Chronic Inflammatory Diseases and Green Tea Polyphenols
Abstract
:1. Introduction
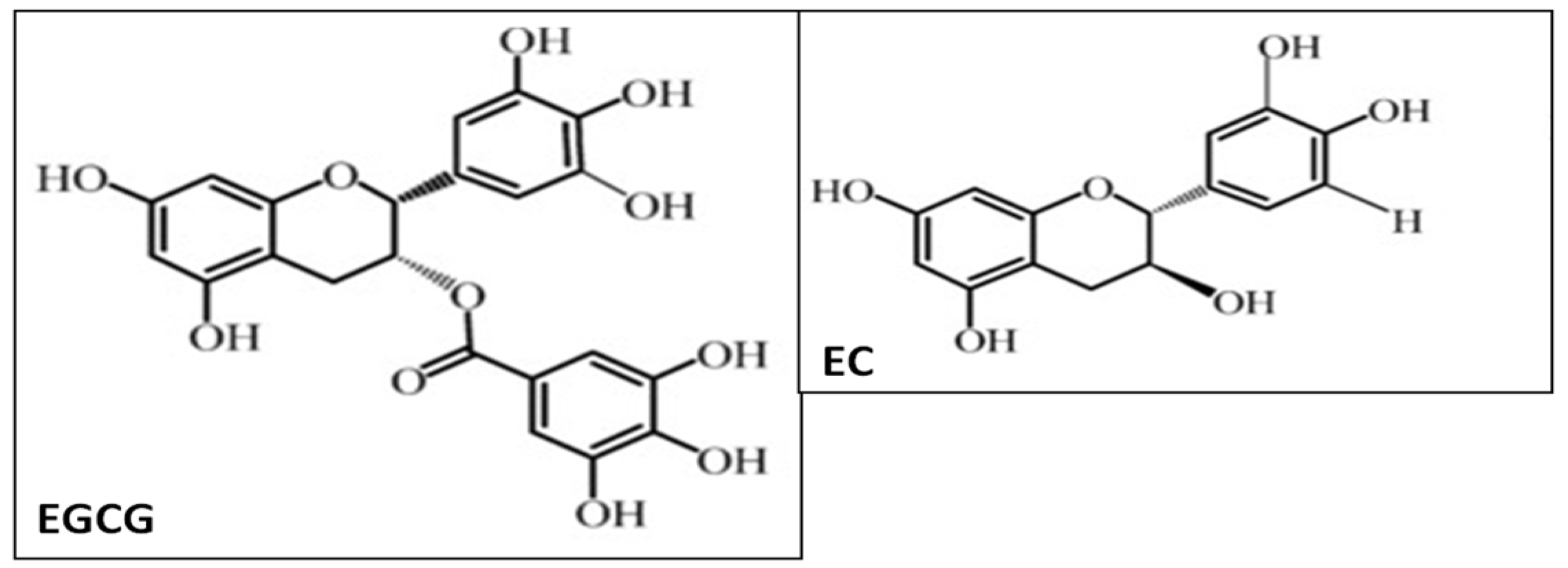
2. Green Tea and Polyphenols
3. Inflammatory Bowel Disease and Green Tea Polyphenols
4. Gastrointestinal Associated Malignancies and Green Tea Polyphenols
5. Hepatic Complications and Green Tea Polyphenols
6. Neurodegenerative Disorders and Green Tea Polyphenols
7. Tea Polyphenols and Possible Side Effects
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gregersen, R.; Lambertsen, K.; Finsen, B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J. Cereb. Blood Flow Metab. 2000, 20, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S. Multiorgan chronic inflammatory hepatobiliary pancreatic murine model deficient in tumor necrosis factor receptors 1 and 2. World J. Gastroenterol. 2016, 22, 4988–4998. Available online: http://www.wjgnet.com/1007-9327/full/v22/i21/4988.htm (accessed on 7 June 2016). [CrossRef] [PubMed]
- Westlund, K.N.; Zhang, L.; Ma, F.; Oz, H.S. Chronic inflammation and pain in a tumor necrosis factor receptor (TNFR) (p55/p75-/-) dual deficient murine model. Transl. Res. 2012, 160, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Uçeyler, N.; Schäfers, M.; Sommer, C. Mode of action of cytokines on nociceptive neurons. Exp. Brain Res. 2009, 196, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Marchand, F.; Perretti, M.; McMahon, S.B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 2005, 6, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Schmidt, C.; George, A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp. Neurol. 1998, 151, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhang, L.; Oz, H.S.; Mashni, M.; Westlund, K.N. Dysregulated TNFα promotes cytokine proteome profile increases and bilateral orofacial hypersensitivity. Neuroscience 2015, 300, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Malleo, G.; Mazzon, E.; Genovese, T.; Di Paola, R.; Muià, C.; Centorrino, T.; Siriwardena, A.K.; Cuzzocrea, S. Etanercept attenuates the development of cerulein-induced acute pancreatitis in mice: A comparison with TNF-alpha genetic deletion. Shock 2007, 27, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.; de Villiers, W.J. Green Tea Polyphenols and Sulfasalazine have Parallel Anti-Inflammatory Properties in Colitis Models. Front Immunol. 2013, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Stub, T.; Quandt, S.A.; Arcury, T.A.; Sandberg, J.C.; Kristoffersen, A.E.; Musial, F.; Salamonsen, A. Perception of risk and communication among conventional and complementary health care providers involving cancer patients’ use of complementary therapies: A literature review. BMC Complement. Altern. Med. 2016, 16, 353. [Google Scholar] [CrossRef] [PubMed]
- Saraceno, R.; Chimenti, S. How to manage infections in the era of biologics? Dermatol. Ther. 2008, 21, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; McClain, C.J.; Nagasawa, H.T.; Ray, M.B.; de Villiers, W.J.; Chen, T.S. Diverse antioxidants protect against acetaminophen hepatotoxicity. J. Biochem. Mol. Toxicol. 2004, 18, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.S.; McClain, C.J.; de Villiers, W.J. Antioxidants as novel therapy in a murine model of colitis. J. Nutr. Biochem. 2005, 16, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Oz, H.S.; Barve, S.; de Villiers, W.J.; McClain, C.J.; Varilek, G.W. The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol. Pharmacol. 2001, 60, 528–533. [Google Scholar] [PubMed]
