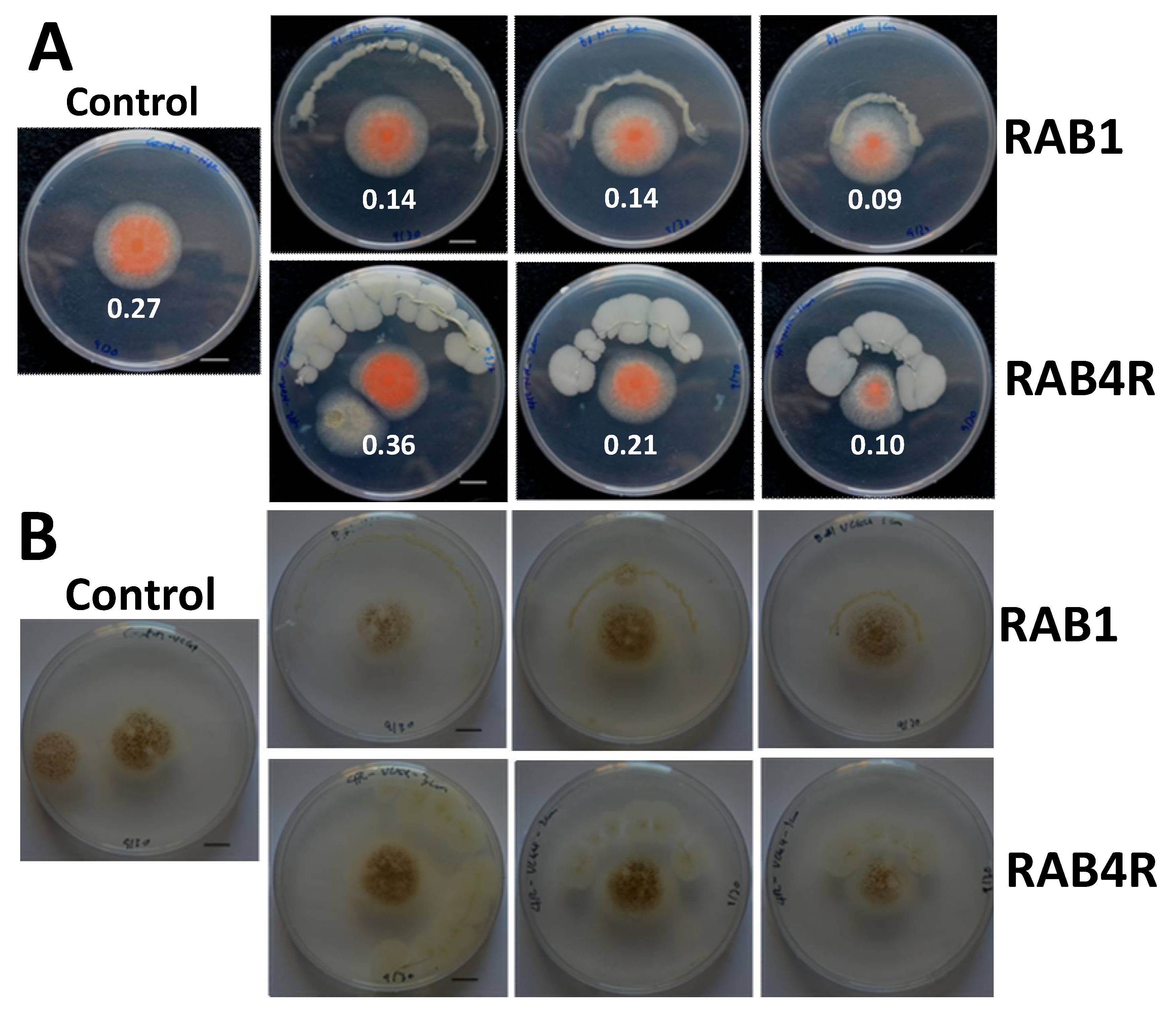
2.4. RAB1 Shows Anti-Aflatoxigenic Activity in Spite of Lacking Fungistatic Activity
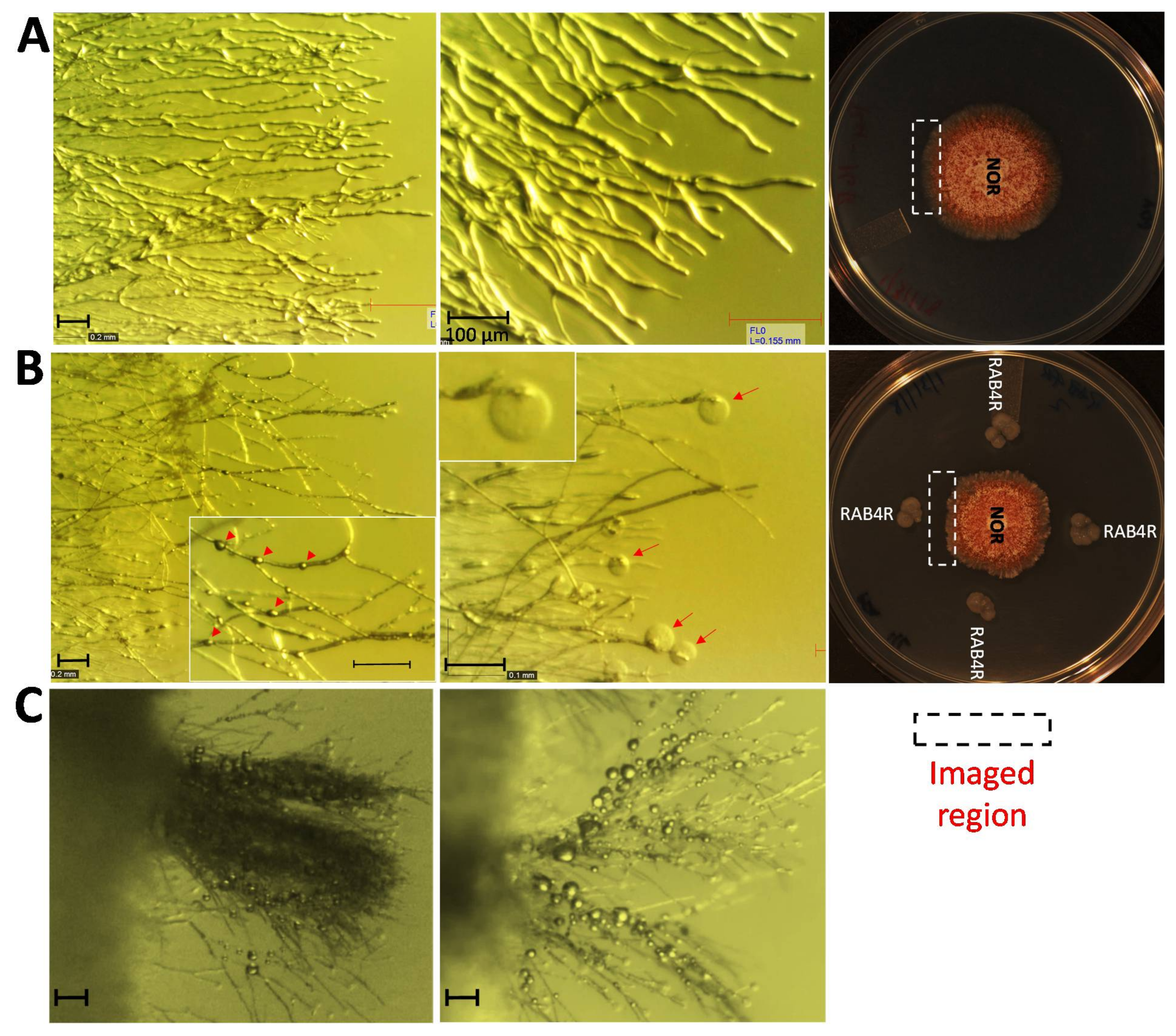
2.5. RAB4R Effects on Hyphal Growth
2.6. Total and HPLC-Fractionated Lipopeptides Provide Effective AF Biocontrol
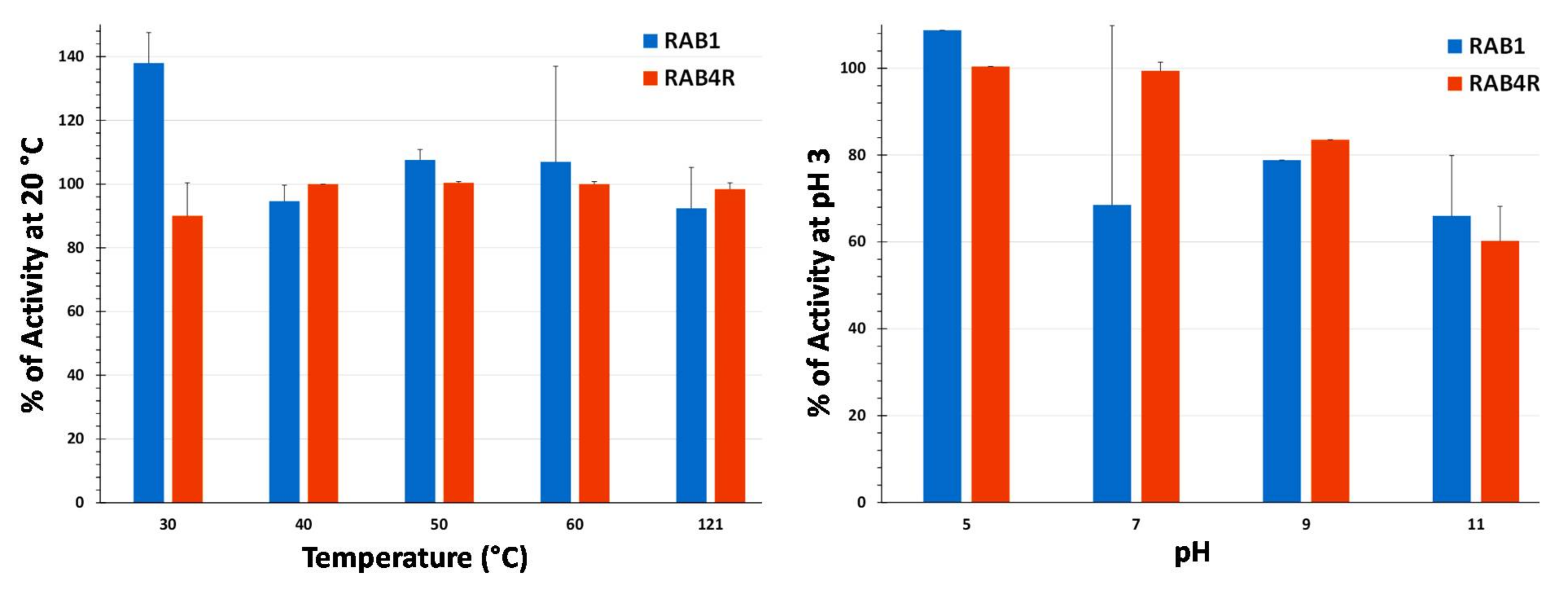
2.7. Temperature and pH Stability of Lipopeptide Extracts
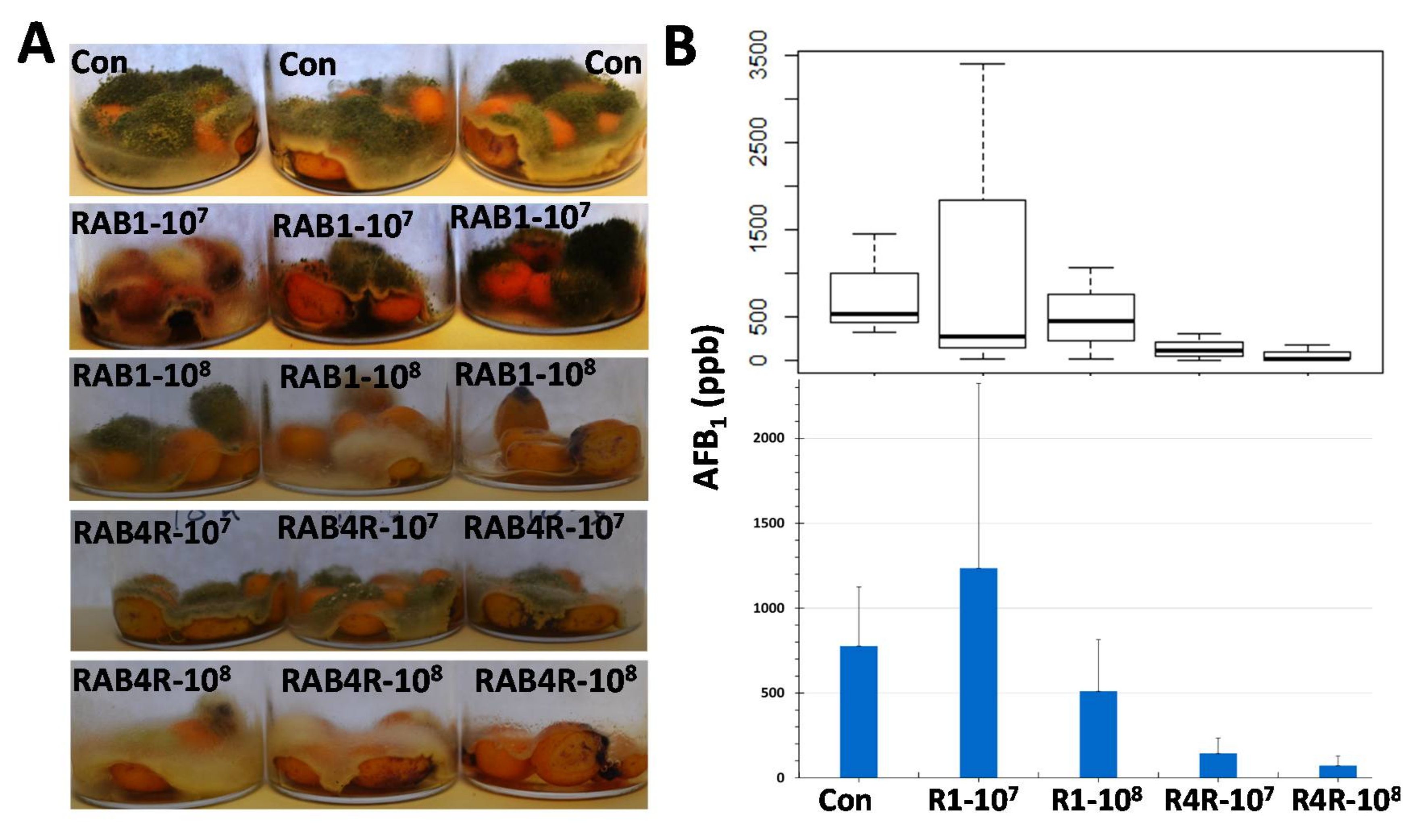
2.8. Postharvest AF Contamination in RAB1 and RAB4R Pretreated Maize Seeds
2.9. Hemolytic Activity of RABs
3. Discussion
3.1. Bacillus Strains from the Phyllosphere of a Non-Host Crop Strongly Inhibit A. flavus Growth and AF Synthesis Both In Vitro and in the Host
3.2. RAB4R Provided Effective AF Biocontrol Both in Preharvest and Postharvest Infection Studies
3.3. RAB4R Inhibition of A. flavus Growth Involved Diffusible Anti-Fungal Compounds
3.4. RAB1 Showed Promising Anti-Aflatoxigenic Activity In Vitro and in Mature Maize Seeds
3.5. In Vitro Studies Indicate the Potential of Other RABs as Independent AF-BCAs or as Helper BCAs in Combination with Intraspecific Biocontrol Strains
4. Conclusions
5. Materials and Methods
5.1. Strains and Media
5.2. Measurement of Fungistatic Activity of RABs
5.3. Field Evaluation of RAB Isolates for Reduction of AF Contamination in Maize
5.4. Quantification of NOR
5.5. Microscopy
5.6. Isolation and High Performance Liquid Chromatography (HPLC) Fractionation of Lipopeptides (LPs)
5.7. Bioassay of LPs for Biocontrol Activity
5.8. Analysis of Temperature and pH Stability of Total LP Extracts
