The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.2.1. Types of Studies
2.2.2. Types of Participants
2.2.3. Types of Intervention
2.2.4. Comparators
2.2.5. Types of Outcome Measures
2.2.6. Limiters
2.3. Information Sources
2.4. Search
2.5. Study Selection
2.6. Data Collection Process
2.7. Data Items
2.8. Quality Assessment
2.9. Method of Analysis
3. Results
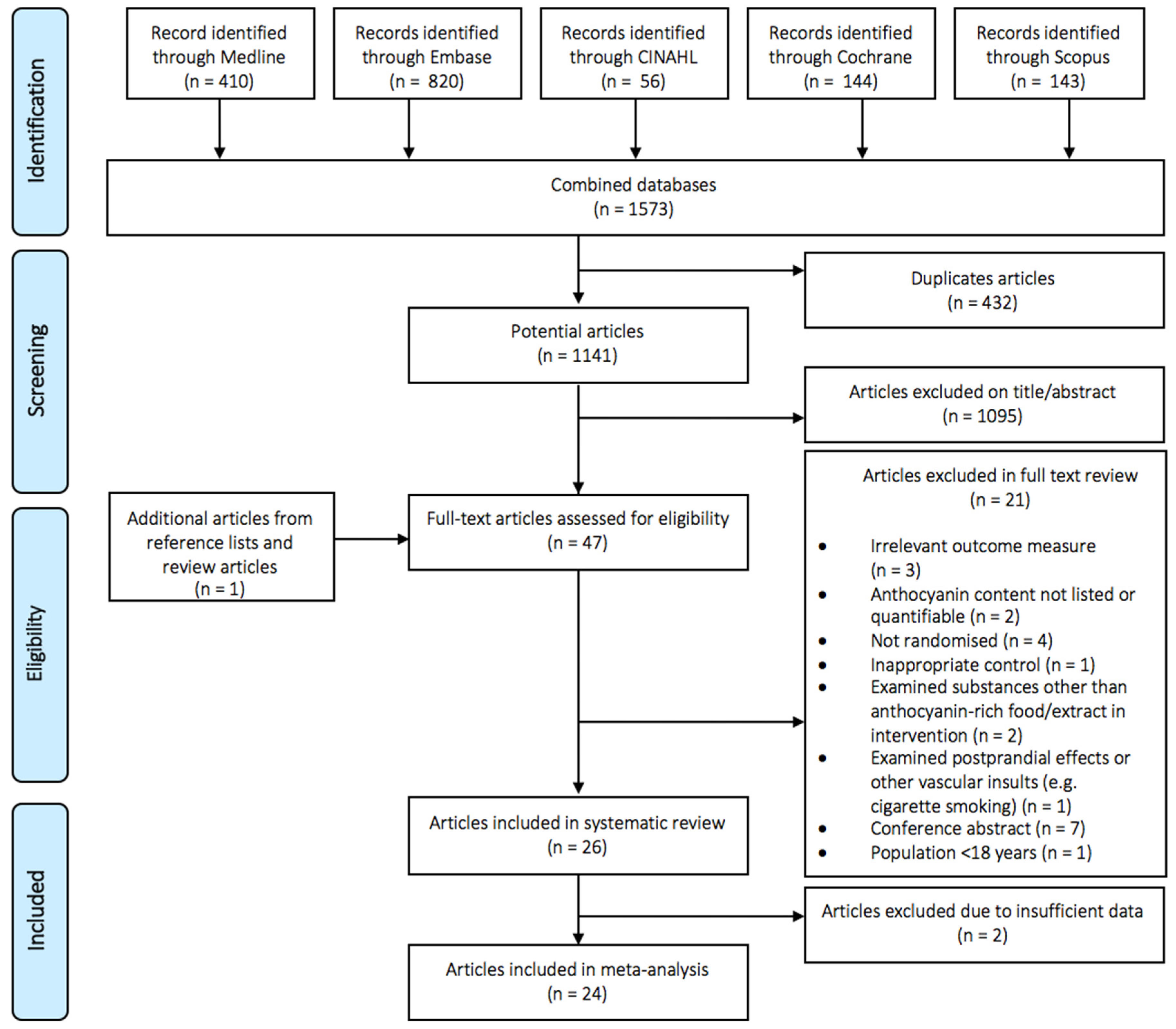
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Effects of Anthocyanins on Vascular Function
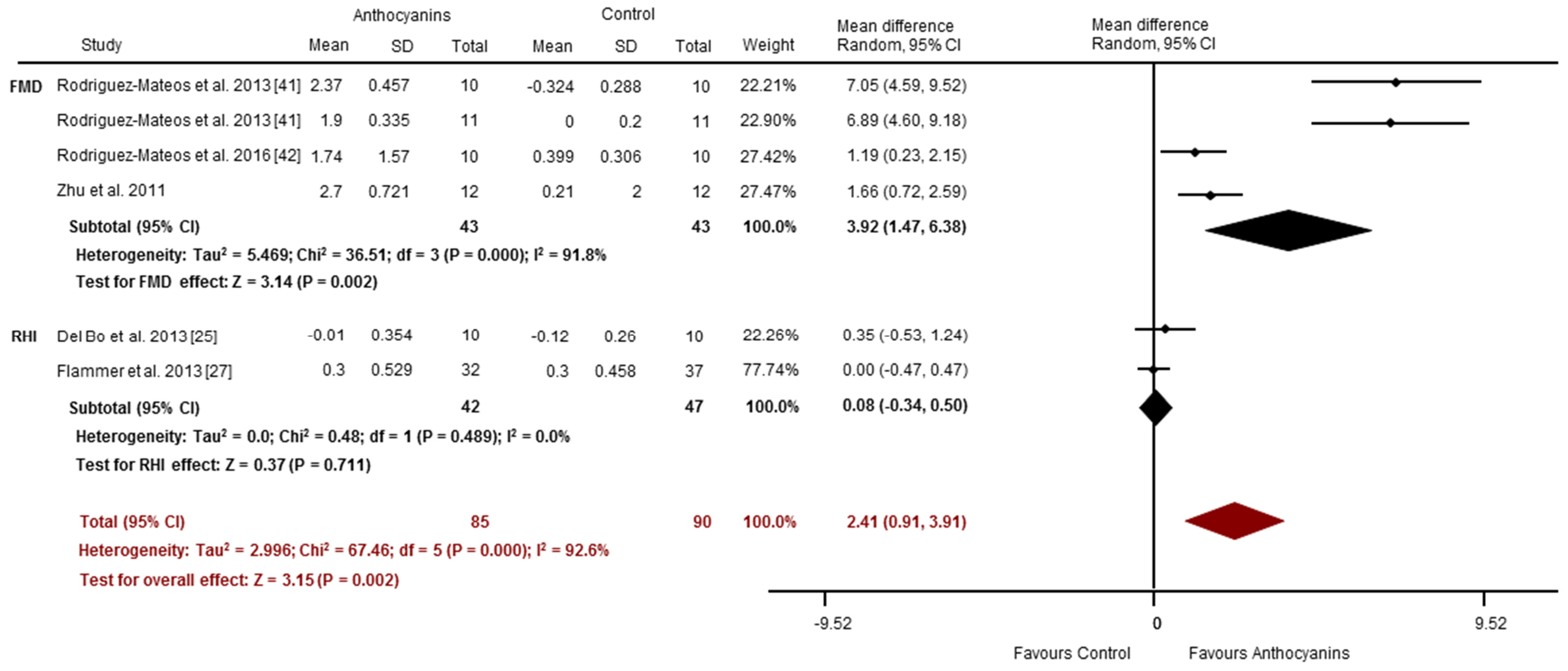
3.4.1. Acute Studies
3.4.2. Chronic Studies
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, M.; Barnes, J.N.; Devan, A.E.; Sugawara, J.; Tanaka, H. Comparison of augmentation index derived from multiple devices. Artery Res. 2011, 5, 112–114. [Google Scholar] [CrossRef]
- Arrebola-Moreno, A.L.; Laclaustra, M.; Kaski, J.C. Noninvasive assessment of endothelial function in clinical practice. Rev. Esp. Cardiol. 2012, 65, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Campbell, F.; Moore, T.; Jayson, M.I.V.; King, T.A.; Herrick, A.L. Laser doppler imaging—A new technique for quantifying microcirculatory flow in patients with primary raynaud's phenomenon and systemic sclerosis. Microvasc. Res. 1999, 57, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Goor, D.A.; Sheffy, J.; Schnall, R.P.; Arditti, A.; Caspi, A.; Bragdon, E.E.; Sheps, D.S. Peripheral arterial tonometry: A diagnostic method for detection of myocardial ischemia induced during mental stress tests: A pilot study. Clin. Cardiol. 2004, 27, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.A.; Seifalian, A.M.; Baker, D. A review of methods currently used for assessment of in vivo endothelial function. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Harten, J.M.; Booth, M.G.; Berry, C.; McConnachie, A.; Rankin, A.C.; Kinsella, J. The cardiovascular effects of normobaric hyperoxia in patients with heart rate fixed by permanent pacemaker. Anaesthesia 2010, 65, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Crouse, J.R.; Hsu, F.C.; Burke, G.L.; Herrington, D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The cardiovascular health study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic. Biol. Med. 2000, 29, 51–60. [Google Scholar] [CrossRef]
- Serraino, I.; Dugo, L.; Dugo, P.; Mondello, L.; Mazzon, E.; Dugo, G.; Caputi, A.P.; Cuzzocrea, S. Protective effects of cyanidin-3-o-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003, 73, 1097–1114. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; McCarthy, D.; Burton-Freeman, B.M. Effect of black currant anthocyanins on the activation of endothelial nitric oxide synthase (enos) in vitro in human endothelial cells. J. Agric. Food Chem. 2011, 59, 8616–8624. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-W.; Ikeda, K.; Yamori, Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension 2004, 44, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Anthocyanins in cardiovascular disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Xue, X. Effects of berries consumption on cardiovascular risk factors: A meta-analysis with trial sequential analysis of randomized controlled trials. Sci. Rep. 2016, 6, 23625. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Slavin, M.; Frankenfeld, C.L. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients 2016, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, J.; Saura-Calixto, F. Grape products and cardiovascular disease risk factors. Nutr. Res. Rev. 2008, 21, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Kraemer, H.C.; Kupfer, D.J. Size of treatment effects and their importance to clinical research and practice. Biol. Psychiatry 2006, 59, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Rosafio, G.; Arcoleo, G.; Mattina, A.; Canino, B.; Montana, M.; Verga, S.; Rini, G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am. J. Clin. Nutr. 2012, 95, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Riso, P.; Campolo, J.; Moller, P.; Loft, S.; Klimis-Zacas, D.; Brambilla, A.; Rizzolo, A.; Porrini, M. A single portion of blueberry (Vaccinium corymbosum L) improves protection against DNA damage but not vascular function in healthy male volunteers. Nutr. Res. 2013, 33, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Martin, E.A.; Gossl, M.; Widmer, R.J.; Lennon, R.J.; Sexton, J.A.; Loeffler, D.; Khosla, S.; Lerman, L.O.; Lerman, A. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. Eur. J. Nutr. 2013, 52, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Hong, S.J.; Lee, T.B.; Kwon, J.W.; Jeong, J.T.; Joo, H.J.; Park, J.H.; Ahn, C.M.; Yu, C.W.; Lim, D.S. Effects of black raspberry on lipid profiles and vascular endothelial function in patients with metabolic syndrome. Phytother. Res. 2014, 28, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Kim, S.; Hong, S.J.; Choi, S.C.; Choi, J.H.; Kim, J.H.; Park, C.Y.; Cho, J.Y.; Lee, T.B.; Kwon, J.W.; et al. Black raspberry extract increased circulating endothelial progenitor cells and improved arterial stiffness in patients with metabolic syndrome: A randomized controlled trial. J. Med. Food 2016, 19, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Hong, S.J.; Cho, J.Y.; Lee, T.B.; Kwon, J.W.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.S. Effects of rubus occidentalis extract on blood pressure in patients with prehypertension: Randomized, double-blinded, placebo-controlled clinical trial. Nutrition 2016, 32, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Alimbetov, D.; George, T.; Gordon, M.H.; Lovegrove, J.A. A randomised trial to investigate the effects of acute consumption of a blackcurrant juice drink on markers of vascular reactivity and bioavailability of anthocyanins in human subjects. Eur. J. Clin. Nutr. 2011, 65, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of montmorency tart cherry (Prunus cerasus L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ray, S.; Craigie, A.M.; Kennedy, G.; Hill, A.; Barton, K.L.; Broughton, J.; Belch, J.J.F. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: A randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radic. Biol. Med. 2014, 72, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Yu, H.M.; Zhang, C.; Yan, F.F.; Liu, Y.; Zhang, Y.; Zhang, M.; Zhao, Y.X. Treatment with rhubarb improves brachial artery endothelial function in patients with atherosclerosis: A randomized, double-blind, placebo-controlled clinical trial. Am. J. Chin. Med. 2007, 35, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Hamadeh, H.; Leung, W.C.; Russell, J.M.; Barker, M.E. Effects of pomegranate juice supplementation on pulse wave velocity and blood pressure in healthy young and middle-aged men and women. Plant Foods Hum. Nutr. 2012, 67, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Mathew, S.; Moore, C.T.; Russell, J.; Robinson, E.; Soumpasi, V.; Barker, M.E. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: A randomised controlled trial. Plant Foods Hum. Nutr. 2014, 69, 122–127. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Naissides, M.; Pal, S.; Mamo, J.C.; James, A.P.; Dhaliwal, S. The effect of chronic consumption of red wine polyphenols on vascular function in postmenopausal women. Eur. J. Clin. Nutr. 2006, 60, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Klimis-Zacas, D.; Del Bo, C.; Martini, D.; Campolo, J.; Vendrame, S.; Moller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Clin. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Boeres, A.; Weber, T.; Dos Santos, C.N.; Ventura, M.R.; Heiss, C. Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study. Mol. Nutr. Food Res. 2016, 60, 2130–2140. [Google Scholar] [CrossRef] [PubMed]
- Ruel, G.; Lapointe, A.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Evidence that cranberry juice may improve augmentation index in overweight men. Nutr. Res. 2013, 33, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tousoulis, D.; Kokkou, E.; Oikonomou, E.; Kollia, M.E.; Verveniotis, A.; Gouliopoulos, N.; Zisimos, K.; Plastiras, A.; Maniatis, K.; et al. Favorable effects of concord grape juice on endothelial function and arterial stiffness in healthy smokers. Am. J. Hypertens. 2014, 27, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, N.; Niv, E. Daily consumption of red grape cell powder in a dietary dose improves cardiovascular parameters: A double blind, placebo-controlled, randomized study. Int. J. Food Sci. Nutr. 2015, 66, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.E.; Myers, S.D.; Gault, M.L.; Cook, M.D. Beneficial physiological effects with blackcurrant intake in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mi, M.; Jin, T.; Ling, W. Purified anthocyanin supplementation improves endothelial function via no-cgmp activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef] [PubMed]
