Resveratrol in Hepatitis C Patients Treated with Pegylated-Interferon-α-2b and Ribavirin Reduces Sleep Disturbance
Abstract
:1. Background
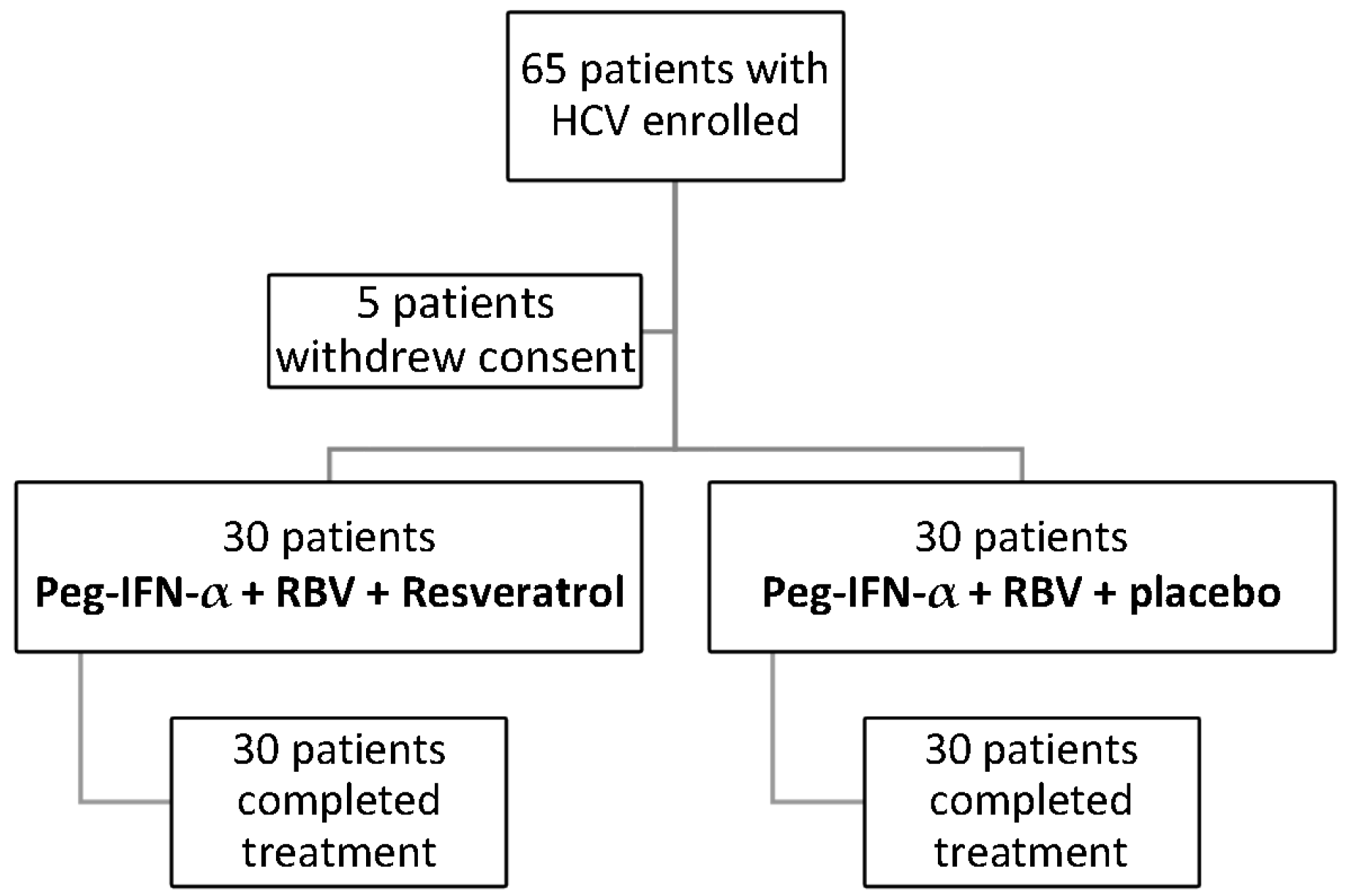
2. Patients and Methods
2.1. Serum Analysis
2.2. Histological Grading Assessment
2.3. General Health Questionnaire (GHQ)
2.4. Profile of Mood States (POMS)
2.5. Pittsburgh Sleep Quality Inventory (PSQI)
2.6. Epworth Sleepiness Scale (ESS)
2.7. Efficacy and Safety Assessment
2.8. Statistical Analysis
3. Results
3.1. Effect of Resveratrol on Transaminases, Viremia and HAI
3.2. Comparison between Patients According to Response Treatment
4. Adverse Events
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, B.D.; Morgan, R.L.; Beckett, G.A.; Falck-Ytter, Y.; Holtzman, D.; Teo, C.-G.; Alter, M. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2012, 61, 1–32. [Google Scholar]
- Hajarizadeh, B.; Grebely, J.; Dore, G.J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Ardiri, A.; Proiti, M.; Rigano, G.; Frazzetto, E.; Demma, S.; Rapisarda, V. Chronic hepatitis C: This and the new era of treatment. World J. Hepatol. 2016, 8, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Catania, V.E.; Francaviglia, A.; Malaguarnera, M.; Drago, F.; Motta, M.; Latteri, S. Lipoprotein (a) in patients with hepatocellular carcinoma and portal vein thrombosis. Aging Clin. Exp. Res. 2017, 29 (Suppl. 1), 185–190. [Google Scholar] [CrossRef] [PubMed]
- Hilsabeck, R.C.; Hassanein, T.I.; Carlson, M.D.; Ziegler, E.A.; Perry, W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J. Int. Neuropsychol. Soc. 2003, 9, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Motta, M.; Vacante, M.; Malaguarnera, G.; Caraci, F.; Nunnari, G.; Bertino, G. Silybin-vitamin E-phospholipids complex reduces liver fibrosis in patients with chronic hepatitis C treated with pegylated interferon α and ribavirin. Am. J. Transl. Res. 2015, 7, 2510–2518. [Google Scholar] [PubMed]