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, R.L.; Menkir, A.; Chen, Z.Y.; Bhatnagar, D.; Yu, J.; Yao, H.; Cleveland, T.E. Breeding aflatoxin-resistant maize lines using recent advances in technologies—A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Cotty, P.J.; Cleveland, T.E. Reduction in aflatoxin content of maize by atoxigenic strains of Aspergillus flavus. J. Food Prot. 1991, 54, 623–626. [Google Scholar] [CrossRef]
- Huang, C.; Jha, A.; Sweany, R.; DeRobertis, C.; Damann, K.E., Jr. Intraspecific Aflatoxin Inhibition in Aspergillus flavus Is Thigmoregulated, Independent of Vegetative Compatibility Group and Is Strain Dependent. PLoS ONE 2011, 6, e23470. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.; Wei, Q.; Brown, R.L.; Bhatnagar, D. Inverse correlation of ability to produce aflatoxin and Aspergillus colonization of maize seed. Food Nutr. Sci. 2011, 2, 486–489. [Google Scholar] [CrossRef]
- Moore, G.G. Sex and recombination in aflatoxigenic Aspergilli: Global implications. Front. Microbiol. 2014, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Olarte, R.A.; Worthington, C.J.; Horn, B.W.; Moore, G.G.; Singh, R.; Monacell, J.W.; Dorner, J.T.; Stone, E.A.; Xie, D.-Y.; Carbone, I. Enhanced diversity and aflatoxigenicity in interspecific hybrids of Aspergillus flavus and Aspergillus parasiticus. Mol. Ecol. 2015, 24, 1889–1909. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W. Biological Control of Aflatoxin Contamination of Crops. J. Toxicol. Toxin Rev. 2004, 23, 425–450. [Google Scholar] [CrossRef]
- Palumbo, J.D.; O’Keeffe, T.L.; Abbas, H.K. Microbial interactions with mycotoxigenic fungi and mycotoxins. Toxin Rev. 2008, 27, 261–285. [Google Scholar] [CrossRef]