- Oz, H.S.; Chen, T.S. Green-tea polyphenols downregulate cyclooxygenase and Bcl-2 activity in acetaminophen-induced hepatotoxicity. Dig. Dis. Sci. 2008, 53, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defense responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Oz, H.S.; Ebersole, J. Green tea polyphenols mediate apoptosis in Intestinal Epithelial Cells. J. Cancer Ther. 2010, 1, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, R.; Kondo, K.; Momiyama, Y. Antioxidant beverages: Green tea intake and coronary artery disease. Clin. Med. Insights Cardiol. 2014, 8, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, M.; Sonego, S.; Gyengesi, E.; Sharman, M.J.; Münch, G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2016, 95, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Yamada, H.; Takuma, N.; Kawasaki, Y.; Harada, S.; Nakase, J.; Ukawa, Y.; Sagesaka, Y.M. Effects of green tea consumption on cognitive dysfunction in an elderly population: A randomized placebo-controlled study. Nutr. J. 2016, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.M.; Papadimitriou, A.; Duarte, D.A.; Lopes de Faria, J.M.; Lopes de Faria, J.B. The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: A double-blind randomised clinical trial. Sci. Rep. 2016, 6, 28282. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Castro-López, M.; López-Vilariño, J.M.; González-Rodríguez, M.V. Analytical determination of flavonoids aimed to analysis of natural samples and active packaging applications. Food Chem. 2014, 150, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Beltran, A.; Valente, A.J.; Jiménez, A.; Garrigós, M.C. Characterization of Poly (ε-caprolactone)-Based Nanocomposites Containing Hydroxytyrosol for Active Food Packaging. J. Agric. Food Chem. 2014, 62, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Development of novel nano-biocomposite antioxidant films based on poly (lactic acid) and thymol for active packaging. Food Chem. 2014, 162, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Perazzo, K.K.; Conceição, A.C.; dos Santos, J.C.; Assis Dde, J.; Souza, C.O.; Druzian, J.I. Properties and antioxidant action of actives cassava starch films incorporated with green tea and palm oil extracts. PLoS ONE 2014, 9, e105199. [Google Scholar] [CrossRef] [PubMed]
- Provvisiero, D.P.; Pivonello, C.; Muscogiuri, G.; Negri, M.; de Angelis, C.; Simeoli, C.; Pivonello, R.; Colao, A. Influence of Bisphenol A on Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2016, 13, E989. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, I.E. Antioxidant polyphenols in tea, cocoa, and wine. Nutrition 2000, 16, 692–694. [Google Scholar] [CrossRef]
- Vickery, M.L.; Vickery, B. Secondary Plant Metabolism; Macmillan Press: London, UK, 1981; p. 335. [Google Scholar]
- Farzaei, M.H.; Rahimi, R.; Abdollahi, M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Varilek, G.W.; Yang, F.; Lee, E.Y.; deVilliers, W.J.; Zhong, J.; Oz, H.S.; Westberry, K.F.; McClain, C.J. Green tea polyphenol extract attenuates inflammation in interleukin-2-deficient mice, a model of autoimmunity. J. Nutr. 2001, 131, 2034–2039. [Google Scholar] [PubMed]