- Calland, N.; Sahuc, M.-E.; Belouzard, S.; Pène, V.; Bonnafous, P.; Mesalam, A.A.; Lambert, O. Polyphenols Inhibit Hepatitis C Virus Entry by a New Mechanism of Action. J. Virol. 2015, 89, 10053–10063. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Heebøll, S.; Thomsen, K.L.; Pedersen, S.B.; Vilstrup, H.; George, J.; Grønbæk, H. Effects of resveratrol in experimental and clinical non-alcoholic fatty liver disease. World J. Hepatol. 2014, 6, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta 2015, 1852, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Geny, B. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.T.; Rodgers, J.T.; Arlow, D.H.; Vazquez, F.; Mootha, V.K.; Puigserver, P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 2007, 450, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.E.; Laskus, T.; Radkowski, M.; Wilkinson, J.; Balan, V.; Douglas, D.D.; Harrison, M.E.; Mulligan, D.C.; Olden, K.; Adair, D.; et al. Detection of hepatitis C virus sequences in brain tissue obtained in recurrent hepatitis C after liver transplantation. Liver Transpl. 2002, 8, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Bertino, G.; Chisari, G.; Motta, M.; Vecchio, M.; Vacante, M.; Caraci, F.; Greco, C.; Drago, F.; Nunnari, G.; et al. Silybin supplementation during HCV therapy with pegylated interferon-α plus ribavirin reduces depression and anxiety and increases work ability. BMC Psychiatry 2016, 16, 398. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Pennisi, M.; Gagliano, C.; Vacante, M.; Malaguarnera, M.; Salomone, S.; Drago, F.; Bertino, G.; Caraci, F.; Nunnari, G.; et al. Acetyl-l-carnitine supplementation during HCV therapy with pegylated interferon-α 2b plus ribavirin: Effect on work performance; a randomized clinical trial. Hepat. Mon. 2014, 14, e11608. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, M.; Gracies, J.-M.; Panza, F.; Fortunato, F.; Vitaliti, G.; Malaguarnera, G.; Santamato, A. Change in Coefficient of Fatigability Following Rapid, Repetitive Movement Training in Post-Stroke Spastic Paresis: A Prospective Open-Label Observational Study. J. Stroke Cerebrovasc. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M. Acetyl-l-carnitine in hepatic encephalopathy. Metab. Brain Dis. 2013, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Kim, M.K. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Sockalingam, S.; Abbey, S.E.; Alosaimi, F.; Novak, M. A review of sleep disturbance in hepatitis C. J. Clin. Gastroenterol. 2010, 44, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.A.; Conrad, S.; Garrett, L.; Battistutta, D.; Cooksley, W.G.; Dunne, M.P.; Macdonald, G.A. Symptom prevalence and clustering of symptoms in people living with chronic hepatitis C infection. J. Pain Symptom Manag. 2006, 31, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Cacoub, P.; Ratziu, V.; Myers, R.P.; Dezailles, M.H.; Mercadier, A.; Ghillani, P.; Charlotte, F.; Piette, J.C.; Moussalli, J.; et al. Fatigue in patients with chronic hepatitis C. J. Viral Hepat. 2002, 9, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lotrich, F.E.; Ferrell, R.E.; Rabinovitz, M.; Pollock, B.G. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biol. Psychiatry 2009, 65, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Vacante, M.; Drago, F.; Bertino, G.; Motta, M.; Giordano, M.; Malaguarnera, M. Endozepine-4 levels are increased in hepatic coma. World J. Gastroenterol. 2015, 21, 9103–9110. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Declaration of Helsinki. Recommendations guiding doctors in clinical research. Adopted by the World Medical Association in 1964. Wis. Med. J. 1967, 66, 25–26.
- Stuyver, L.; Wyseur, A.; van Arnhem, W.; Hernandez, F.; Maertens, G. Second-generation line probe assay for hepatitis C virus genotyping. J. Clin. Microbiol. 1996, 34, 2259–2266. [Google Scholar] [PubMed]