- Miguel, G.; Fontes, C.; Antunes, D.; Neves, A.; Martins, D. Anthocyanin concentration of “assaria” pomegranate fruits during different cold storage conditions. J. Biomed. Biotechnol. 2004, 2004, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Kim, H.L.; Kim, S.J.; Park, K.-S. Fruit quality, anthocyanin and total phenolic contents, and antioxidant activities of 45 blueberry cultivars grown in suwon, korea. J. Zhejiang Univ. Sci. B 2013, 14, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Bitsch, R.; Netzel, M.; Frank, T.; Strass, G.; Bitsch, I. Bioavailability and biokinetics of anthocyanins from red grape juice and red wine. J. Biomed. Biotechnol. 2004, 2004, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Gerhard-Herman, M.; Creager, M.A.; Hurley, S.; Mitra, D.; Ganz, P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J. Appl. Physiol. 2006, 101, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ohishi, M.; Takagi, T.; Terai, M.; Shiota, A.; Hayashi, N.; Rakugi, H.; Ogihara, T. Clinical usefulness and limitations of brachial-ankle pulse wave velocity in the evaluation of cardiovascular complications in hypertensive patients. Hypertens. Res. 2006, 29, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cassidy, A. Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomised controlled trials of flavonoid-rich food products. Mol. Nutr. Food Res. 2012, 56, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, T.; Schlinzig, T.; Krohn, K.; Meinertz, T.; Münzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001, 104, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.H.; Hornig, B.; Buser, P.T.; Olschewski, M.; Magosaki, N.; Pfisterer, M.; Nitzsche, E.U.; Solzbach, U.; Just, H. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Rein, M.; Ollilainen, V.; Vahermo, M.; Yli-Kauhaluoma, J.; Heinonen, M. Identification of novel pyranoanthocyanins in berry juices. Eur. Food Res. Technol. 2005, 220, 239–244. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Percival, S.S. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005, 218, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Peyrat-Maillard, M.; Cuvelier, M.; Berset, C. Antioxidant activity of phenolic compounds in 2,2′-azobis (2-amidinopropane) dihydrochloride (aaph)-induced oxidation: Synergistic and antagonistic effects. JAOCS 2003, 80, 1007–1012. [Google Scholar] [CrossRef]
