A Single Day of Excessive Dietary Fat Intake Reduces Whole-Body Insulin Sensitivity: The Metabolic Consequence of Binge Eating
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Pre-Testing
2.3. Experimental Design
2.4. Experimental Protocol
2.5. Blood Sampling
2.6. Analytical Procedures
2.7. Calculations
2.8. Statistics
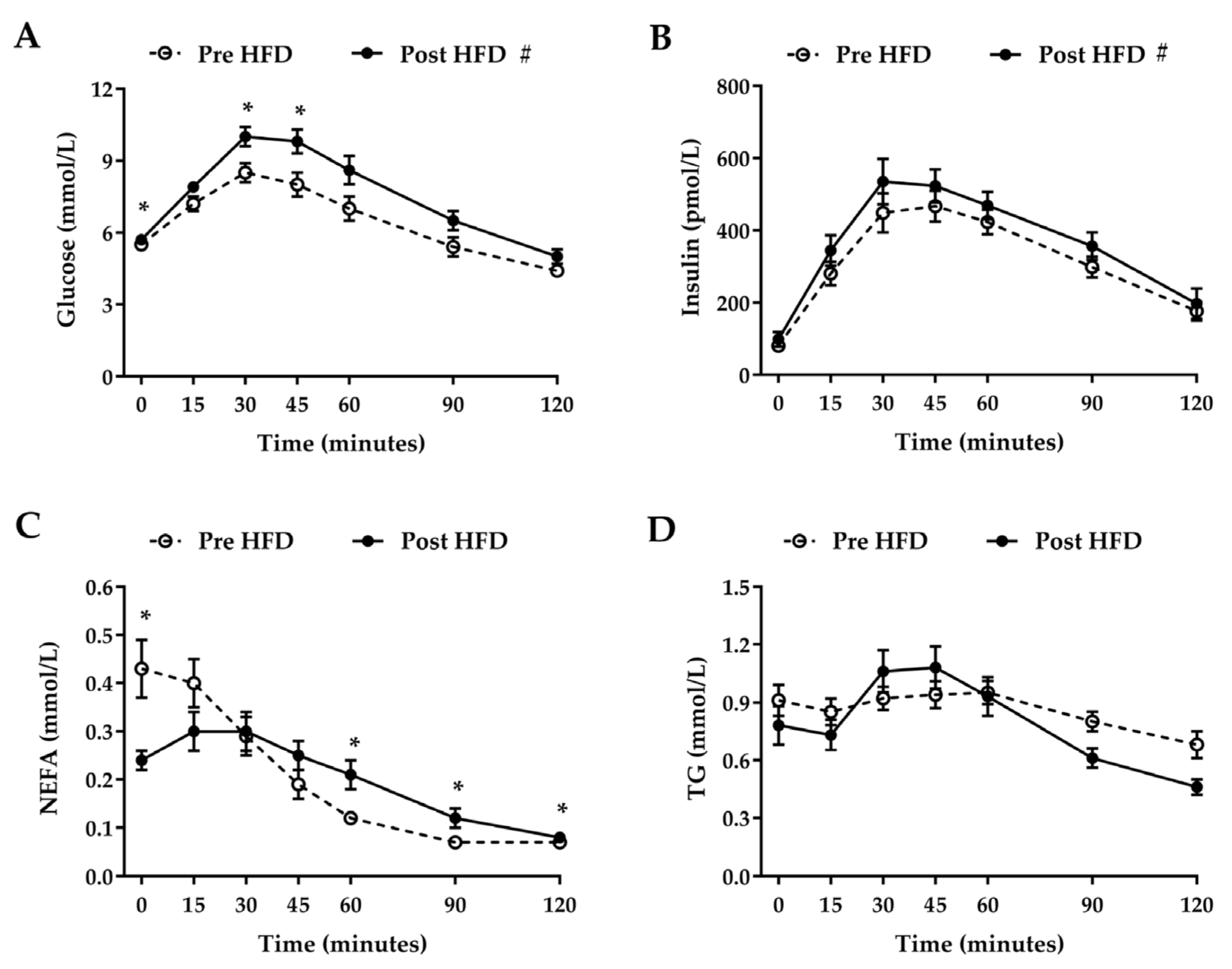
3. Results
3.1. Diet Intervention
3.2. Subject Characteristics
3.3. Oral Glucose Tolerance Test
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A.