- Simmonds, P.; Alberti, A.; Alter, H.J.; Bonino, F.; Bradley, D.W.; Brechot, C.; Brouwer, J.T.; Chan, S.W.; Chayama, K.; Chen, D.S.; et al. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology 1994, 19, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Latteri, S.; Malaguarnera, G.; Mannino, M.; Pesce, A.; Currò, G.; Tamburrini, S.; Scuderi, M. Ultrasound as point of care in management of polytrauma and its complication. J. Ultrasound 2017, 20, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D. The Recognition of Psychiatric Illness by Non-Psychiatrists. Aust. N. Z. J. Psychiatry 1984, 18, 128–133. [Google Scholar] [CrossRef] [PubMed]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Manual for the Profile of Mood States; Educational and Industrial Testing Services: San Diego, CA, USA, 1971. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.D.; Hilsabeck, R.C.; Barakat, F.; Perry, W. Role of Sleep Disturbance in Chronic Hepatitis C Infection. Curr. Hepat. Rep. 2010, 9, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Chisari, G.; Stagni, E.; Rampello, L.; Malaguarnera, M.; Chisari, C.G. The ocular surface in Patients video display terminal (VDT). Acta Med. Mediterr. 2013, 3, 369–373. [Google Scholar]
- Kallman, J.; O’Neil, M.M.; Larive, B.; Boparai, N.; Calabrese, L.; Younossi, Z.M. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig. Dis. Sci. 2007, 52, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Russo, C.; Gargante, M.P.; Giordano, M.; Bertino, G.; Volti, G.L. Rosuvastatin reduces nonalcoholic fatty liver disease in patients with chronic hepatitis C treated with α-interferon and ribavirin. Hepat. Mon. 2011, 11, 92–98. [Google Scholar] [PubMed]
- Malaguarnera, M.; Vacante, M.; Condorelli, G.; Leggio, F.; Di Rosa, M.; Motta, M.; Malaguarnera, G.; Alessandria, I.; Rampello, L.; Chisari, G. Probiotics and prebiotics in the management of constipation in the elderly. Acta Med. Mediterr. 2013, 29, 791–798. [Google Scholar]
- Malaguarnera, M.; Cristaldi, E.; Romano, G.; Malaguarnera, L. Autoimmunity in the elderly: Implications for cancer. J. Cancer Res. Ther. 2012, 8, 520–527. [Google Scholar] [PubMed]