- Zhou, B.; Jia, Z.-S.; Chen, Z.-H.; Yang, L.; Wu, L.-M.; Liu, Z.-L. Synergistic antioxidant effect of green tea polyphenols with α-tocopherol on free radical initiated peroxidation of linoleic acid in micelles. J. Chem. Soc. Perkin Trans. 2 2000, 785–791. [Google Scholar] [CrossRef]
- Warner, E.F.; Zhang, Q.; Raheem, K.S.; O’Hagan, D.; O’Connell, M.A.; Kay, C.D. Common phenolic metabolites of flavonoids, but not their unmetabolized precursors, reduce the secretion of vascular cellular adhesion molecules by human endothelial cells. J. Nutr. 2016, 146, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Grima, N.A.; Sarris, J. The effects of dietary and nutrient interventions on arterial stiffness: A systematic review. Am. J. Clin. Nutr. 2011, 93, 446. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Byrne, G.J. A systematic review of insomnia and complementary medicine. Sleep. Med. Rev. 2011, 15, 99–106. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Description |
|---|---|
| P—Population | Healthy or diseased adults |
| I—Intervention/variable of interest | Anthocyanin-rich foods or extracts or purified anthocyanins |
| C—Comparator | Control or placebo |
| O—Outcome | Vascular function as indicated by measures of arterial stiffness or vascular reactivity |
| S—Study design | Randomised controlled trials |
| Reference | Country | Study Design | No. Randomised | No. Completed | Gender | Mean Age ± SD (Years) | BMI Status, Mean BMI (kg/m2) | Health Status | Anthocyanin Source (Food/Extract) | Intake | Anthocyanin (mg/dose) | Control | Duration | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Del Bo et al. 2013 [25] | Italy | A, CR | 10 | 10 | M | 20.8 ± 1.6 | Healthy, 22.5 | Healthy | Homogenised blueberries | 300 g | 348 | Placebo jelly | 1 h | PAT-RHI |
| Flammer et al. 2013 [27] | USA | A, CR | 84 | 69 | M, F | Pl: 51 ± 15.1, I: 45 ± 17.5 | Overweight/obese, Pl: 37.2 ± 5.5 I: 27.7 ± 5.9 | Endothelial dysfunction, CVD risk, or CVD | Cranberry juice | 460 mL | 69 | Placebo drink | 1 h | PAT-RHI PAT-AI |
| Jin et al. 2011 [31] | UK | A, CR | 20 | 20 | M, F | 44.6 ± 13.3 | Healthy, 23.8 ± 2.5 | Healthy | Black currant juice | 250 mL | 50 | Placebo drink | 2 h | LDI |
| Keane et al. 2016 [33] | UK | A, CR | 15 | 15 | M | 31 ± 9 | 27.0 ± 3.8 | Hypertension | Cherry juice | 60 mL | 73.5 | Control drink | 1, 2, 3, 5, 8 h | AI (%) PWV (m/s) LDI DVP-SI (m/s) |
| Rodriguez-Mateos et al. 2013 [41] | UK | A, CR | 11 | 10 | M | 27 ± 1.3 | Healthy, 25 ± 0.8 | Healthy | Blueberry drink | 500 mL | 310, 517, or 724 | Placebo drink | 1, 2, 4, 6 h | FMD (%) PWV (m/s) AI (%) DVP-SI (m/s) |
| Rodriguez-Mateos et al. 2013 [41] | UK | A, CR | 11 | 11 | M | 27 ± 1.0 | Healthy, 22 ± 0.9 | Healthy | Blueberry drink | 500 mL | 129, 258, 310, 517, or 724 | Placebo drink | 1 h | FMD (%) |
| Rodriguez-Mateos et al. 2016 [42] | UK | A, CR | 10 | 10 | M | 24 ± 2 | Healthy, 24 ± 2 | Healthy | Cranberry juice | 450 mL | 7, 16, 23, 26, 32 | Control drink | 1, 2, 4, 6, 8 h | FMD (%) |
