Adherence to Hunger Training over 6 Months and the Effect on Weight and Eating Behaviour: Secondary Analysis of a Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Outcome Measures
2.4. Hunger Training Intervention
2.5. Adherence to Hunger Training
2.6. Statistical Analysis
3. Results
3.1. Participants
3.2. Weight Loss
3.3. Blood Glucose Monitoring
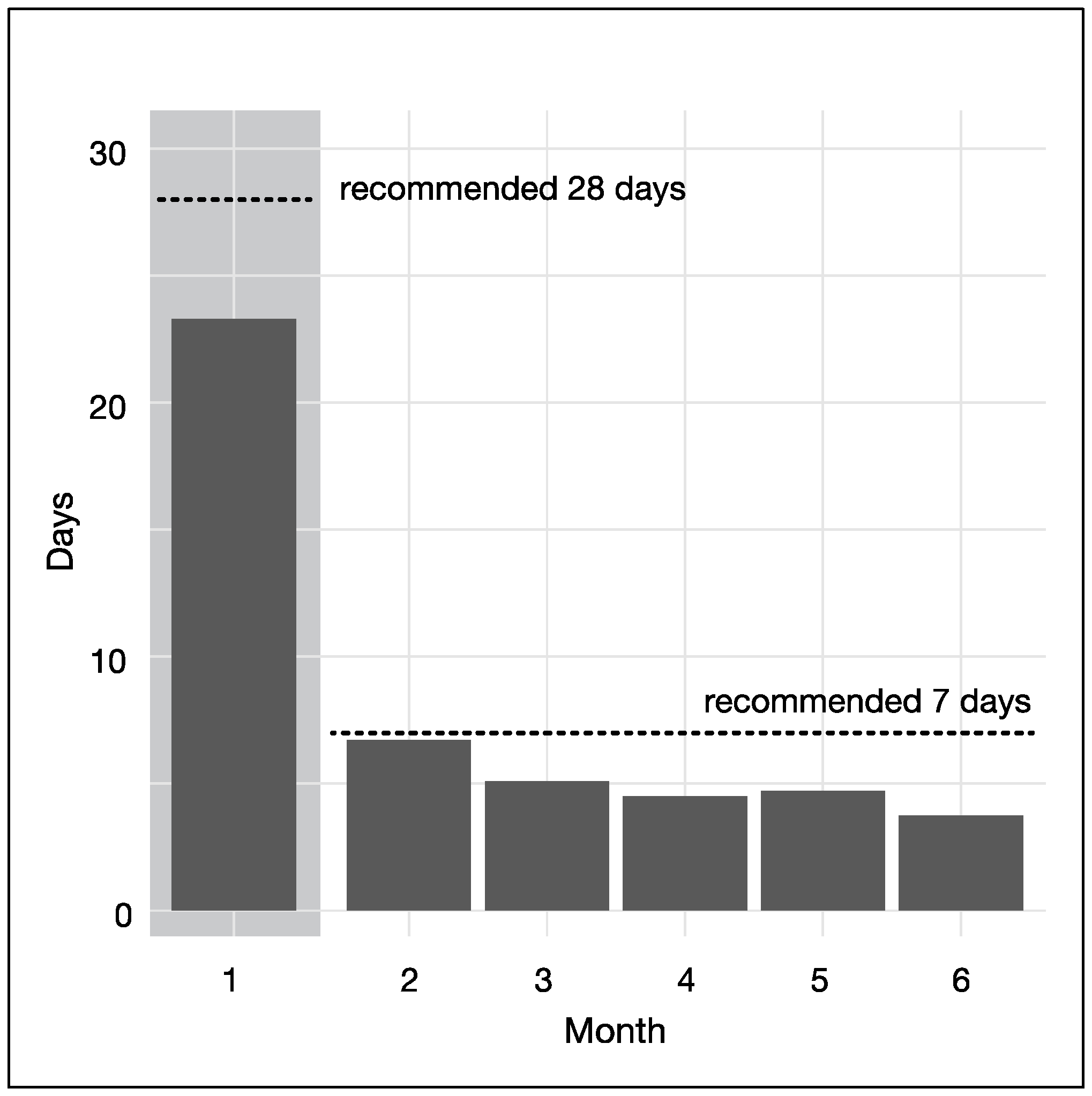
3.4. Adherence to Hunger Training Booklet
3.5. Hunger Training Adherence and Weight Loss
3.6. Hunger Training Frequency and Eating Behaviours
3.7. Types of Hunger
3.8. Predictors of Adherence
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baños, R.M.; Cebolla, A.; Moragrega, I.; Van Strien, T.; Fernández-Aranda, F.; Agüera, Z.; de la Torre, R.; Casanueva, F.F.; Fernández-Real, J.M.; Fernández-García, J.C.; et al. Relationship between eating styles and temperament in an Anorexia Nervosa, Healthy Control, and Morbid Obesity female sample. Appetite 2014, 76, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Van Strien, T.; Winkens, L.; Toft, M.B.; Pedersen, S.; Brouwer, I.; Visser, M.; Lähteenmäki, L. The mediation effect of emotional eating between depression and body mass index in the two European countries Denmark and Spain. Appetite 2016, 105, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Madden, C.E.L.; Leong, S.L.; Gray, A.; Horwath, C.C. Eating in response to hunger and satiety signals is related to BMI in a nationwide sample of 1601 mid-age New Zealand women. Public Health Nutr. 2012, 15, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Tylka, T.L.; Kroon Van Diest, A.M. The Intuitive Eating Scale-2: Item refinement and psychometric evaluation with college women and men. J. Couns. Psychol. 2013, 60, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Denny, K.N.; Loth, K.; Eisenberg, M.E.; Neumark-Sztainer, D. Intuitive eating in young adults. Who is doing it, and how is it related to disordered eating behaviors? Appetite 2013, 60, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Iannantuono, A.C.; Tylka, T.L. Interpersonal and intrapersonal links to body appreciation in college women: An exploratory model. Body Image 2012, 9, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, N.; Drinkwater, E.J. Review Article Relationships between intuitive eating and health indicators: Literature review. Public Health Nutr. 2014, 17, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Borkoles, E.; Carroll, S.; Clough, P.; Polman, R.C.J. Effect of a non-dieting lifestyle randomised control trial on psychological well-being and weight management in morbidly obese pre-menopausal women. Maturitas 2016, 83, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Provencher, V.; Bégin, C.; Tremblay, A.; Mongeau, L.; Corneau, L.; Dodin, S.; Boivin, S.; Lemieux, S. Health-At-Every-Size and Eating Behaviors: 1-Year Follow-Up Results of a Size Acceptance Intervention. J. Am. Diet Assoc. 2009, 109, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, V.; Provencher, V.; Bégin, C.; Corneau, L.; Tremblay, A.; Lemieux, S. Impact of a Health-At-Every-Size intervention on changes in dietary intakes and eating patterns in premenopausal overweight women: Results of a randomized trial. Clin. Nutr. 2012, 31, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Bacon, L.; Keim, N.L.; Van Loan, M.D.; Derricote, M.; Gale, B.; Kazaks, A.; Stern, J.S. Evaluating a “non-diet” wellness intervention for improvement of metabolic fitness, psychological well-being and eating and activity behaviors. Int. J. Obes. 2002, 26, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Barkeling, B.; King, N.A.; Naslund, E.; Blundell, J.E. Characterization of obese individuals who claim to detect no relationship between their eating pattern and sensations of hunger or fullness. Int. J. Obes. 2007, 31, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Stubbs, R.J.; Golding, C.; Croden, F.; Alam, R.; Whybrow, S.; Le Noury, J.; Lawton, C.L. Resistance and susceptibility to weight gain: Individual variability in response to a high-fat diet. Physiol. Behav. 