- Suganuma, M.; Okabe, S.; Oniyama, M.; Tada, Y.; Ito, H.; Fujiki, H. Wide distribution of [3H] (−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 1998, 10, 1771–1776. [Google Scholar] [CrossRef]
- Hendry, J.H.; Potten, C.S. Cryptogenic cells and proliferative cells in intestinal epithelium. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1974, 25, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Hermos, J.A.; Mathan, M.; Trier, J.S. DNA synthesis and proliferation by villous epithelial cells in fetal rats. J. Cell Biol. 1971, 50, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Ebersole, J.L. Application of prodrugs to inflammatory diseases of the gut. Molecules 2008, 13, 452–474. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef] [PubMed]
- Doering, J.; Begue, B.; Lentze, M.J.; Rieux-Laucat, F.; Goulet, O.; Schmitz, J.; Cerf-Bensussan, N.; Ruemmele, F.M. Induction of T lymphocyte apoptosis by sulphasalazine in patients with Crohn’s disease. Gut 2004, 53, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Najafzadeh, M.; Reynolds, P.D.; Baumgartner, A.; Anderson, D. Flavonoids inhibit the genotoxicity of hydrogen peroxide (H2O2) and of the food mutagen 2-amino-3-methylimadazo[4,5-f]-quinoline (IQ) in lymphocytes from patients with inflammatory bowel disease (IBD). Mutagenesis 2009, 24, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Miao, J.; Tang, Y.; Nan, Q.; Liu, Y.; Yang, G.; Dong, X.; Huang, Q.; Xia, S.; Wang, K.; et al. Identification of Environmental Factors Associated with Inflammatory Bowel Disease in a Southwestern Highland Region of China: A Nested Case-Control Study. PLoS ONE 2016, 11, e0153524. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Liu, Y.; Hong, J.; Huang, M.T.; Conney, A.H.; Yang, C.S. Effects of green tea and high-fat diet on arachidonic acid metabolism and aberrant crypt foci formation in an azoxymethane-induced colon carcinogenesis mouse model. Nutr. Cancer 2003, 46, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Metz, N.; Lobstein, A.; Schneider, Y.; Gossé, F.; Schleiffer, R.; Anton, R.; Raul, F. Suppression of azoxymethane-induced preneoplastic lesions and inhibition of cyclooxygenase-2 activity in the colonic mucosa of rats drinking a crude green tea extract. Nutr. Cancer 2000, 38, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.Y.; Volate, S.R.; Muga, S.J.; Nitcheva, D.; Smith, T.; Wargovich, M.J. Green tea selectively targets initial stages of intestinal carcinogenesis in the AOM-ApcMin mouse model. Carcinogenesis 2007, 28, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Kishimoto, Y.; Miura, N.; Shiota, G.; Kohri, T.; Hara, Y.; Hasegawa, J.; Isemura, M. Synergistic effects of (−)-epigallocatechin gallate with sulindac against colon carcinogenesis of rats treated with azoxymethane. Cancer Lett. 2002, 177, 49–56. [Google Scholar] [CrossRef]
- Isemura, M.; Saeki, K.; Kimura, T.; Hayakawa, S.; Minami, T.; Sazuka, M. Tea catechins and related polyphenols as anti-cancer agents. Biofactors 2000, 13, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.P.; Kuo, S.C.; Huang, W.W.; Yang, J.S.; Lai, K.C.; Chen, H.J.; Lin, K.L.; Chiu, Y.J.; Huang, L.J.; Chung, J.G. (−)-Epigallocatechingallate induced apoptosis in human adrenal cancer NCI-H295 cells through caspase-dependent and caspase-independent pathway. Anticancer Res. 2009, 29, 1435–1442. [Google Scholar] [PubMed]