- Asara, Y.; Melis, A.; De Luca, L.M.; Bozzo, C.; Castiglia, P.; Chessa, G.; Marchal, J.A. Influence of metals on rhinosinusal polyposis in Sardinian population (Italy). Environ. Sci. Pollut. Res. Int. 2016, 23, 21726–21732. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Khaoustov, V.I.; Xie, Q.; Pan, T.; Le, W.; Yoffe, B. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J. Hepatol. 2005, 42, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Z.; You, W.; Zhang, X.; Li, S.; Barish, P.A.; Ogle, W.O. Antidepressant-like effect of trans-resveratrol: Involvement of serotonin and noradrenaline system. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2010, 20, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Opp, M.R. Cytokines and sleep. Sleep Med. Rev. 2005, 9, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Bertino, G.; Greco, C.; Gagliano, C.; Motta, M.; Chisari, G.; Malaguarnera, M. Job performance in chronic hepatitis C virus patients treated with pegylated interferon-α2b plus ribavirin: An observational study. Transl. Med. Commun. 2017, 2, 2. [Google Scholar] [CrossRef]
- Imeri, L.; Opp, M.R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009, 10, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Chisari, G.; Rampello, L.; Chisari, E.; Catania, V.; Greco, C.; Stagno, E.; Chisari, C. Microbiology synergism between tear substitutes and symbiotic treatment of patients with irritable bowel syndrome. Acta Med. Mediterr. 2016, 32, 865–870. [Google Scholar]
- Beloborodova, N.; Bairamov, I.; Olenin, A.; Shubina, V.; Teplova, V.; Fedotcheva, N. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J. Biomed. Sci. 2012, 19, 89. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Group A 30 pt (Peg-IFN-α + RBV + Placebo) | Group B 30 pt (Peg-IFN-α + RBV + Resveratrol) | p-Value |
|---|---|---|---|
| Male | 18 | 17 | NS |
| Female | 12 | 13 | NS |
| Route of transmission of HCV (No. of patients) | |||