2005, 86, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, V.; Blundell, J.; Gallant, A.R.; Arguin, H.; Després, J.P.; Lamarche, B.; Tremblay, A. Behavioural and metabolic characterisation of the low satiety phenotype. Appetite 2013, 70, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Gregersen, N.T.; Gluud, L.L.; Møller, B.K.; Raben, A.; Tetens, I.; Verdich, C.; Astrup, A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: A meta-analysis of test meal studies. Br. J. Nutr. 2007, 98, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, S.G.; Martens, E.A.; Kester, A.D.; Westerterp-Plantenga, M.S. Changes in gut hormone and glucose concentrations in relation to hunger and fullness. Am. J. Clin. Nutr. 2011, 94, 717–725. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Ciampolini, M.; Bianchi, R. Training to estimate blood glucose and to form associations with initial hunger. Nutr. Metab. 2006, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Jospe, M.R.; Brown, R.C.; Roy, M.; Taylor, R.W. Adherence to hunger training using blood glucose monitoring: A feasibility study. Nutr. Metab. 2015, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Ciampolini, M.; Lovell-Smith, D.; Sifone, M. Sustained self-regulation of energy intake. Loss of weight in overweight subjects. Maintenance of weight in normal-weight subjects. Nutr. Metab. 2010, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Jospe, M.R.; Roy, M.; Brown, R.C.; Williams, S.M.; Osborne, H.R.; Meredith-Jones, K.A.; McArthur, J.R.; Williams, E.E.; Taylor, R.W. The effect of different types of monitoring strategies on weight loss: A randomised controlled trial. Obesity 2017, 25, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Adherence to Long-Term Therapies; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Taylor, R.W.; Roy, M.; Jospe, M.R.; Osborne, H.R.; Meredith-Jones, K.J.; Williams, S.M.; Brown, R.C. Determining how best to support overweight adults to adhere to lifestyle change: Protocol for the SWIFT study. BMC Public Health 2015, 15, 861. [Google Scholar] [CrossRef] [PubMed]
- Gosling, S.D.; Rentfrow, P.J.; Swann, W.B., Jr. A very brief measure of the Big-Five personality domains. J. Res. Personal. 2003, 37, 504–528. [Google Scholar] [CrossRef]
- Smith, B.W.; Dalen, J.; Wiggins, K.; Tooley, E.; Christopher, P.; Bernard, J. The brief resilience scale: Assessing the ability to bounce back. Int. J. Behav. Med. 2008, 15, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Witt, A.A.; Katterman, S.N.; Lowe, M.R. Assessing the three types of dieting in the Three-Factor Model of dieting. The Dieting and Weight History Questionnaire. Appetite 2013, 63, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Van Strien, T.; Frijters, J.E.R.; Bergers, G.P.A.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- Van Strien, T.; Peter Herman, C.; Verheijden, M.W. Eating style, overeating and weight gain. A prospective 2-year follow-up study in a representative Dutch sample. Appetite 2012, 59, 782–789. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Bacon, L.; Stern, J.S.; Van Loan, M.D.; Keim, N.L. Size Acceptance and Intuitive Eating Improve Health for Obese, Female Chronic Dieters. J. Am. Diet. Assoc. 2005, 105, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Gagnon-Girouard, M.