- Basu, A.; Haldar, S. Combinatorial effect of epigallocatechin-3-gallate and TRAIL on pancreatic cancer cell death. Int. J. Oncol. 2009, 34, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Mercier, I.; Vuolo, M.; Jasmin, J.F.; Medina, C.M.; Williams, M.; Mariadason, J.M.; Qian, H.; Xue, X.; Pestell, R.G.; Lisanti, M.P.; et al. ARC (apoptosis repressor with caspase recruitment domain) is a novel marker of human colon cancer. Cell Cycle 2008, 7, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, N.; Zhuang, W.; Liu, G.; Wu, T.; Yao, X.; Du, L.; Wei, M.; Wu, X. Green tea and gastric cancer risk: Meta-analysis of epidemiologic studies. Asia Pac. J. Clin. Nutr. 2008, 17, 159–165. [Google Scholar] [PubMed]
- Wang, Y.; Duan, H.; Yang, H. A case-control study of stomach cancer in relation to Camellia sinensis in China. Surg. Oncol. 2015, 24, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.; Babar, A.; Choudhary, M.; Kutner, M.; Pyrsopoulos, N. Acetaminophen-Induced Hepatotoxicity: A Comprehensive Update. J. Clin. Transl. Hepatol. 2016, 4, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Elias, R.J.; Vijay-Kumar, M.; Lambert, J.D. (−)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol. Nutr. Food Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; Carvalho-Silva, M.; Gomes, L.M.; Okuda, M.H.; Santana, A.A.; Streck, E.L.; Seelaender, M.; do Nascimento, C.M.; Ribeiro, E.B.; Lira, F.S.; et al. Decaffeinated green tea extract rich in epigallocatechin-3-gallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. J. Nutr. Biochem. 2015, 26, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zheng, S.; Lu, S.C.; Chen, A. Epigallocatechin-3-gallate inhibits growth of activated hepatic stellate cells by enhancing the capacity of glutathione synthesis. Mol. Pharmacol. 2008, 73, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.Y.; Mah, E.; Masterjohn, C.; Noh, S.K.; Park, H.J.; Clark, R.M.; Park, Y.K.; Lee, J.Y.; Bruno, R.S. Green Tea Lowers Hepatic Cox-2 and Prostaglandin E2 in Rats with Dietary Fat-Induced Nonalcoholic Steatohepatitis. J. Med. Food 2015, 18, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Safi, S.; Feizi, A.; Askari, G.; Karami, F. The Effect of Green Tea Extract Supplementation on Liver Enzymes in Patients with Nonalcoholic Fatty Liver Disease. Int. J. Prev. Med. 2016, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Deczkowska, A. Neurological Disease as a Failure of Brain-Immune Crosstalk: The Multiple Faces of Neuroinflammation. Trends Immunol. 2016, 37, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Chung Wei, J.; Huang, H.C.; Chen, W.J.; Huang, C.N.; Peng, C.H.; Lin, C.L. Epigallocatechin gallate attenuates amyloid β-induced inflammation and neurotoxicity in EOC 13.31 microglia. Eur. J. Pharmacol. 2016, 770, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Nabavi, S.F.; Daglia, M.; Nabavi, S.M.; Martinoli, M.G. Epigallocatechin-3-Gallate, a Promising Molecule for Parkinson’s Disease? Rejuvenation Res. 2015, 18, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Bitu Pinto, N.; da Silva Alexandre, B.; Neves, K.R.; Silva, A.H.; Leal, L.K.; Viana, G.S. Neuroprotective Properties of the Standardized Extract from Camellia sinensis (Green Tea) and Its Main Bioactive Components, Epicatechin and Epigallocatechin Gallate, in the 6-OHDA Model of Parkinson’s Disease. Evid. Based Complement. Altern. Med. 2015, 2015, 161092. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, N.; Konstantinov, A.; Anavi, S.; Aronis, A.; Hagay, Z.; Madar, Z.; Tirosh, O. Prolonged Feeding with Green Tea Polyphenols Exacerbates Cholesterol-induced Fatty Liver Disease in Mice. Mol. Nutr. Food Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; Valverde, A.M. Modulation of microglia in the retina: New insights into diabetic retinopathy. Acta Diabetol. 2017, 54, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, Y.J.; Yip, Y.W.; Chan, K.P.; Chu, K.O.; Chu, W.K.; Ng, T.K.; Pang, C.P.; Chan, S.O. Green tea catechins are potent anti-oxidants that ameliorate sodium iodate-induced retinal degeneration in rats. Sci. Rep. 2016, 6, 29546. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Lyu, Z.; Yang, X.; Pan, Z.; Lou, J.; Dong, T. Epigallocatechin-3-gallate promotes angiogenesis via up-regulation of Nrf2 signaling pathway in a mouse model of ischemic stroke. Behav. Brain Res. 2017, 321, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Xu, H.; Yuan, Y.; Chen, J.Y.; Zhang, Y.J.; Lin, Y.; Yuan, S.Y. Delayed Treatment with Green Tea Polyphenol EGCG Promotes Neurogenesis After Ischemic Stroke in Adult Mice. Mol. Neurobiol. 2017, 54, 3652–3664. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zhang, Z.; Zheng, T.Z.; Bassig, B.A.; Mao, C.; Liu, X.; Zhu, Y.; Shi, K.; Ge, J.; Yang, Y.J.; et al. Green tea consumption and risk of cardiovascular and ischemic related diseases: A meta-analysis. Int. J. Cardiol. 2016, 202, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Banji, D.; Banji, O.J.; Abbagoni, S.; Hayath, M.S.; Kambam, S.; Chiluka, V.L. Amelioration of behavioral aberrations and oxidative markers by green tea extract in valproate induced autism in animals. Brain Res. 2011, 1410, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and Safety of Green Tea Polyphenols after Multiple-Dose Administration of Epigallocatechin Gallate and Polyphenon E in Healthy Individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Ting, A.; Chow, Y.; Tan, W. Microbial and heavy metal contamination in commonly consumed traditional Chinese herbal medicines. J. Tradit. Chin. Med. 2013, 33, 119–124. [Google Scholar] [CrossRef]
- Lessa, F.C.; Mu, Y.; Bamberg, W.M. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.O., II; Starley, B.; Galagan, J.C.; Yabes, J.M.; Evans, S.; Salama, J.J. Tea and Recurrent Clostridium difficile Infection. Gastroenterol. Res. Pract. 2016, 2016. [Google Scholar] [CrossRef]
- Vossoughinia, H.; Salari, M.; Mokhtari Amirmajdi, E.; Saadatnia, H.; Abedini, S.; Shariati, A.; Shariati, M.; Khosravi Khorashad, A. An epidemiological study of gastroesophageal reflux disease and related risk factors in urban population of Mashhad, Iran. Iran. Red Crescent Med. J. 2014, 16, e15832. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, B.S.; Aguilera Olvera, R.; Singh, V.; Xiao, X.; Kennett, M.J.; Joe, B.; Lambert, J.D.; Vijay-Kumar, M. Epigallocatechin-3-Gallate Inhibition of Myeloperoxidase and Its Counter-Regulation by Dietary Iron and Lipocalin 2 in Murine Model of Gut Inflammation. Am. J. Pathol. 2016, 186, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Rice-Evans, C. Polyphenolicflavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Chen, D.; Sun, H.P.; Yan, N.; Xu, Y.; Pan, C.W. Regular Chinese Green Tea Consumption is Protective for Diabetic Retinopathy: A Clinic-Based Case-Control Study. J. Diabetes Res. 2015, 2015, 231570. [Google Scholar] [CrossRef] [PubMed]