| Blood transfusion | 16 | 12 | NS |
| Intravenous drug abuse | 3 | 5 | NS |
| Occupational | 1 | 3 | NS |
| Unknown | 11 | 11 | NS |
| HCV genotype | |||
| 1a | 1 | 2 | NS |
| 1b | 23 | 23 | NS |
| 2a | 3 | 3 | NS |
| 3a | 1 | 2 | NS |
| Mean age (years) | 46.8 ± 4.4 | 46.4 ± 4.1 | NS |
| HCV exposure time (years) | 3.87 ± 3.4 | 3.91 ± 3.8 | NS |
| Body Mass Index (BMI) (kg/m2) | 25.8 ± 3.4 | 25.4 ± 3.6 | NS |
| Plasma glucose (mmol/L) (normal 3.9–6.4) | 5.4 ± 0.74 | 6.3 ± 0.71 | NS |
| Aspartate Aminotrasferase (AST) (IU/L) (normal 15–50) | 143.4 ± 33.2 | 162.4 ± 32.1 | NS |
| Alanine Aminotrasferase (ALT) (IU/L) (normal 15–50) | 155.4 ± 36.1 | 162.4 ± 32.1 | NS |
| Viremia (106 copies/mL) | 3.4 ± 2.1 | 3.7 ± 2.8 | NS |
| C-Reactive Protein (CRP) (mg/dL) (normal < 1.0) | 6.4 ± 0.9 | 6.5 ± 0.8 | NS |
| HAI | 10.4 ± 2.8 | 10.5 ± 2.2 | NS |
| Group A Peg-IFN α + RBV + Placebo (n = 30) | Group B Peg-IFN α + RBV + Resveratrol (n = 30) | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Before Treatment | After 6 Months | After 12 Months | Follow-Up | Before Treatment | After 6 Months | After 12 Months | Follow-Up |
| Aspartate Aminotransferase (AST) (IU/L) | 143.4 ± 33.2 | 107.1 ± 34.6 A*** | 74.2 ± 22.1 C*** | 66 ± 22.4 C*** | 144 ± 33.8 | 96.1 ± 35.6 A*** | 51.6 ± 14.2 C*** | 44.2 ± 16.2 C*** |
| Alanine Aminotransferase (ALT) (IU/L) | 155.4 ± 36.1 | 122 ± 31.4 A*** | 68.1 ± 16.4 A*** | 64.1 ± 18.2 A*** | 162.4 ± 2.8 | 104.1 ± 36.4 A*** | 61.8 ± 13.2 A*** | 56.2 ± 14.4 A*** |
| Bilirubin (mmol/L) | 10.5 ± 7.1 | 10.2 ± 7.3 A* | 10.2 ± 6.6 A* | 10.2 ± 6.8 A* | 10.5 ± 4.9 | 10.1 ± 4.8 A* | 10.2 ± 3.0 A* | 10.3 ± 3.6 A* |
| Albumin (g/dL) | 4.2 ± 0.7 | 4.2 ± 0.94 A* | 4.1 ± 0.9 A* | 4.1 ± 0.7 A* | 4.2 ± 0.6 | 4.3 ± 0.7 A* | 4.2 ± 0.9 A* | 4.1 ± 0.7 A* |
| Viremia (106 copies/mL) | 3.4 ± 2.1 | 3.3 ± 2.4 A* | 2.96 ± 0.9 C* | 2.98 ± 0.8 C* | 3.7 ± 2.14 | 3.1 ± 2.5 A* | 1.8 ± 1.2 C*** | 1.9 ± 1.7 C*** |
