Peripheral Endocannabinoid Responses to Hedonic Eating in Binge-Eating Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics Statement
2.3. Experimental Procedures
2.4. Statistics
3. Results
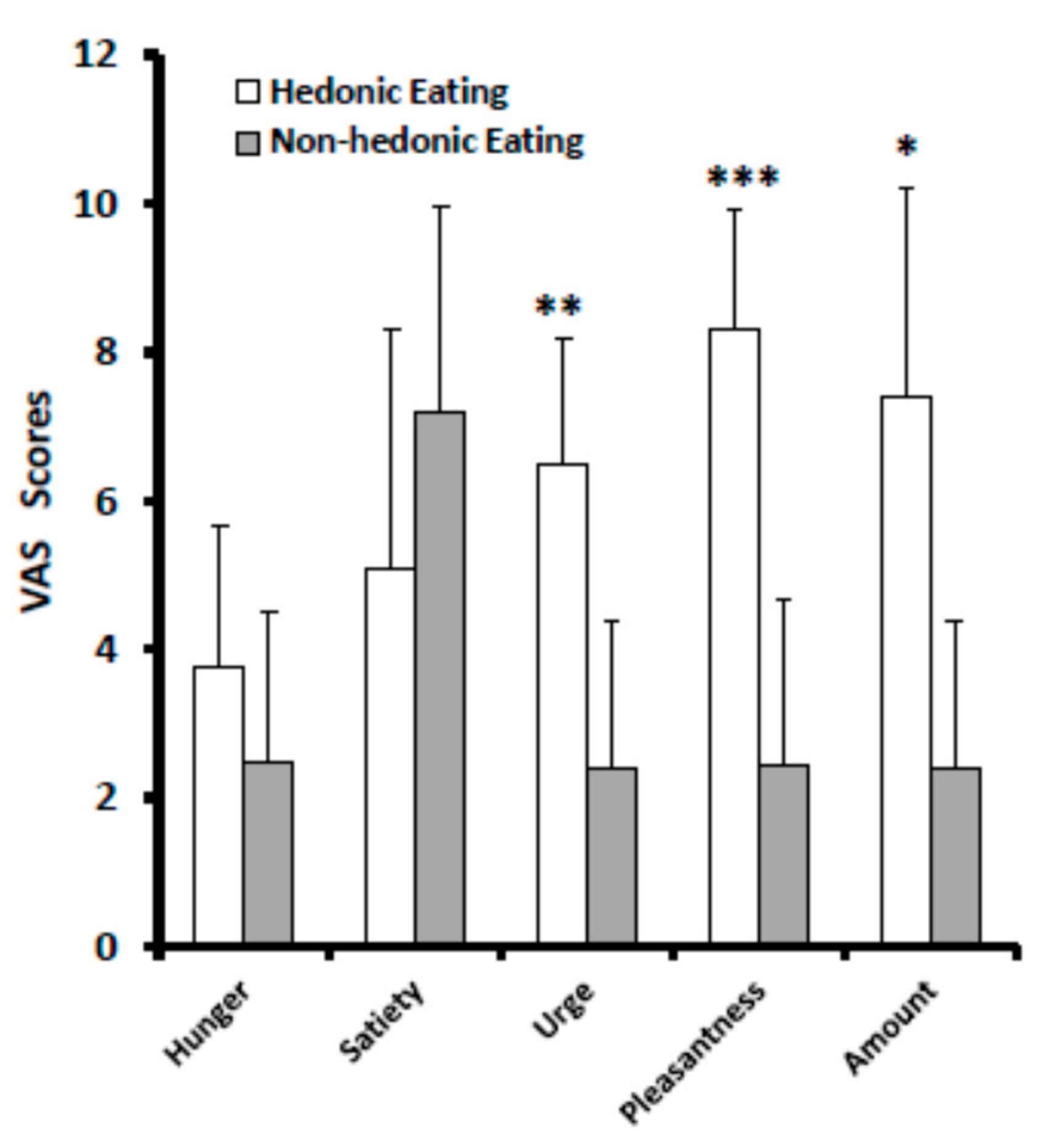
3.1. VAS Scores
3.2. Calories and Nutrients
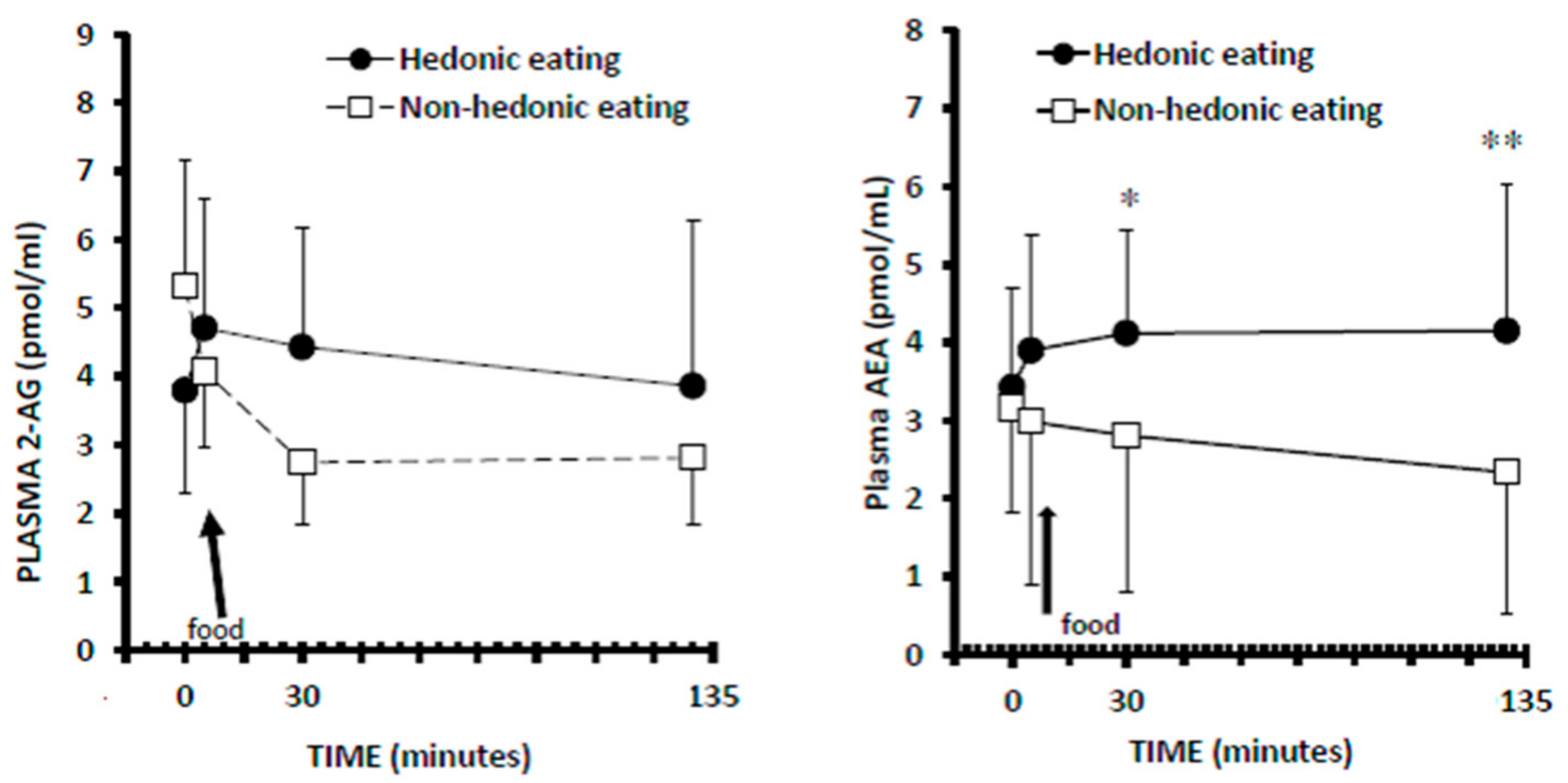
3.3. Plasma 2-AG Levels
3.4. Plasma AEA Levels
3.5. Correlations
4. Discussions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Association (APA): Washington, DC, USA, 2013. [Google Scholar]
- Franklin, J.C.; Schiele, B.C.; Brozek, J.; Keys, A. Observations of human behavior in experimental semistarvation and rehabilitation. J. Clin. Psychol. 1948, 4, 4–28. [Google Scholar] [CrossRef]
- Lowe, M.R.; Butryn, M.L. Hedonic hunger: A new dimension of appetite? Physiol. Behav. 2007, 91, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Maj, M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: Beyond the homeostatic control of food intake. Psychoneuroendocrinology 2013, 38, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Becker, T.; Di Marzo, V. Endocannabinoids and energy homeostasis: An update. Biofactors 2014, 40, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Gonthier, M.P.; Orlando, P.; Martiadis, V.; De Petrocellis, L.; Cervino, C.; Petrosino, S.; Hoareau, L.; Festy, F.; Pasquali, R.; et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 2006, 91, 3171–3180. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Piscitelli, F.; Scognamiglio, P.; Monteleone, A.M.; Canestrelli, B.; Di Marzo, V.; Maj, M. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: A pilot study. J. Clin. Endocrinol. Metab. 2012, 97, E917–E924. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, A.M.; Di Marzo, V.; Monteleone, P.; Dalle Grave, R.; Aveta, T.; El Ghoch, M.; Piscitelli, F.; Volpe, U.; Calugi, S.; Maj, M. Responses of peripheral endocannabinoids and endocannabinoid-related compounds to hedonic eating in obesity. Eur. J. Nutr. 2016, 55, 1789–1795. [Google Scholar]
- Scherma, M.; Fattore, L.; Satta, V.; Businco, F.; Pigliacampo, B.; Goldberg, S.R.; Dessi, C.; Fratta, W.; Fadda, P. Pharmacological modulation of the endocannabinoid signalling alters binge-type eating behaviour in female rats. Br. J. Pharmacol. 2013, 169, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Annuzzi, G.; Piscitelli, F.; Di Marino, L.; Patti, L.; Giacco, R.; Costabile, G.; Bozzetto, L.; Riccardi, G.; Verde, R.; Petrosino, S.; et al. Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutaneous adipose tissue of obese diabetic patients. Lipids Health Dis. 2010, 9, 43. [Google Scholar]
- Dixon, J. BMDP Statistical Software; University of California Press: Berkley, CA, USA, 1985. [Google Scholar]
- Mahler, S.V.; Smith, K.S.; Berridge, K.C. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology 2007, 32, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Mahler, S.V.; Pecina, S.; Berridge, K.C. Hedonic hotspots: Generating sensory pleasure in the brain. In Pleasures of the Brain; Kringelbach, M.L., Berridge, K.C., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 27–49. [Google Scholar]