| Breakfast | |
|---|---|
| Foods | Pork sausages (230 g), streaky bacon (120 g), fried eggs (180 g), fried white bread (36 g), whole milk (300 mL) |
| Protein (g) | 86 |
| Carbohydrate (g) | 52 |
| Fat (g) | 127 |
| Energy (kJ) | 7045 |
| % of the days intake | 26 |
| Lunch | |
| Foods | White bread (72 g), butter (15 g), cheddar cheese (70 g), mayonnaise (20 g), sausage roll (90 g) |
| Protein (g) | 31 |
| Carbohydrate (g) | 65 |
| Fat (g) | 86 |
| Energy (kJ) | 4814 |
| % of the days intake | 17 |
| Snack | |
| Foods | Pork pie (200 g) |
| Protein (g) | 22 |
| Carbohydrate (g) | 47 |
| Fat (g) | 51 |
| Energy (kJ) | 3060 |
| % of the days intake | 11 |
| Dinner | |
| Foods | Beef burgers (300 g), streaky bacon (120 g), cheddar cheese (90 g), coleslaw (150 g) |
| Protein (g) | 95 |
| Carbohydrate (g) | 7 |
| Fat (g) | 173 |
| Energy (kJ) | 8135 |
| % of the days intake | 30 |
| Dessert | |
| Foods | Chocolate chip muffin (70 g), double cream (150 mL) |
| Protein (g) | 6 |
| Carbohydrate (g) | 37 |
| Fat (g) | 98 |
| Energy (kJ) | 4357 |
| % of the days intake | 16 |
| Total intake | |
| Protein (g) | 240 |
| Carbohydrate (g) | 209 |
| Fat (g) | 535 |
| Energy (kJ) | 27,411 |
References
- Brownley, K.A.; Berkman, N.D.; Sedway, J.A.; Lohr, K.N.; Bulik, C.M. Binge eating disorder treatment: A systematic review of randomized controlled trials. Int. J. Eat. Disord. 2007, 40, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Levitan, R.D.; Carter, J.; Kaplan, A.S.; Reid, C.; Curtis, C.; Patte, K.; Kennedy, J.L. Personality and eating behaviors: A case-control study of binge eating disorder. Int. J. Eat. Disord. 2008, 41, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.M.; Massaro, J.M.; Hoffmann, U.; Yanovski, J.A.; Fox, C.S. Metabolic characterization of adults with binge eating in the general population: The framingham heart study. Obesity 2014, 22, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- De Zwaan, M. Binge eating disorder and obesity. Int. J. Obes. Relat. Metab. Disord. 2001, 25, S51–S55. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.I.; Pope, H.G., Jr. Genetic epidemiology of eating disorders and co-occurring conditions: The role of endophenotypes. Int. J. Eat. Disord. 2007, 40, S76–S78. [Google Scholar] [CrossRef]
- Bray, G.A. Medical consequences of obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Mathes, W.F.; Brownley, K.A.; Mo, X.; Bulik, C.M. The biology of binge eating. Appetite 2009, 52, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Heatherton, T.F.; Baumeister, R.F. Binge eating as escape from self-awareness. Psychol. Bull. 1991, 110, 86–108. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.B.; Jones, F.; Conner, M.; McMillan, B.; Ferguson, E. Effects of daily hassles and eating style on eating behavior. Health Psychol. 2008, 27, S20–S31. [Google Scholar] [CrossRef] [PubMed]
- Deer, J.; Koska, J.; Ozias, M.; Reaven, P. Dietary models of insulin resistance. Metabolism 2015, 64, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jarvinen, H. Nutritional modulation of non-alcoholic fatty liver disease and insulin resistance. Nutrients 2015, 7, 9127–9138. [Google Scholar] [CrossRef] [PubMed]
- Vessby, B.; Uusitupa, M.; Hermansen, K.; Riccardi, G.; Rivellese, A.A.; Tapsell, L.C.; Nalsen, C.; Berglund, L.; Louheranta, A.; Rasmussen, B.M.; et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The kanwu study. Diabetologia 2001, 44, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, G.; Giacco, R.; Rivellese, A.A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin. Nutr. 2004, 23, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Gordon, E.S. Non-esterified fatty acids in the blood of obese and lean subjects. Am. J. Clin. Nutr. 1960, 8, 740–747. [Google Scholar]
