Bacillus subtilis Type I antitoxin SR6 Promotes Degradation of Toxin yonT mRNA and Is Required to Prevent Toxic yoyJ Overexpression
Abstract
:1. Introduction
2. Results
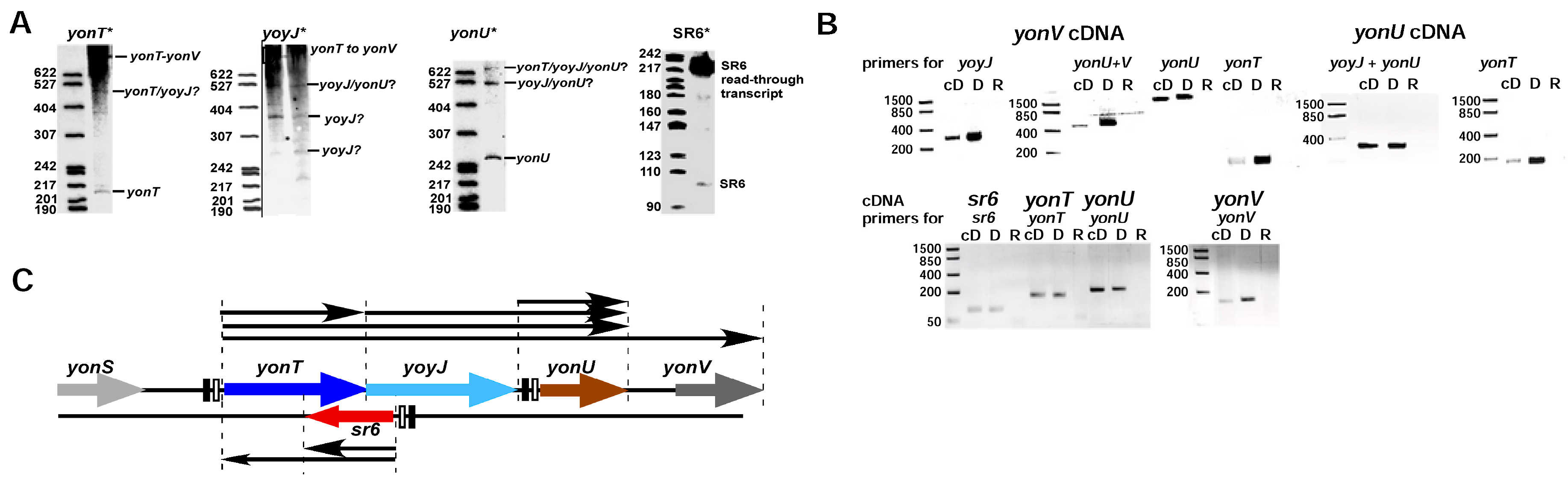
2.1. yonT Is Located in an Operon, and Transcripts of Different Length Originate from pyonT
2.2. Mapping of 5′ and 3′ Ends of yonT RNA, SR6 and yonU RNA
2.3. Expression Profiles of yonT mRNA, yoyJ mRNA, yonU mRNA and SR6 in Complex TY and Minimal CSE Medium
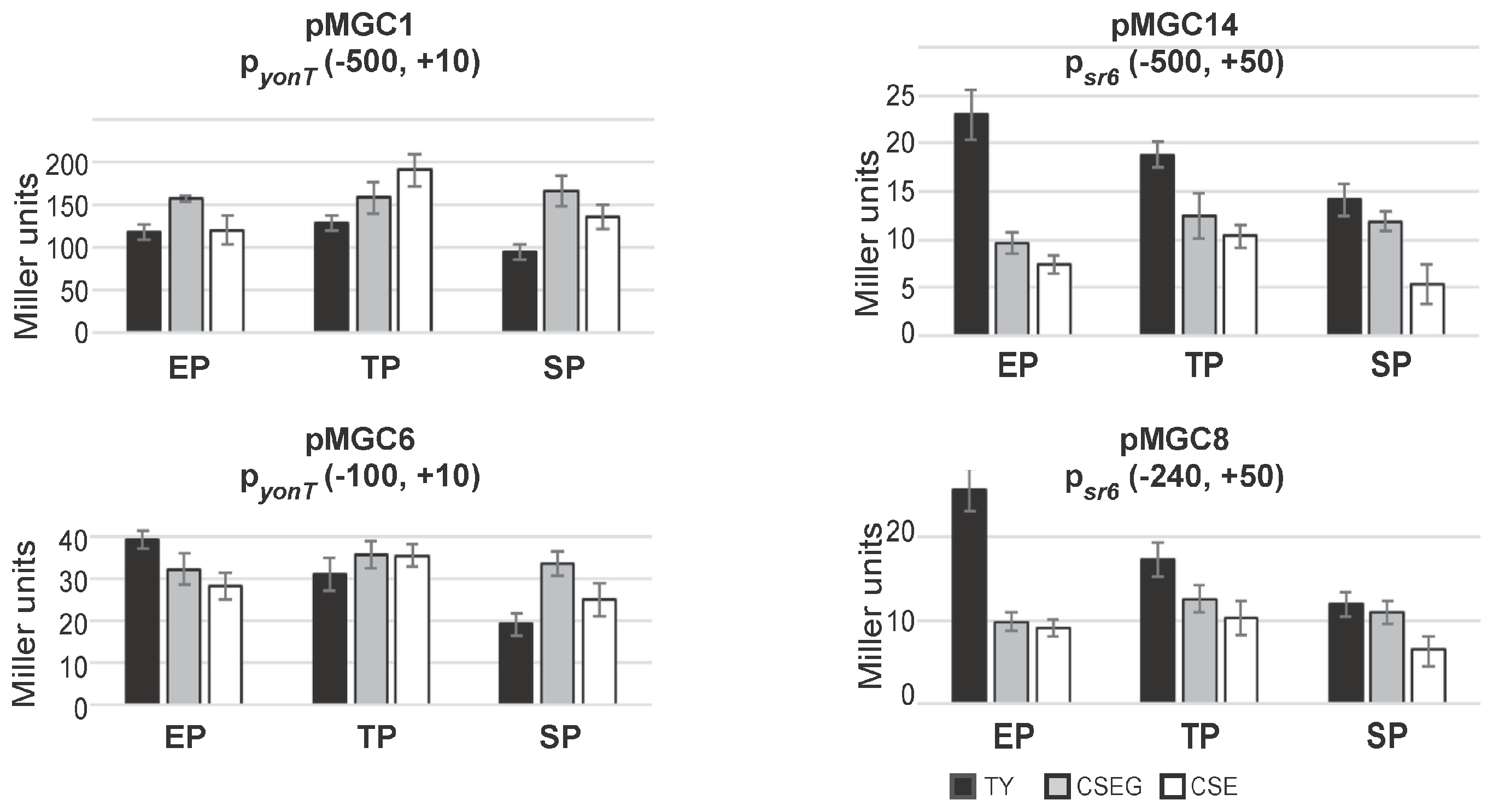
2.4. The sr6 Promoter Is Weaker than the yonT Promoter
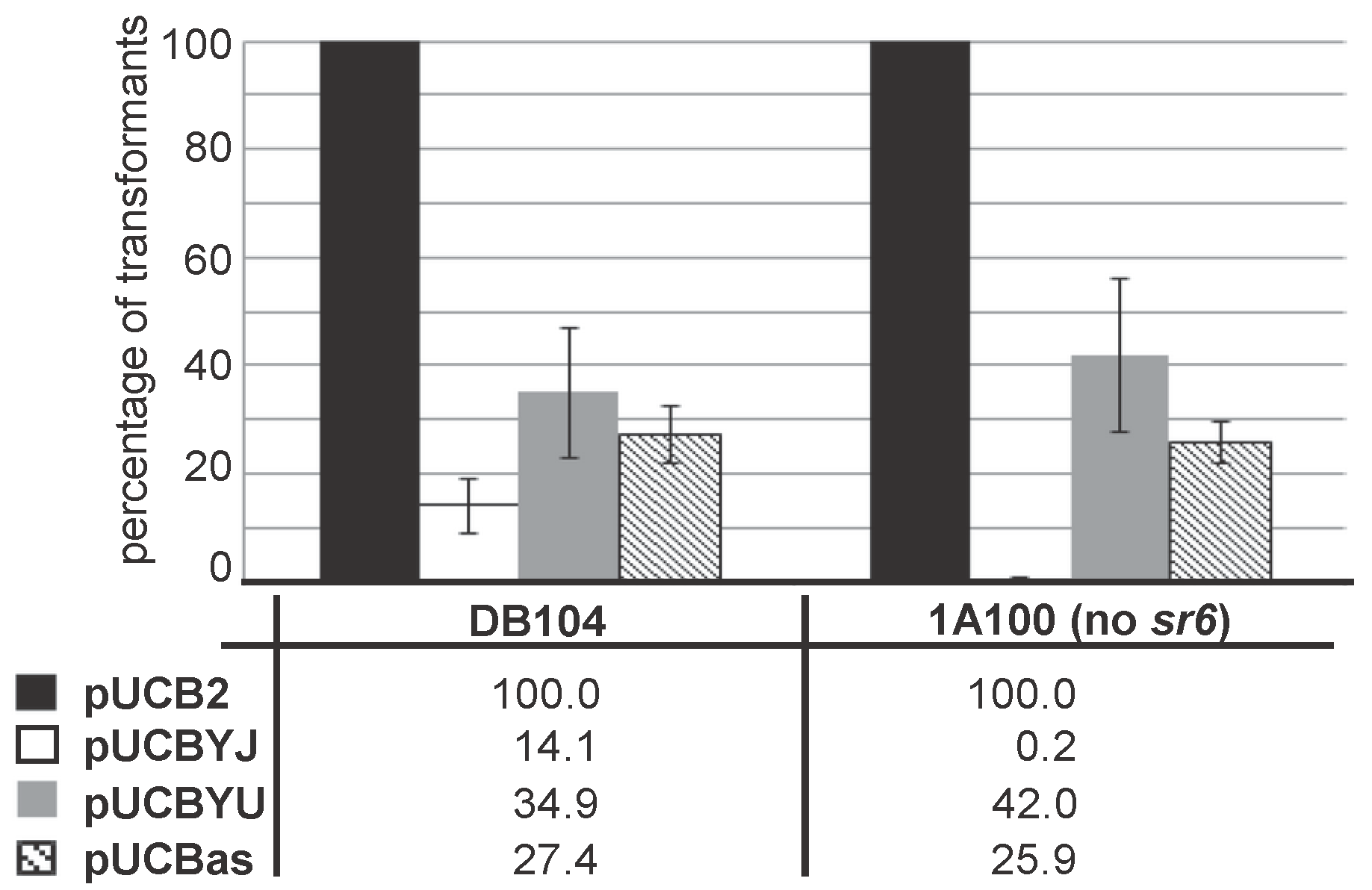
2.5. Overexpression of yoyJ Impairs the Establishment of Transformants in B. subtilis Indicating That It Is Also a Toxin
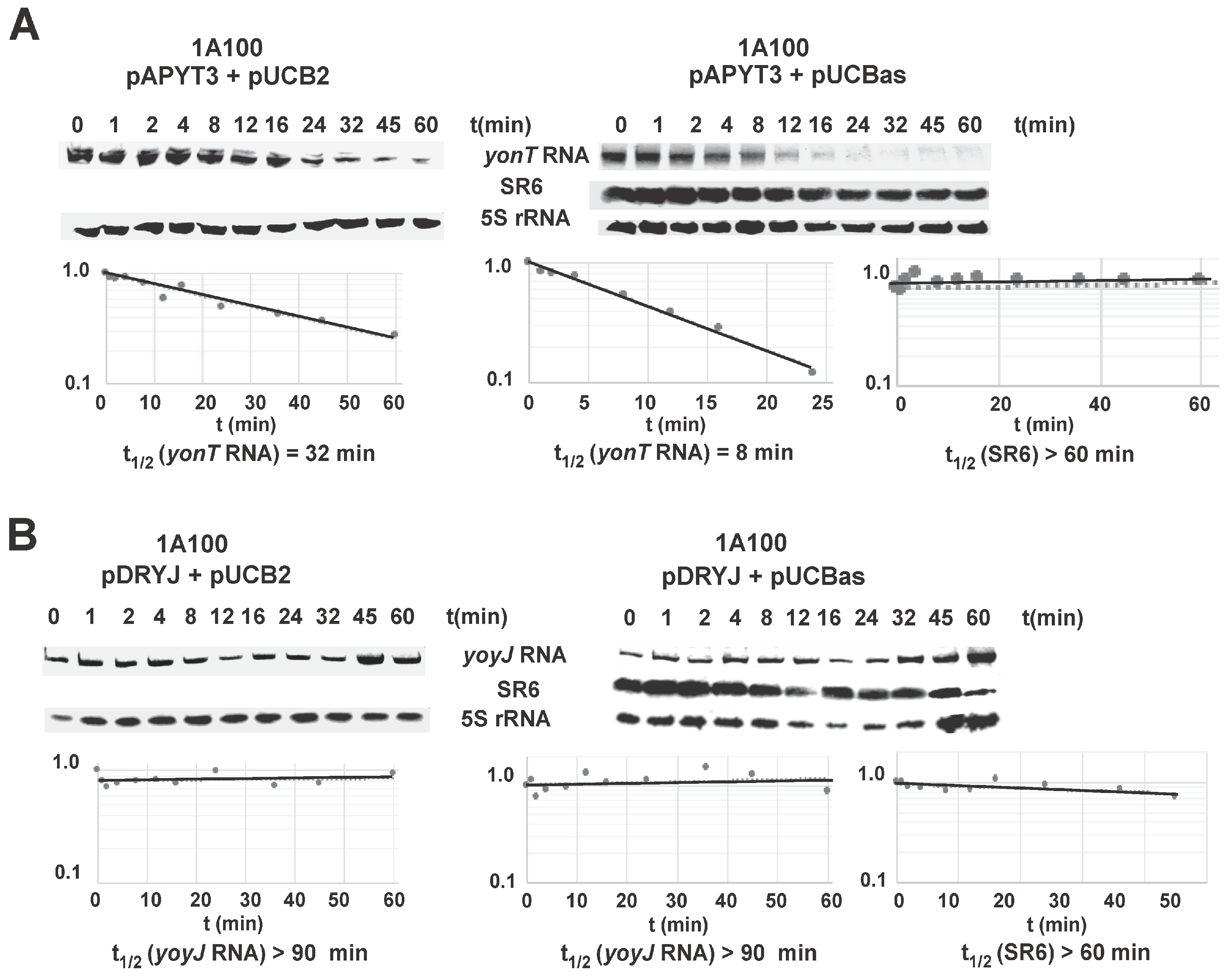
2.6. SR6 Is Significantly More Stable than yonT mRNA
2.7. SR6 Promotes Degradation of yonT RNA, but Does Not Affect the Stability of yoyJ RNA
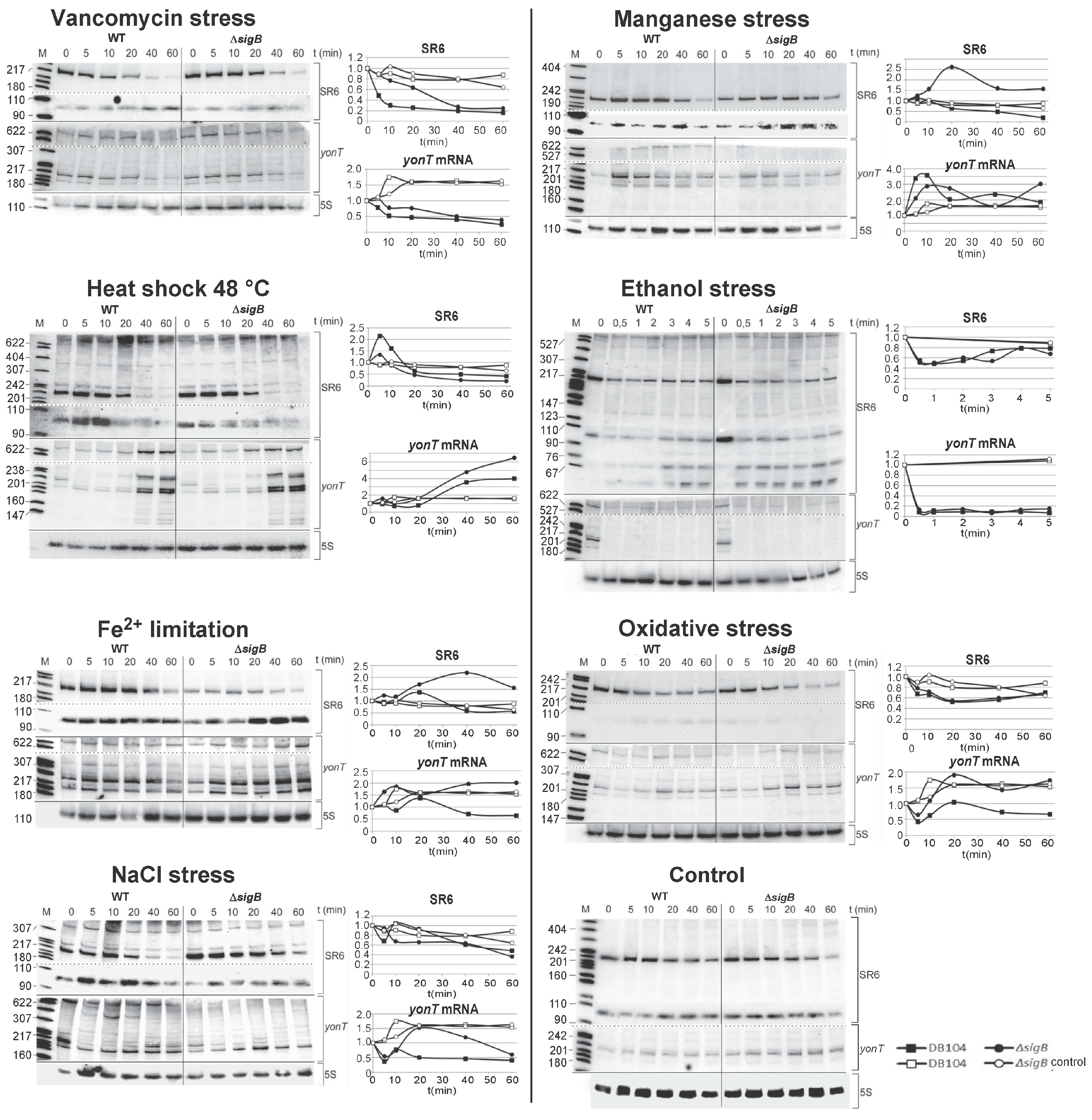
2.8. The Amounts of yonT RNA and SR6 Are Affected by Different Stress Conditions
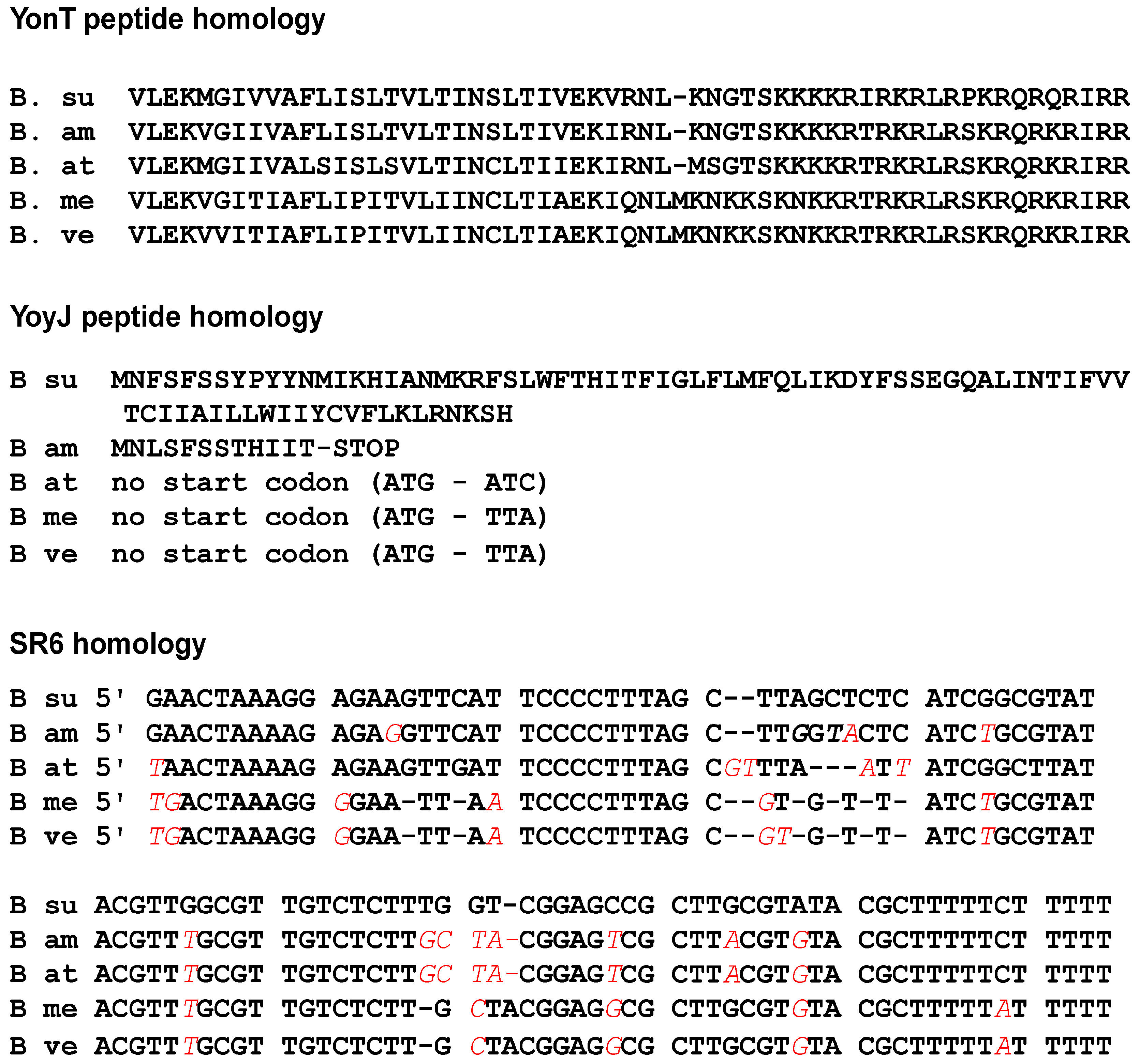
2.9. Homologues of the yonT, yoyJ and sr6 Are Present in Some Other Bacillus Species
3. Discussion
3.1. yonT/SR6 Is a Type I TA System That Encodes Two Toxins Regulated by Different Mechanisms
3.2. Antitoxin/toxin RNA Ratios and Half-Lives
3.3. yonT/SR6 Is a Multistress-Responsive TA System
4. Materials and Methods
4.1. Enzymes and DNA Manipulations
4.2. Strains, Media and Isolation of Chromosomal DNA
4.3. Primer Extension