- Dalton, M.; Blundell, J.; Finlayson, G.S. Examination of food reward and energy intake under laboratory and free-living conditions in a trait binge eating subtype of obesity. Front. Psychol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Montoya, M.L.; Neyrinck, A.M.; Delzenne, N.M.; Lambert, D.M. Potential modulation of plasma ghrelin and glucagon-like peptide-1 by anorexigenic cannabinoid compounds, SR141716A (rimonabant) and oleoylethanolamide. Br. J. Nutr. 2004, 92, 757–761. [Google Scholar] [CrossRef] [PubMed]
| Favorite Food | Non-Favorite Food | ||||||
|---|---|---|---|---|---|---|---|
| Kcal | Carbohydrates | Proteins | Lipids | Kcal | Carbohydrates | Proteins | Lipids |
| 724.06 ± 381.24 | 101.60 ± 66.49 | 12.40 ± 7.80 | 26.35 ± 16.15 | 688.97 ± 326.31 | 91.20 ± 57.52 | 12.22 ± 9.17 | 31.77 ± 13.34 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteleone, A.M.; Piscitelli, F.; Dalle Grave, R.; El Ghoch, M.; Di Marzo, V.; Maj, M.; Monteleone, P. Peripheral Endocannabinoid Responses to Hedonic Eating in Binge-Eating Disorder. Nutrients 2017, 9, 1377. https://doi.org/10.3390/nu9121377
Monteleone AM, Piscitelli F, Dalle Grave R, El Ghoch M, Di Marzo V, Maj M, Monteleone P. Peripheral Endocannabinoid Responses to Hedonic Eating in Binge-Eating Disorder. Nutrients. 2017; 9(12):1377. https://doi.org/10.3390/nu9121377
Chicago/Turabian StyleMonteleone, Alessio Maria, Fabiana Piscitelli, Riccardo Dalle Grave, Marwan El Ghoch, Vincenzo Di Marzo, Mario Maj, and Palmiero Monteleone. 2017. "Peripheral Endocannabinoid Responses to Hedonic Eating in Binge-Eating Disorder" Nutrients 9, no. 12: 1377. https://doi.org/10.3390/nu9121377
APA StyleMonteleone, A. M., Piscitelli, F., Dalle Grave, R., El Ghoch, M., Di Marzo, V., Maj, M., & Monteleone, P. (2017). Peripheral Endocannabinoid Responses to Hedonic Eating in Binge-Eating Disorder. Nutrients, 9(12), 1377. https://doi.org/10.3390/nu9121377