| Zhu et al. 2011 [48] | China | A, CR | 12 | 12 | M, F | NR | NR | HC | Purified anthocyanins | 320 mg | 320 | Placebo capsules | 1 h | FMD (%) |
| Reference | Country | Study Design | No. Randomised | No. Completed | Gender | Mean Age ± SD (Years) | BMI Status, Mean BMI (kg/m2) | Health Status | Anthocyanin Source (Food/Extract) | Intake | Anthocyanin (mg/day) | Control | Duration | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barona et al. 2012 [23] | USA | C, CR | 25 | 24 | M | 51.3 ± 9.6 | Obese, 31.6 | Metabolic syndrome | Grape powder | 46 g | 35 | Placebo capsule | 30 day | FMD (%) |
| Buscemi et al. 2012 [24] | Italy | C, CR | 21 | 19 | M, F | 48 ± 13 | Obese, 32.1 ± 4.9 | CVD risk factors | Red orange juice | 500 mL | 36 | Placebo drink | 7 day | FMD (%) |
| Dohadwala et al. 2011 [26] | USA | C, CR | 47 | 44 | M, F | Pl (1st): 63 ± 9 I (1st): 61 ± 11 | Overweight, NR | CHD | Cranberry juice | 480 mL | 94 | Placebo drink | 4 week | FMD (%) PAT-RHI crPWV (m/s) cfPWV (m/s) |
| Flammer et al. 2013 [27] | USA | C, P | 84 | 69 | M, F | Pl: 51 ± 15.1 I: 44.8 ± 17.5 | Overweight/obese, Pl: 37.2 ± 5.5 I: 27.7 ± 5.9 | Endothelial dysfunction, CVD risk factors, CVD | Cranberry juice | 460 mL | 69 | Placebo drink | 4 month | PAT-AI PAT-RHI |
| Jeong et al. 2014 [28] | Korea | C, P | 77 | 77 | M, F | Pl: 60.1 ± 9.5 I: 58.0 ± 9.2 | Overweight, Pl: 25.1 ± 4.0 I: 26.3 ± 4.3 | Metabolic syndrome | Black raspberry powder | 750 mg | 26 a | Placebo capsule | 12 week | PWV (m/s) FMD (%) |
| Jeong et al. 2016 [29] | Korea | C, P | 51 | 50 | M, F | Pl: 60.7 ± 10.4 I: 56.4 ± 9.2 | Healthy, Pl: 24.7 ± 3.9 I: 25.9 ± 4.6 | Metabolic syndrome | Black raspberry powder | 750 mg | 26 a | Placebo capsule | 12 week | AI (%) |
| Jeong et al. 2016 [30] | Korea | C, P | 45 | 45 | M, F | Pl: 55.9 ± 12.8 MD: 60.2 ± 11.2 HD: 55.5 ± 12.3 | Healthy, Pl: 25.8 ± 3.0 MD: 24.5 ± 2.9 HD: 23.5 ± 2.4 | Pre-hypertension | MD & HD black raspberry powder | MD: 1500 mgHD: 2500 mg | MD: 52HD: 87 a | Placebo capsule | 8 week | AI (%) AI p75 (%) baPWV- (m/s) |
| Johnson et al. 2015 [32] | USA | C, P | 48 | 40 | PF | Pl: 53.7 ± 4.8 I: 59.7 ± 4.6 | NR, NR | Pre- and stage 1- hypertension | Blueberry powder | 22 g | 470 | Control powder | 8 week | cfPWV (cm/s) baPWV (cm/s) |
| Khan et al. 2014 [34] | UK | C, P | 66 | 64 | M, F | Pl: 51 ± 8 LD: 55 ± 10 HD: 51 ± 11 | Overweight, Pl: 28.9 ± 6.5 LD: 28.4 ± 5.4 HD: 29.2 ± 6.9 | Healthy | LD or HD black current juice | 250 mL | LD: 10 HD: 36 | Flavoured water | 6 week | FMD (%) |
| Liu et al. 2007 [35] | China | C, P | 103 | 83 | M, F | Pl: 59.8 ± 7.8 I: 58.5 ± 8.6 | Healthy, Pl: 23.8 ± 3.6, I: 24.8 ± 3.4 | Healthy | Rhubarb capsule | 50 mg | 12 | Placebo capsule | 6 month | FMD (%) |