- Opie, L.H.; Walfish, P.G. Plasma free fatty acid concentrations in obesity. N. Engl. J. Med. 1963, 268, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty acids, obesity, and insulin resistance: Time for a reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Jadali, F. Effects of lipid on basal carbohydrate metabolism in normal men. Diabetes 1991, 40, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Jadali, F.; White, J.; Liang, Y.; Mozzoli, M.; Chen, X.; Coleman, E.; Smith, C. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J. Clin. Investig. 1991, 88, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; She, P.; Mozzoli, M.; Cheung, P.; Gumireddy, K.; Reddy, P.; Xiang, X.; Luo, Z.; Ruderman, N. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappab pathway in rat liver. Diabetes 2005, 54, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Reeds, D.N.; Stuart, C.A.; Perez, O.; Klein, S. Adipose tissue, hepatic, and skeletal muscle insulin sensitivity in extremely obese subjects with acanthosis nigricans. Metabolism 2006, 55, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M.; Hollenbeck, C.; Jeng, C.Y.; Wu, M.S.; Chen, Y.D. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with niddm. Diabetes 1988, 37, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Lagerpusch, M.; Bosy-Westphal, A.; Kehden, B.; Peters, A.; Muller, M.J. Effects of brief perturbations in energy balance on indices of glucose homeostasis in healthy lean men. Int. J. Obes. (Lond). 2012, 36, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.A.; Smith, J.R.; Corbett, T.R.; Woods, R.M.; Hulston, C.J. Short-term, high-fat overfeeding impairs glycaemic control but does not alter gut hormone responses to a mixed meal tolerance test in healthy, normal-weight individuals. Br. J. Nutr. 2017, 117, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Wulan, S.N.; Westerterp, K.R.; Plasqui, G. Metabolic profile before and after short-term overfeeding with a high-fat diet: A comparison between south asian and white men. Br. J. Nutr. 2014, 111, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Brons, C.; Jensen, C.B.; Storgaard, H.; Hiscock, N.J.; White, A.; Appel, J.S.; Jacobsen, S.; Nilsson, E.; Larsen, C.M.; Astrup, A.; et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J. Physiol. 2009, 587, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Cornford, A.S.; Hinko, A.; Nelson, R.K.; Barkan, A.L.; Horowitz, J.F. Rapid development of systemic insulin resistance with overeating is not accompanied by robust changes in skeletal muscle glucose and lipid metabolism. Appl. Physiol. Nutr. Metab. 2013, 38, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Hulston, C.J.; Churnside, A.A.; Venables, M.C. Probiotic supplementation prevents high-fat, overfeeding-induced insulin resistance in human subjects. Br. J. Nutr. 2015, 113, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, B.; Zahiragic, L.; Krog, D.; Nowotny, P.J.; Herder, C.; Carstensen, M.; Yoshimura, T.; Szendroedi, J.; Phielix, E.; Schadewaldt, P.; et al. Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes 2013, 62, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.J.; Hill, A.J. Breakdown of dietary restraint following mere exposure to food stimuli: Interrelationships between restraint, hunger, salivation, and food intake. Addict. Behav. 1989, 14, 387–397. [Google Scholar] [CrossRef]
- Waters, A.; Hill, A.; Waller, G. Internal and external antecedents of binge eating episodes in a group of women with bulimia nervosa. Int. J. Eat. Disord. 2001, 29, 17–22. [Google Scholar] [CrossRef]
- Adochio, R.L.; Leitner, J.W.; Gray, K.; Draznin, B.; Cornier, M.A. Early responses of insulin signaling to high-carbohydrate and high-fat overfeeding. Nutr. Metab. (Lond). 2009, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Bjorkman, O.; Reichard, G.A., Jr.; Pilo, A.; Olsson, M.; Wahren, J.; DeFronzo, R.A. The disposal of an oral glucose load in healthy subjects: A quantitative study. Diabetes 1985, 34, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Woerle, H.J.; Meyer, C.; Dostou, J.M.; Gosmanov, N.R.; Islam, N.; Popa, E.; Wittlin, S.D.; Welle, S.L.; Gerich, J.E. Pathways for glucose disposal after meal ingestion in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E716–E725. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.M.; Firth, R.G.; Rizza, R.A. Assessment of the postprandial pattern of glucose metabolism in nondiabetic subjects and patients with non-insulin-dependent diabetes mellitus using a simultaneous infusion of [2(3)h] and [3(3)h] glucose. Metabolism 1989, 38, 38–45. [Google Scholar] [CrossRef]