- Yin, Y.-N.; Yan, L.-Y.; Jiang, J.-H.; Ma, Z.-H. Biological control of aflatoxin contamination of crops. J. Zhejiang Univ. Sci. B 2008, 9, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Beattie, G.A.; Lindow, S.E. The Secret Life of Foliar Bacterial Pathogens on Leaves. Annu. Rev. Phytopathol. 1995, 33, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Misaghi, I.J.; Cotty, P.J.; Decianne, D.M. Bacterial Antagonists of Aspergillus flavus. Biocontrol Sci. Technol. 1995, 5, 387–392. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Karki, H.S.; Groth, D.E.; Jungkhun, N.; Ham, J.H. Biological Control Activities of Rice-Associated Bacillus sp. Strains against Sheath Blight and Bacterial Panicle Blight of Rice. PLoS ONE 2016, 11, e0146764. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.M.; Thomashow, L.S. Current challenges in introducing beneficial microorganisms into the rhizosphere. In Molecular Ecology of Rhizosphere Microorganisms; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007; pp. 1–18. [Google Scholar]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Hamdache, A.; Lamarti, A.; Aleu, J.; Collado, I.G. Non-peptide Metabolites from the Genus Bacillus. J. Nat. Prod. 2011, 74, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Papa, K.E. Norsolorinic acid mutant of Aspergillus flavus. J. Gen. Microbiol. 1982, 128, 1345–1348. [Google Scholar] [CrossRef]
- López, Y.; Keller, N.P.; Sarr, B.; Phillips, T.D.; Cuero, R.G.; Smith, O.D. Visual Estimation of Aflatoxin Production in Peanut with Aspergillus Norsolorinic Acid Mutants. Peanut Sci. 1998, 25, 92–99. [Google Scholar] [CrossRef]