| HAI | 10.4 ± 2.8 | - | 9.7 ± 2.4 C* | - | 10.9 ± 3.4 | - | 8.1 ± 0.9 C*** | - |
| C-Reactive Protein (CRP) | 6.4 ± 0.9 | 6.0 ± 0.8 C* | 5.9 ± 0.8 C** | 5.4 ± 0.7 C*** | 6.5 ± 0.8 | 5.2 ± 0.7 C*** | 3.5 ± 0.8 C*** | 3.8 ± 0.7 C*** |
| Before Treatment | After 1 Month | After 6 Months | After 12 Months | Follow-Up | |
|---|---|---|---|---|---|
| Group A Peg-IFN α + RBV + Placebo (n = 30) | |||||
| General Health Questionnaire (GHQ) | 28.1 ± 12.4 | 52.1 ± 14.1 A*** | 48.2 ± 14.4 A*** | 42.3 ± 13.4 C*** | 40.2 ± 14.4 B*** |
| Profile of Mood States (POMS) | 184.2 ± 13.7 | 290 ± 13.8 C*** | 260.1 ± 13.9 C*** | 256.4 ± 14.1 C*** | 250.1 ± 10.8 C*** |
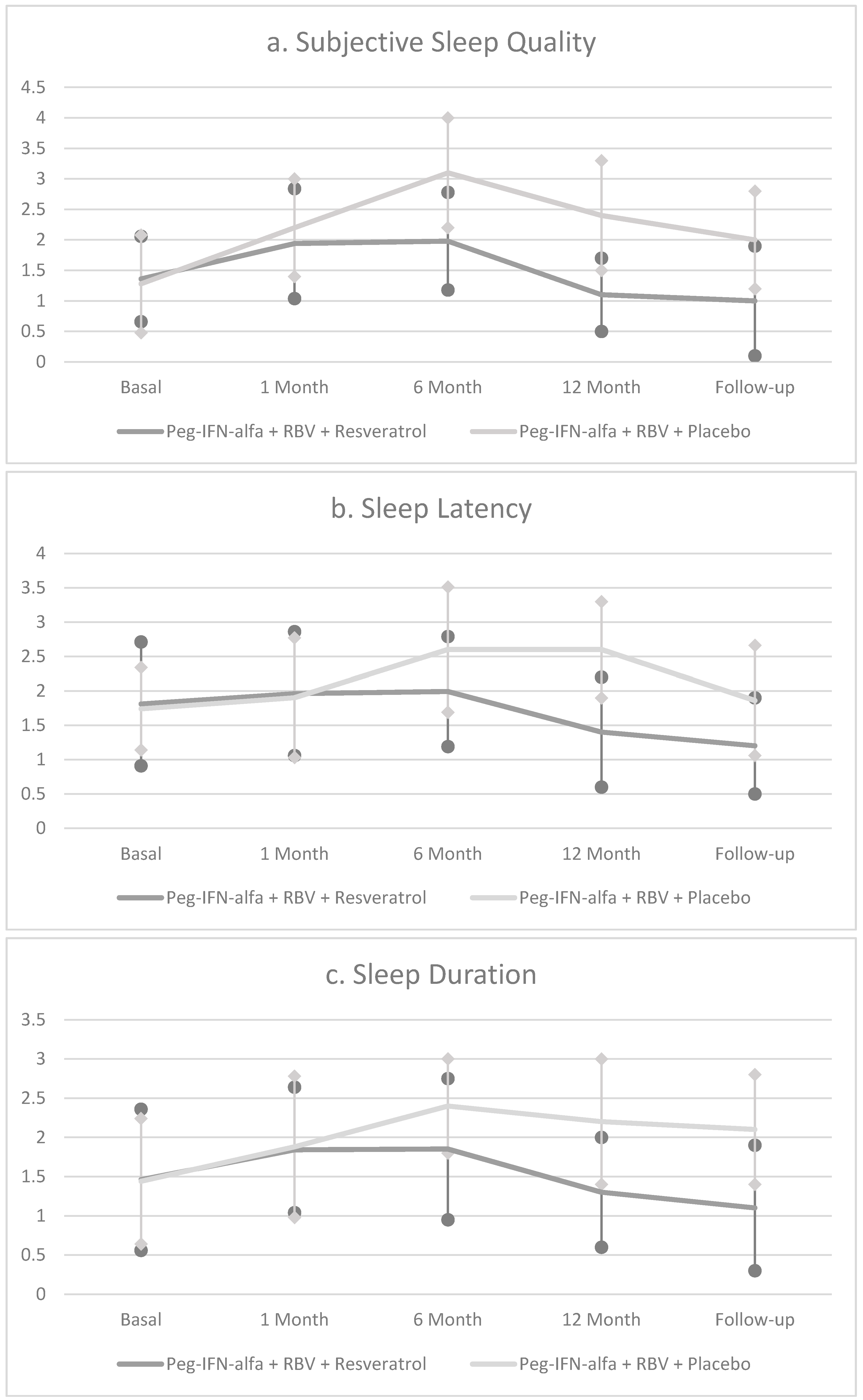
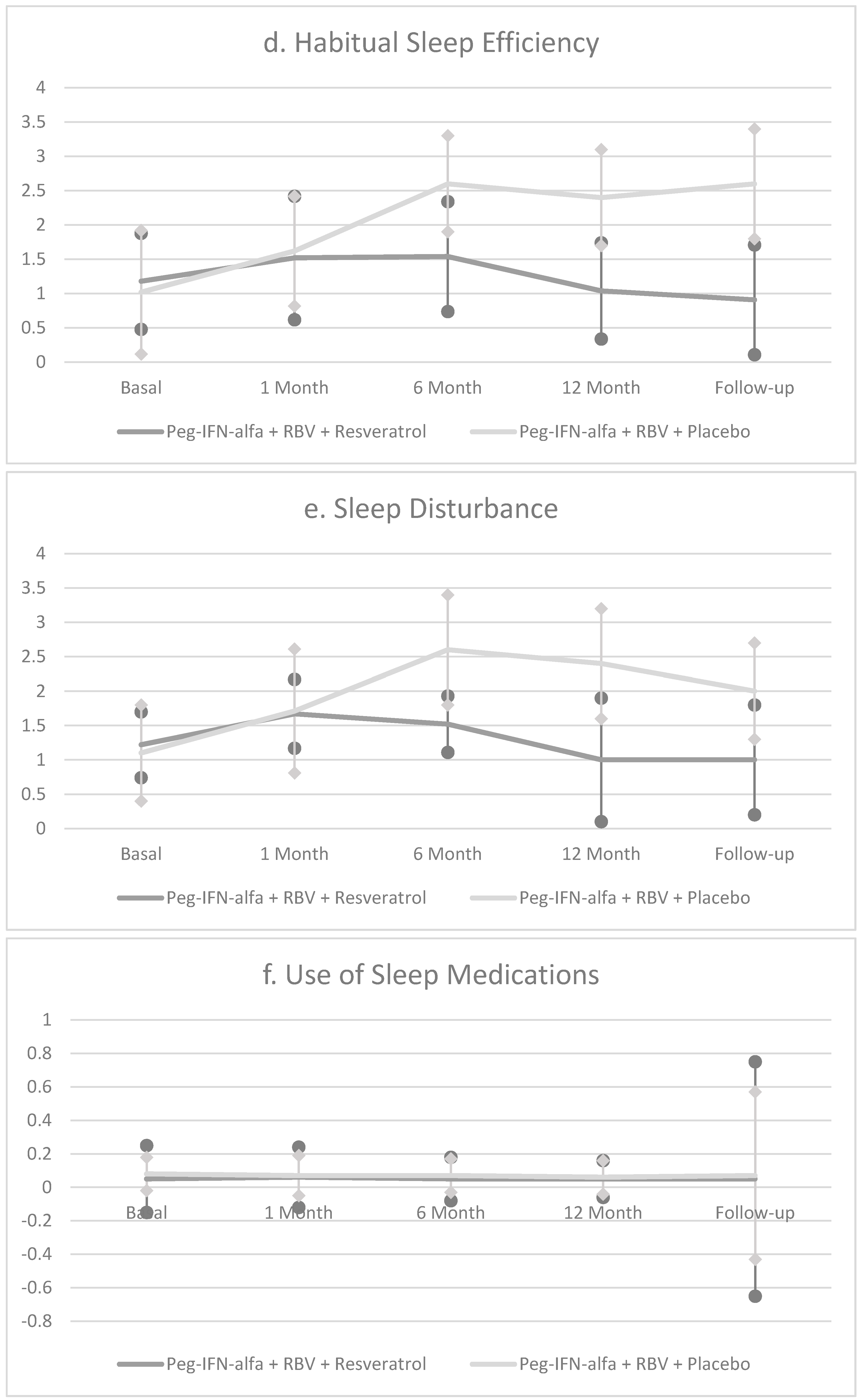
| Pittsburgh Sleep Quality Inventory (PSQI) | 8.0 ± 3.1 | 11.8 ± 3.4 A*** | 16.1 ± 3.2 C*** | 15.4 ± 3.6 C*** | 12.8 ± 3.6 C*** |
| Epworth Sleepiness Scale (ESS) | 12.1 ± 2.2 | 16.4 ± 3.0 A*** | 15.4 ± 3.0 B*** | 12.9 ± 3.0 B* | 12.2 ± 2.4 B* |
| Group B Peg-IFN-α + RBV + Resveratrol (n = 30) | |||||
| General Health Questionnaire (GHQ) | 29.1 ± 16.1 | 48.1 ± 13.2 A*** | 44.2 ± 15.2 A*** | 30.4 ± 12.8 C* | 30.2 ± 12.6 B* |
| Profile of Mood States (POMS) | 187.2 ± 12.4 | 208.4 ± 13.2 C*** | 191 ± 13.8 C* | 170 ± 12.6 C*** | 168 ± 12.9 C*** |
| Pittsburgh Sleep Quality Inventory (PSQI) | 8.2 ± 2.9 | 10.8 ± 3.6 A** | 10.2 ± 2.4 C** | 7.1 ± 2.1 C* | 7.0 ± 2.4 C* |
| Epworth Sleepiness Scale (ESS) | 12.8 ± 2.4 | 16.4 ± 3.2 A*** | 13.4 ± 3.1 B* | 10.4 ± 2.7 B** | 10.6 ± 2.8 B** |
| Group A 30 pt (Peg-IFN-α + RBV + Placebo) | Group B 30 pt (Peg-IFN-α + RBV + Resveratrol) | |||||
|---|---|---|---|---|---|---|
| Pretreatment | SVR | Non R | Pretreatment | SVR | Non R | |
| 14 | 16 | 19 | 11 | |||
| General Health Questionnaire (GHQ) | 28.1 ± 12.4 | 26.7 ± 10.9 °A* | 29.7 ± 11.8 °A* | 29.1 ± 16.1 | 20.8 ± 14.2 °°A* | 32.8 ± 14.9 °°A* |
| Profile of Mood States (POMS) | 184.2 ± 13.7 | 175.1 ± 12.0 °A** | 180.0 ± 13.8 °B* | 187.2 ± 12.4 | 174.4 ± 11.8 °°°A*** | 194.0 ± 12.1 °°°B* |