4.4. Vector Construction
4.5. β-Galactosidase Assay
4.6. Determination of the Intracellular Concentrations of yonT mRNA, yoyJ mRNA and SR6
4.7. Preparation of total RNA, RNA Gel Electrophoresis and Northern Blotting
4.8. Stress Conditions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Brantl, S. Bacterial type I toxin-antitoxin systems. RNA Biol. 2012, 9, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Page, R.; Peti, W. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat. Chem. Biol. 2016, 12, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Aakre, C.D.; Phung, T.N.; Huang, D.; Laub, M.T. A bacterial toxin inhibits DNA replication elongation through a direct interaction with the β sliding clamp. Mol. Cell 2013, 52, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Brantl, S.; Jahn, N. sRNAs in bacterial type I and type III toxin/antitoxin systems. FEMS Microbiol. Rev. 2015, 39, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, K.; Wagner, E.G.H. RNA antitoxins. Curr. Opin. Microbiol. 2007, 10, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Fozo, E.M.; Makarova, K.S.; Shabalina, S.A.; Yutin, N.; Koonin, E.V.; Storz, G. Abundance of type I toxin-antitoxin systems in bacteria: Searches for new candidates and discovery of novel families. Nucleic Acids Res. 2010, 38, 3743–3759. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.E. The par toxin-antitoxin system from Enterococcus faecalis plasmid pAD1 and its chromosomal homologs. RNA Biol. 2012, 9, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.E. The type I toxin-antitoxin par locus from Enterococcus faecalis plasmid pAD1: RNA regulation by both cis- and trans-acting elements. Plasmid 2014, 78, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, K.; Christensen, S.K.; Løbner-Olesen, A. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 2005, 3, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Silvaggi, J.M.; Perkins, J.B.; Losick, R. Small untranslated RNA antitoxin in Bacillus subtilis. J. Bacteriol. 2005, 187, 6641–6650. [Google Scholar] [CrossRef] [PubMed]
- Durand, S.; Gilet, L.; Condon, C. The essential function of B. subtilis RNase III is to silence foreign toxic genes. PLoS Genet. 2012, 8, e1003181. [Google Scholar] [CrossRef] [PubMed]
- Arnion, H.; Korkut, D.N.; Masachis Gelo, S.; Chabas, S.; Reignier, J.; Iost, I.; Darfeuille, F. Mechanistic insights into type I toxin antitoxin systems in Helicobacter pylori: The importance of mRNA folding in controlling toxin expression. Nucleic Acids Res. 2017, 45, 4782–4795. [Google Scholar] [PubMed]
- Wagner, E.G.H.; Unoson, C. The toxin-antitoxin system tisB-istR1. Expression, regulation and biological role in persister phenotypes. RNA Biol. 2012, 9, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Fozo, E.M. New type I toxin-antitoxin families from “wild” and laboratory strains of E. coli. Ibs-Sib, ShoB-OhsC and Zor-Orz. RNA Biol. 2012, 9, 1504–1512. [Google Scholar] [PubMed]