- Butler, P.C.; Rizza, R.A. Contribution to postprandial hyperglycemia and effect on initial splanchnic glucose clearance of hepatic glucose cycling in glucose-intolerant or niddm patients. Diabetes 1991, 40, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Simonson, D.C.; Katz, L.D.; Reichard, G., Jr.; Bevilacqua, S.; Barrett, E.J.; Olsson, M.; DeFronzo, R.A. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988, 37, 79–85. [Google Scholar] [CrossRef]
- Ferrannini, E.; Wahren, J.; Felig, P.; DeFronzo, R.A. The role of fractional glucose extraction in the regulation of splanchnic glucose metabolism in normal and diabetic man. Metabolism 1980, 29, 28–35. [Google Scholar] [CrossRef]
- Firth, R.G.; Bell, P.M.; Marsh, H.M.; Hansen, I.; Rizza, R.A. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J. Clin. Investig. 1986, 77, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Marsh, H.M.; Rizza, R.A. Effects of basal insulin supplementation on disposition of mixed meal in obese patients with niddm. Diabetes 1989, 38, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Woerle, H.J.; Dostou, J.M.; Welle, S.L.; Gerich, J.E. Abnormal renal, hepatic, and muscle glucose metabolism following glucose ingestion in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E1049–E1056. [Google Scholar] [CrossRef] [PubMed]
- Woerle, H.J.; Szoke, E.; Meyer, C.; Dostou, J.M.; Wittlin, S.D.; Gosmanov, N.R.; Welle, S.L.; Gerich, J.E. Mechanisms for abnormal postprandial glucose metabolism in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E67–E77. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, E.; Fra, G.P.; Carnevale Schianca, G.P. The oral glucose tolerance test (ogtt) revisited. Eur. J. Intern. Med. 2011, 22, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Van Haeften, T.; Fritsche, A.; Gerich, J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 2001, 24, 796–797. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [PubMed]
- Finegood, D.T.; Bergman, R.N.; Vranic, M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps: Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 1987, 36, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Hother-Nielsen, O.; Henriksen, J.E.; Holst, J.J.; Beck-Nielsen, H. Effects of insulin on glucose turnover rates in vivo: Isotope dilution versus constant specific activity technique. Metabolism 1996, 45, 82–91. [Google Scholar] [CrossRef]
- Knudsen, S.H.; Hansen, L.S.; Pedersen, M.; Dejgaard, T.; Hansen, J.; Hall, G.V.; Thomsen, C.; Solomon, T.P.; Pedersen, B.K.; Krogh-Madsen, R. Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. J. Appl. Physiol. (1985) 2012, 113, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zick, Y. Ser/Thr phosphorylation of IRS proteins: A molecular basis for insulin resistance. Sci STKE 2005, 2005, pe4. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Zhang, Y.L.; Hernandez-Ono, A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch. Med. Res. 2005, 36, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Pramfalk, C.; Pavlides, M.; Banerjee, R.; McNeil, C.A.; Neubauer, S.; Karpe, F.; Hodson, L. Fasting plasma insulin concentrations are associated with changes in hepatic fatty acid synthesis and partitioning prior to changes in liver fat content in healthy adults. Diabetes 2016, 65, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Banerjee, R.; Rial, B.; Arlt, W.; Adiels, M.; Boren, J.; Marinou, K.; Fisher, C.; Mostad, I.L.; Stratton, I.M.; et al. Menopausal status and abdominal obesity are significant determinants of hepatic lipid metabolism in women. J. Am. Heart Assoc. 