- Windham, G.L.; Williams, W.P. Systemic infection of stalks and ears of corn hybrids by Aspergillus parasiticus. Mycopathologia 2007, 164, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.S.T.; Baker, J.L.; Flores-Espiritu, M. Interactions of Saprophytic Yeasts with a nor Mutant of Aspergillus flavus. Appl. Environ. Microbiol. 1999, 65, 2738–2740. [Google Scholar] [PubMed]
- Sweany, R.R.; Damann, K.E.; Kaller, M.D. Comparison of Soil and Corn Kernel Aspergillus flavus Populations: Evidence for Niche Specialization. Phytopathology 2011, 101, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Yokota, A.; Yamazoe, A.; Fujiwara, T. Proposal of Lysinibacillus boronitolerans gen. nov. sp. nov.; and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.Y. Lysinibacillus sphaericus TC1 Strain and Method for Controlling Plant Diseases Using the Same. Patent Application No. KR20140051676A, 2 May 2014. [Google Scholar]
- Saito, M.; Machida, S. A rapid identification method for aflatoxin-producing strains of Aspergillus flavus and Aspergillus parasiticus by ammonia vapour. Mycoscience 1999, 40, 205–208. [Google Scholar] [CrossRef]
- Kope, H.H.; Fortin, J.A. Inhibition of phytopathogenic fungi in vitro by cell free culture media of ectomycorrhizal fungi. New Phytol. 1989, 113, 57–63. [Google Scholar] [CrossRef]
- Blacutt, A.A.; Mitchell, T.R.; Bacon, C.W.; Gold, S.E. Bacillus mojavensis RRC101 Lipopeptides Provoke Physiological and Metabolic Changes During Antagonism Against Fusarium verticillioides. Mol. Plant-Microbe Interact. 2016, 29, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Zhao, M.; Chen, D.; Cheng, J.; Li, J.; Feng, Z.; Ma, Z.; An, D. Biocontrol of rice blast by the phenaminomethylacetic acid producer of Bacillus methylotrophicus strain BC79. Crop Prot. 2013, 44, 29–37. [Google Scholar] [CrossRef]