- Jahn, N.; Preis, H.; Wiedemann, C.; Brantl, S. BsrG/SR4 from Bacillus subtilis—The first temperature-dependent type I toxin-antitoxin system. Mol. Microbiol. 2012, 83, 579–598. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M. Divergently overlapping cis-encoded antisense RNA regulating toxin-antitoxin systems from E. coli: Hok/sok, ldr/rdl, symE/symR. RNA Biol. 2012, 9, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Weel-Sneve, R.; Kristiansen, K.; Odsbu, I.; Dalhus, B.; Booth, J.; Rognes, T.; Skarstad, K.; Bjoras, M. Single transmembrane peptide DinQ modulates membrane-dependent activities. PLoS Genet. 2013, 8, e1003260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Quiroga, C.; Chen, Q.; McAnulty, M.J.; Benedik, M.J.; Wood, T.K.; Wang, X. RalR (a DNase) and RalA (a small RNA) form a type I toxin-antitoxin system in Escherichia coli. Nucleic Acids Res. 2014, 42, 6448–6462. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, B.A.; Hoekzema, M.; Aulbach, L.; Wagner, E.G.H. Two regulatory RNA elements affect TisB-dependent depolarization and persister formation. Mol. Microbiol. 2017, 103, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Durand, S.; Jahn, N.; Condon, C.; Brantl, S. Type I toxin-antitoxin systems in Bacillus subtilis. RNA Biol. 2012, 9, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Jahn, N.; Ring, C.; Maiwald, C.; Neubert, R.; Meißner, C.; Brantl, S. A multistress responsive type I toxin-antitoxin system: BsrE/SR5 from the B. subtilis chromosome. RNA Biol. 2016, 13, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Meißner, C.; Jahn, N.; Brantl, S. In vitro characterization of the type I toxin-antitoxin system bsrE/SR5 from Bacillus subtilis. J. Biol. Chem. 2016, 291, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Jahn, N.; Brantl, S. One antitoxin—two functions: SR4 controls toxin mRNA decay and translation. Nucleic Acids Res. 2013, 41, 9870–9880. [Google Scholar] [CrossRef] [PubMed]
- Jahn, N.; Brantl, S.; Strahl, H. Against the mainstream: The membrane associated type I toxin BsrG from Bacillus subtilis interferes with cell envelope biosynthesis without increasing membrane permeability. Mol. Microbiol. 2015, 98, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Brantl, S. The copR gene product of plasmid pIP501 acts as a transcriptional repressor at the essential repR promoter. Mol. Microbiol. 1994, 14, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Brantl, S. Regulatory mechanisms employed by cis-encoded antisense RNAs. Curr. Opin. Microbiol. 2007, 10, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Jahn, N.; Brantl, S. Heat shock induced refolding entails rapid degradation of bsrG toxin mRNA by RNases Y and J1. Microbiology 2016, 162, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Li, G.W.; Oh, E.; Weissman, J.S. The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature 2012, 484, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.; Jousselin, A.; Felden, B. A cis-antisense RNA acts in trans in Staphylococcus aureus to control translation