| Pittsburgh Sleep Quality Inventory (PSQI) | 8.0 ± 3.1 | 7.5 ± 2.4 °°°A* | 11.0 ± 2.5 °°°A** | 8.2 ± 2.9 | 6.0 ± 2.1 °°°A* | 10.8 ± 2.4 °°°A** |
| Epworth Sleepiness Scale (ESS) | 12.1 ± 2.2 | 9.2 ± 2.3 °°B*** | 12.0 ± 2.0 °°A* | 12.8 ± 2.4 | 7.4 ± 2.2 °°°B*** | 11.9 ± 2.1 °°°A* |
| Group A (n = 30) (Peg-IFNa + RBV + Placebo) | Group B (n = 30) (Peg-IFNa + RBV + Resveratrol) | |
|---|---|---|
| Psychological disorders | 18% | 15% |
| Hypercholesterolemia | 16% | 20% |
| Fatigue | 48% | 44% |
| Headache | 36% | 30% |
| Musculoskeletal pain | 51% | 28% |
| Myalgia | 44% | 32% |
| Hypertriglyceridemia | 48% | 27% |
| Nausea | 15% | 18% |
| Anorexia | 10% | 12% |
| Irritability | 22% | 18% |
| Hyperglycemia | 15% | 7% |
| Weight loss | 13% | 14% |
| Decrease of hemoglobin values at the end of treatment | from 13.4 g/dL (range 11.4–14.4) to 11.4 g/dL (range 10.4–14.0 g/dL) | from 13.5 g/dL (range 11.6–15.3 g/dL) to 10.6 (range 10.4–13.8 g/dL) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennisi, M.; Bertino, G.; Gagliano, C.; Malaguarnera, M.; Bella, R.; Borzì, A.M.; Madeddu, R.; Drago, F.; Malaguarnera, G. Resveratrol in Hepatitis C Patients Treated with Pegylated-Interferon-α-2b and Ribavirin Reduces Sleep Disturbance. Nutrients 2017, 9, 897. https://doi.org/10.3390/nu9080897
Pennisi M, Bertino G, Gagliano C, Malaguarnera M, Bella R, Borzì AM, Madeddu R, Drago F, Malaguarnera G. Resveratrol in Hepatitis C Patients Treated with Pegylated-Interferon-α-2b and Ribavirin Reduces Sleep Disturbance. Nutrients. 2017; 9(8):897. https://doi.org/10.3390/nu9080897
Chicago/Turabian StylePennisi, Manuela, Gaetano Bertino, Caterina Gagliano, Michele Malaguarnera, Rita Bella, Antonio Maria Borzì, Roberto Madeddu, Filippo Drago, and Giulia Malaguarnera. 2017. "Resveratrol in Hepatitis C Patients Treated with Pegylated-Interferon-α-2b and Ribavirin Reduces Sleep Disturbance" Nutrients 9, no. 8: 897. https://doi.org/10.3390/nu9080897