2015, 4, e002258. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Parks, E.J. Postprandial metabolism of meal triglyceride in humans. Biochim. Biophys. Acta 2012, 1821, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, N.; Adiels, M.; Soderlund, S.; Stennabb, S.; Ahola, T.; Hakkarainen, A.; Boren, J.; Taskinen, M.R. Hepatic lipogenesis and a marker of hepatic lipid oxidation, predict postprandial responses of triglyceride-rich lipoproteins. Obesity 2014, 22, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D.; Parkes, M.; Warren, B.F.; Ferguson, D.J.; Jackson, K.G.; Jewell, D.P.; Frayn, K.N. Mobilisation of enterocyte fat stores by oral glucose in humans. Gut 2003, 52, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lee, B.; Buhman, K.K.; Cheng, J.X. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-stokes raman scattering imaging. J. Lipid Res. 2009, 50, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Fielding, B.A.; Callow, J.; Owen, R.M.; Samra, J.S.; Matthews, D.R.; Frayn, K.N. Postprandial lipemia: The origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am. J. Clin. Nutr. 1996, 63, 36–41. [Google Scholar] [PubMed]
- Jackson, K.G.; Robertson, M.D.; Fielding, B.A.; Frayn, K.N.; Williams, C.M. Olive oil increases the number of triacylglycerol-rich chylomicron particles compared with other oils: An effect retained when a second standard meal is fed. Am. J. Clin. Nutr. 2002, 76, 942–949. [Google Scholar] [PubMed]
- Robertson, M.D.; Henderson, R.A.; Vist, G.E.; Rumsey, R.D. Extended effects of evening meal carbohydrate-to-fat ratio on fasting and postprandial substrate metabolism. Am. J. Clin. Nutr. 2002, 75, 505–510. [Google Scholar] [PubMed]
- Nathan, D.M.; Davidson, M.B.; DeFronzo, R.A.; Heine, R.J.; Henry, R.R.; Pratley, R.; Zinman, B.; American Diabetes, A. Impaired fasting glucose and impaired glucose tolerance: Implications for care. Diabetes Care 2007, 30, 753–759. [Google Scholar] [CrossRef] [PubMed]
| Estimated Energy Requirement | Experimental Energy Intake | |
|---|---|---|
| Energy (kJ) | 14,028 ± 433 | 24,949 ± 797 * |
| Carbohydrate (g) | 192 ± 6 | |
| Protein (g) | 278 ± 8 | |
| Fat (g) | 449 ± 15 | |
| Fatty acid composition (%) | ||
| SFA | 42 ± 0.6 | |
| MUFA | 40 ± 0.4 | |
| PUFA | 10 ± 0.2 |
| Before Overfeeding | After Overfeeding | |
|---|---|---|
| Males/Females | 13/2 | |
| Age (years) | 22.1 ± 0.5 | |
| Weight (kg) | 86.0 ± 3.2 | 86.8 ± 3.2 * |
| BMI (kg/m2) | 26.4 ± 1.1 | 26.6 ± 1.1 * |
| Fasting plasma biochemical parameters and Matsuda ISI | ||
| Glucose (mmol/L) | 5.5 ± 0.1 | 5.7 ± 0.1 |
| Insulin (pmol/L) | 80 ± 9 | 98 ± 20 |
| NEFA (mmol/L) | 0.43 ± 0.06 | 0.24 ± 0.02 * |
| TG (mmol/L) | 0.91 ± 0.08 | 0.78 ± 0.10 |
| Matsuda ISI | 5.0 ± 0.5 | 3.6 ± 0.4 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parry, S.A.; Woods, R.M.; Hodson, L.; Hulston, C.J. A Single Day of Excessive Dietary Fat Intake Reduces Whole-Body Insulin Sensitivity: The Metabolic Consequence of Binge Eating. Nutrients 2017, 9, 818. https://doi.org/10.3390/nu9080818
Parry SA, Woods RM, Hodson L, Hulston CJ. A Single Day of Excessive Dietary Fat Intake Reduces Whole-Body Insulin Sensitivity: The Metabolic Consequence of Binge Eating. Nutrients. 2017; 9(8):818. https://doi.org/10.3390/nu9080818
Chicago/Turabian StyleParry, Siôn A., Rachel M. Woods, Leanne Hodson, and Carl J. Hulston. 2017. "A Single Day of Excessive Dietary Fat Intake Reduces Whole-Body Insulin Sensitivity: The Metabolic Consequence of Binge Eating" Nutrients 9, no. 8: 818. https://doi.org/10.3390/nu9080818
APA StyleParry, S. A., Woods, R. M., Hodson, L., & Hulston, C. J. (2017). A Single Day of Excessive Dietary Fat Intake Reduces Whole-Body Insulin Sensitivity: The Metabolic Consequence of Binge Eating. Nutrients, 9(8), 818. https://doi.org/10.3390/nu9080818