of a human cytolytic peptide. Nat. Struct. Mol. Biol. 2011, 19, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domka, J.; Lee, J.; Bansal, T.; Wood, T.K. Temporal gene-expression in Escherichia coli K-12 biofilms. Environ. Microbiol. 2007, 9, 332–3346. [Google Scholar] [CrossRef] [PubMed]
- Brantl, S.; Behnke, D. Characterization of the minimal origin required for replication of the streptococcal plasmid pIP501 in Bacillus subtilis. Mol. Microbiol. 1992, 6, 3501–3510. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, N.; Chinali, A.; Gerth, U.; Brantl, S. The small untranslated RNA SR1 from the B. subtilis genome is involved in the regulation of arginine catabolism. Mol. Microbiol. 2006, 62, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Licht, A.; Preis, S.; Brantl, S. Implication of CcpN in the regulation of a novel untranslated RNA (SR1) in B. subtilis. Mol. Microbiol. 2005, 58, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Kawamura, F.; Doi, R.H. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J. Bacteriol. 1984, 160, 442–444. [Google Scholar] [PubMed]
- Preis, H.; Eckart, R.A.; Gudipati, R.K.; Brantl, S. CodY activates transcription of a small RNA in Bacillus subtilis. J. Bacteriol. 2009, 191, 5446–5457. [Google Scholar] [CrossRef] [PubMed]