- Frikha-Gargouri, O.; Ben Abdallah, D.; Ghorbel, I.; Charfeddine, I.; Jlaiel, L.; Triki, M.A.; Tounsi, S. Lipopeptides from a novel Bacillus methylotrophicus 39b strain suppress Agrobacterium crown gall tumours on tomato plants. Pest Manag. Sci. 2017, 73, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Shabanamol, S.; Sreekumar, J.; Jisha, M.S. Bioprospecting endophytic diazotrophic Lysinibacillus sphaericus as biocontrol agents of rice sheath blight disease. 3 Biotech 2017, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, S.A.; Mou, H.; Ma, Y.; Li, M.; Hu, X. Characterization of Lipopeptide Biosurfactants Produced by Bacillus licheniformis MB01 from Marine Sediments. Front. Microbiol. 2017, 8, 871. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.; Edwards, S.G.; Markellou, E.; Malathrakis, N.E. Bacterial antagonist-fungal pathogen interactions on the plant aerial surface. In Multitrophic Interactions in Terrestrial Systems: The 36th Symposium of the British Ecological Society, Royal Holloway College, University of London; Blackwell Science Ltd.: Oxford, UK, 1997; pp. 5–25. [Google Scholar]
- Renwick, A.; Campbell, R.; Coe, S. Assessment of in vivo screening systems for potential biocontrol agents of Gaeumannomyces graminis. Plant Pathol. 1991, 40, 524–532. [Google Scholar] [CrossRef]