| Applied Investigations | Tea Extract, GrTP, EGCG | In Vitro/Animal/Human Trial | References |
|---|---|---|---|
| Inflammatory Bowel Disease | |||
| GrTP | DSS-WT mouse model | Oz et al. [13] | |
| GrTP, EGCG | IL-10-/- spontaneous and DSS-WT | Oz et al. [9] | |
| GrTP | IL-2-/-spontaneous | Varilek et al. [31] | |
| Tea consumption | Patients | Niu J et al. [39] | |
| in vitro (patients with lymphocytes) | Najafzadeh et al. [38] | ||
| GrTP, EGCG, EGC, ECG | in vitro IEC | Yang et al. [14] | |
| GI malignancy/prevention | Tea extract | WT-mice, rats | Ju et al. [40], Metz et al. [41]; Issa et al. [42], Ohishi et al. [43] |
| GrTP, EGCG | in vitrocell lines | Oz et al. [17], Isemura et al. [44], Wu [45], Basu et al. [46] | |
| Tea consumption | Human subjects | Zhou et al. [48] | |
| Hepatic complications | GrTP | WT-mice and APAP toxicity | Oz et al. [12,15] |
| NASH | GrTP | Rat model | Chung et al. [54] |
| Diabetic | Tea extract | Patients | Borges et al. [22] |
| Metabolicweight loss, | EGCG | WT-mice | Oz et al. [9], Bitzer et al. [51], Santamarina et al. [52] |
| Fatty liver disease | WT-mice | Hirsch et al. [60] | |
| Neurodegenerative Disorders | |||
| Alzheimer‘s. | EGCG | in vitro neuronal cells | Cheng-Chung et al. [57] |
| Parkinson‘s disease | EGCG | Patients | Renaud et al. [58] |
| EGCG | Rat model | Bitu et al. [59] | |
| Cognitive function | Tea extract | Elderly | Ide et al. [21] |
| Diabetic retinopathy | Green tea | Human subjects | Ma et al. [61] |
| Retinalneurodegeneration | EGCG | Tat retina | Yang et al. [62] |
| Stroke | EGCG | WT-mice | Bai et al. [63], Zhang et al. [64] |
| Tea consumption | Human subjects | Pang et al. [65] | |
| Autism spectrum | Tea extract | WT-mice pups | Banji D et al. [66] |
| Tea and Side effects | |||
| EGCG | Human subjects | Chow et al. [67] | |
| Weight loss | GrTP, EGCG | WT-mice | Oz et al. [9], Bitzer et al. [51] |
| Microbia, toxic metal contaminant | Tea | Ting et al. [68] | |
| Microbial contaminant/ provocation | Tea consumption | Human subjects | Lessa et al. [69], Evans et al. [70] |
| Gastroesophageal reflux disease | Tea consumption | Human subjects | Vossoughinia et al. [71] |
| Iron deficiency | EGCG | WT-mice | Yeoh et al. [72] |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oz, H.S. Chronic Inflammatory Diseases and Green Tea Polyphenols. Nutrients 2017, 9, 561. https://doi.org/10.3390/nu9060561
Oz HS. Chronic Inflammatory Diseases and Green Tea Polyphenols. Nutrients. 2017; 9(6):561. https://doi.org/10.3390/nu9060561
Chicago/Turabian StyleOz, Helieh S. 2017. "Chronic Inflammatory Diseases and Green Tea Polyphenols" Nutrients 9, no. 6: 561. https://doi.org/10.3390/nu9060561
APA StyleOz, H. S. (2017). Chronic Inflammatory Diseases and Green Tea Polyphenols. Nutrients, 9(6), 561. https://doi.org/10.3390/nu9060561