- Van Ooij, C.; Losick, R. Subcellular localization of a small sporulation protein in Bacillus subtilis. J. Bacteriol. 2003, 185, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Gimpel, M.; Heidrich, N.; Mäder, U.; Krügel, H.; Brantl, S. A dual-function sRNA from Bacillus subtilis: SR1 acts as a peptide-encoding mRNA on the gapA operon. Mol. Microbiol. 2010, 76, 990–1009. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, N.; Brantl, S. Antisense-RNA mediated transcriptional attenuation: Importance of a U-turn loop structure in the target RNA of plasmid pIP501 for the efficient inhibition by the antisense RNA. J. Mol. Biol. 2003, 333, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Brantl, S.; Wagner, E.G.H. An unusually long-lived antisense RNA in plasmid copy number control: In vivo RNAs encoded by the streptococcal plasmid pIP501. J. Mol. Biol. 1996, 255, 275–288. [Google Scholar] [CrossRef] [PubMed]


| Strain | Genotype | Reference |
|---|---|---|
| E. coli DH5α | fhu2, Δ(argF-lacZ), U169, phoA, glnV44, Φ80, Δ(lacZ)M15, gyrA96, recA1, relA1, endA1, thi-1, hsdR17 | [34] |
| B. subtilis DB104 | His, nprR, 2 nprE18, ΔaprA3 | [35] |
| B. subtilis 1A100 | 168 ΔSPβ, trpC2 | Ohio strain collection |
| Plasmid | Description | Reference |
|---|---|---|
| pUCB2 | Shuttle vector of pUC19 and pUB110, NeoR, PhleoR | [38] |
| pDR111 | Vector for IPTG-inducible overexpression and integration into the B. subtilis amyE locus, SpecR | [37] |
| pAPNC213cat | Vector for IPTG-inducible overexpression and integration into the B. subtilis AP locus, CmR | [24] |
| pMG16 | Vector for integration of transcriptional lacZ fusions into B. subtilis amyE locus, SpecR | M. Gimpel, unpublished |