- Moreno-Velásquez, S.D.; Seidel, C.; Juvvadi, P.R.; Steinbach, W.J.; Read, N.D. Caspofungin-mediated growth inhibition and paradoxical growth in Aspergillus fumigatus involve fungicidal hyphal tip lysis coupled with regenerative intrahyphal growth and dynamic changes in β-1,3-glucan synthase localization. Antimicrob. Agents Chemother. 2017, 61, e00710–e00717. [Google Scholar] [CrossRef] [PubMed]
- Pramudito, T.E.; Agustina, D.; Nguyen, T.K.N.; Suwanto, A. A Novel Variant of Narrow-Spectrum Antifungal Bacterial Lipopeptides That Strongly Inhibit Ganoderma boninense. Probiotics Antimicrob. Proteins 2018, 10, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Asari, S.; Matzén, S.; Petersen, M.A.; Bejai, S.; Meijer, J. Multiple effects of Bacillus amyloliquefaciens volatile compounds: Plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol. Ecol. 2016, 92, fiw070. [Google Scholar] [CrossRef] [PubMed]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Guidi, V.; Lehner, A.; Lüthy, P.; Tonolla, M. Dynamics of Bacillus thuringiensis var. israelensis and Lysinibacillus sphaericus Spores in Urban Catch Basins after Simultaneous Application against Mosquito Larvae. PLoS ONE 2013, 8, e55658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allievi, M.C.; Palomino, M.M.; Prado Acosta, M.; Lanati, L.; Ruzal, S.M.; Sánchez-Rivas, C. Contribution of S-Layer Proteins to the Mosquitocidal Activity of Lysinibacillus sphaericus. PLoS ONE 2014, 9, e111114. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced Systemic Resistance and Promotion of Plant Growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Rehman, N.U.; Hussain, H.; Hussain, J.; Gilani, S.A.; Al Housni, S.K.; Mabood, F.; Khan, A.L.; Farooq, S.; Abbas, G.; et al. Exploring the Potentials of Lysinibacillus sphaericus ZA9 for Plant Growth Promotion and Biocontrol Activities against Phytopathogenic Fungi. Front. Microbiol. 2017, 8, 1477. [Google Scholar] [CrossRef] [PubMed]