| Lynn et al. 2012 [36] | UK | C, P | 51 | 48 | M, F | C: 36.1 ± 0.92 I: 39.0 ± 1.24 | Healthy, C: 25 ± 1.1 I: 25 ± 1.3 | Healthy | Pomegranate juice | 330 mL | 127 b | Lemon drink | 4 week | bkPWV (m/s) |
| Lynn et al. 2014 [37] | UK | C, P | 47 | 46 | M, F | C: 37.2 ± 5.8 I: 38.3 ± 6.2 | Healthy, C: 24.6 I: 23.5 | Healthy | Cherry juice concentrate | 30 mL | 274 | Lemon drink | 6 week | bkPWV (m/s) |
| McAnulty et al. 2014 [38] | USA | C, P | 25 | NR | M, F | Pl: 39.9 ± 13.4 I: 46.2 ± 11.9 | Healthy, Pl: 24.2 ± 3.4 I: 27.8 ± 5.5 | Pre-hypertension | Blueberry powder | 38 g | 625 c | Placebo powder | 6 week | cfPWV (m/s) AI (%) |
| Naissides et al. 2006 [39] | Australia | C, P | 45 | 43 | PF | Pl: 59.3 ± 1.4 I: 57.6 ± 1.3 | Overweight, Pl: 26.7 ± 1.2 I: 26.3 ± 0.9 | Healthy | Dealcoholised red wine | 400 mL | 283 d | Water | 6 week | AI (%) |
| Riso et al. 2013 [40] | Italy | C, CR | 20 | 18 | M | 47.8 ± 9.7 | Healthy, 24.8 ± 2.6 | Healthy (with 1 CVD risk factor) | Blueberry powder | 25 g | 375 | Placebo drink | 6 week | PAT-RHI PAT-AI |
| Ruel et al. 2013 [43] | Canada | C, CR | 35 | 35 | M | 45 ± 10 | Overweight 28.3 ± 2.4 | Healthy | Cranberry juice | 500 mL | 21 | Placebo drink | 4 week | AI (%) Salbutamol AI (%) |
| Siasos et al. 2014 [44] | Greece | C, CR | 26 | NR | M, F | 26 ± 5 | NR, NR | Healthy, smokers | Concord grape juice | 240 mL | 71 | Grape-fruit juice | 7, 14 day | FMD (%) PWV (m/s) |
| Stull et al. 2015 [45] | USA | C, P | 46 | 44 | M, F | PI: 59 ± 2 I: 55 ± 2 | Obese, Pl: 36.0 ± 1.1 I: 35.2 ± 0.8 | Metabolic syndrome | Blueberry powder | 45 g | 581 | Placebo drink | 6 week | PAT-RHI |
| Vaisman & Niv 2015 [46] | Israel | C, P | 50 | 45 | M, F | Pl: 56.4 ± 7.0 MD: 58.5 ± 7.9 HD: 57.6 ± 7.2 | Overweight, PL:26.3 ± 4.1 MD: 29.7 ± 3.0 HD: 26.4 ± 3.0 | Pre- and stage 1- hypertension | Red grape powder | MD: 200 mg HD: 400 mg | MD: 1.34 HD: 2.68 | Placebo | 12 week | FMD (%) |
| Willems, et al. 2015 [47] | UK | C, CR | 13 | 10 | M, F | 38 ± 8 | NR, NR | Healthy | Black currant powder | 6 g | 139 | Placebo drink | 7 day | TPR |
| Zhu et al. 2011 [48] | China | C, P | 150 | 146 | M, F | Pl: 70.1 ± 9.8 I: 68.9 ± 8.8 | Overweight, Pl: 26.8 ± 2 I: 26.4 ± 2.1 | HC | Purified anthocyanins | 320 mg | 320 | Placebo capsule | 12 week | FMD (%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 908. https://doi.org/10.3390/nu9080908
Fairlie-Jones L, Davison K, Fromentin E, Hill AM. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients. 2017; 9(8):908. https://doi.org/10.3390/nu9080908
Chicago/Turabian StyleFairlie-Jones, Lucy, Kade Davison, Emilie Fromentin, and Alison M. Hill. 2017. "The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials" Nutrients 9, no. 8: 908. https://doi.org/10.3390/nu9080908
APA StyleFairlie-Jones, L., Davison, K., Fromentin, E., & Hill, A. M. (2017). The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients, 9(8), 908. https://doi.org/10.3390/nu9080908