| pGAB1 | Vector for integration of translational lacZ fusions into B. subtilis amyE locus, KanR | S. Brantl, unpublished |
| pMGCR1 | pMG16 with yonT from −500 to +10 * | This study |
| pMGCR6 | pMG16 with yonT from −100 to +10 * | This study |
| pMGCR8 | pMG16 with sr6 from −240 to +50 * | This study |
| pMGCR14 | pMG16 with sr6 from −500 to +50 * | This study |
| pGAY1 | pGAB1 with yonT gene | This study |
| pGAY2 | pGAB1 with yoyJ gene | This study |
| pUCBas | pUCB2 with sr6 gene | This study |
| pUCBYT | pUCB2 with yonT gene | This study |
| pUCBYJ | pUCB2 with yoyJ gene | This study |
| pUCBYU | pUCB2 with yonU gene | This study |
| pAPYT3 | pAPNC213cat with yonT gene (M1Stop) | This study |
| pDRYJ | pDR111 with yoyJ gene | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reif, C.; Löser, C.; Brantl, S. Bacillus subtilis Type I antitoxin SR6 Promotes Degradation of Toxin yonT mRNA and Is Required to Prevent Toxic yoyJ Overexpression. Toxins 2018, 10, 74. https://doi.org/10.3390/toxins10020074
Reif C, Löser C, Brantl S. Bacillus subtilis Type I antitoxin SR6 Promotes Degradation of Toxin yonT mRNA and Is Required to Prevent Toxic yoyJ Overexpression. Toxins. 2018; 10(2):74. https://doi.org/10.3390/toxins10020074
Chicago/Turabian StyleReif, Celine, Charlotte Löser, and Sabine Brantl. 2018. "Bacillus subtilis Type I antitoxin SR6 Promotes Degradation of Toxin yonT mRNA and Is Required to Prevent Toxic yoyJ Overexpression" Toxins 10, no. 2: 74. https://doi.org/10.3390/toxins10020074