- Konstantinovas, C.; de Oliveira Mendes, T.A.; Vannier-Santos, M.A.; Lima-Santos, J. Modulation of Human Immune Response by Fungal Biocontrol Agents. Front. Microbiol. 2017, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Chalivendra, S.C.; DeRobertis, C.; Chang, P.-K.; Damann, K.E. Cyclopiazonic Acid Is a Pathogenicity Factor for Aspergillus flavus and a Promising Target for Screening Germplasm for Ear Rot Resistance. Mol. Plant-Microbe Interact. 2017, 30, 361–373. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalivendra, S.; DeRobertis, C.; Reyes Pineda, J.; Ham, J.H.; Damann, K. Rice Phyllosphere Bacillus Species and Their Secreted Metabolites Suppress Aspergillus flavus Growth and Aflatoxin Production In Vitro and in Maize Seeds. Toxins 2018, 10, 159. https://doi.org/10.3390/toxins10040159
Chalivendra S, DeRobertis C, Reyes Pineda J, Ham JH, Damann K. Rice Phyllosphere Bacillus Species and Their Secreted Metabolites Suppress Aspergillus flavus Growth and Aflatoxin Production In Vitro and in Maize Seeds. Toxins. 2018; 10(4):159. https://doi.org/10.3390/toxins10040159
Chicago/Turabian StyleChalivendra, Subbaiah, Catherine DeRobertis, Jorge Reyes Pineda, Jong Hyun Ham, and Kenneth Damann. 2018. "Rice Phyllosphere Bacillus Species and Their Secreted Metabolites Suppress Aspergillus flavus Growth and Aflatoxin Production In Vitro and in Maize Seeds" Toxins 10, no. 4: 159. https://doi.org/10.3390/toxins10040159