-P.; Bégin, C.; Provencher, V.; Tremblay, A.; Mongeau, L.; Boivin, S.; Lemieux, S. Psychological Impact of a “Health-at-Every-Size” Intervention on Weight-Preoccupied Overweight/Obese Women. J. Obes. 2010, 2010, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hawley, G.; Horwath, C.; Gray, A.; Bradshaw, A.; Katzer, L.; Joyce, J.; O’Brien, S. Sustainability of health and lifestyle improvements following a non-dieting randomised trial in overweight women. Prev. Med. 2008, 47, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.A.; Lv, N.; Xiao, L.; Ma, J. Gender Differences in Weight-Related Attitudes and Behaviors Among Overweight and Obese Adults in the United States. Am. J. Mens. Health 2016, 10, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L.; Wood, L.G.; Collins, C.E.; Callister, R. Effectiveness of weight loss interventions—Is there a difference between men and women: A systematic review. Obes. Rev. 2015, 16, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.; Avenell, A.; Boachie, C.; Stewart, F.; Archibald, D.; Douglas, F.; Hoddinott, P.; van Teijlingen, E.; Boyers, D. Should weight loss and maintenance programmes be designed differently for men? A systematic review of long-term randomised controlled trials presenting data for men and women: The ROMEO project. Obes. Res. Clin. Pract. 2016, 10, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Pagoto, S.L.; Schneider, K.L.; Oleski, J.L.; Luciani, J.M.; Bodenlos, J.S.; Whited, M.C. Male Inclusion in Randomized Controlled Trials of Lifestyle Weight Loss Interventions. Obesity 2011, 20, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and Trends in Obesity among US Adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Boucher, S.; Edwards, O.; Gray, A.; Nada-Raja, S.; Lillis, J.; Tylka, T.L.; Horwath, C.C. Teaching Intuitive Eating and Acceptance and Commitment Therapy Skills Via a Web-Based Intervention: A Pilot Single-Arm Intervention Study. JMIR Res. Protoc. 2016, 5, e180. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, K.; Anderson, D. Dietary restraint and weight loss as risk factors for eating pathology. Eat. Behav. 2016, 23, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.; Lemstra, M.; Bird, Y.; Nwankwo, C.; Moraros, J. Weight-loss intervention adherence and factors promoting adherence: A meta-analysis. Patient Preference Adher. 2016, 10, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- King, A.C.; Ahn, D.F.; Atienza, A.A.; Kraemer, H.C. Exploring Refinements in Targeted Behavioral Medicine Intervention to Advance Public Health. Ann. Behav. Med. 2008, 35, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Vervloet, M.; Linn, A.J.; van Weert, J.C.M.; de Bakker, D.H.; Bouvy, M.L.; van Dijk, L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: A systematic review of the literature. J. Am. Med. Inform. Assoc. 2012, 19, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-Based Noninvasive Glucose Monitoring: A Proof-of-Concept Study. Anal. Chem. 2014, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Farandos, N.M.; Yetisen, A.K.; Monteiro, M.J.; Lowe, C.R.; Yun, S.H. Contact lens sensors in ocular diagnostics. Adv. Healthc. Mater. 2015, 4, 792–810. [Google Scholar] [CrossRef] [PubMed]
| Measure Number | Adherence Measure (Predictors) | Time | Calculation | Categories |
|---|---|---|---|---|
| 1 | Blood glucose measurement before eating | week 1–2 | Eating occasions where blood glucose was reported (%) | 0–70%, 70.1%–90%, 90.1%–100% |
| 2 | Eating only when blood glucose measurement was under cut-off | week 1–2 | Eating occasions where blood glucose was below the assigned cut-off divided by the total number of eating occasions with a noted blood glucose values (%) | 0–70%, 70.1%–90%, 90.1%–100% |
| 3 | Number of blood glucose measurements | month 1–6 | Total blood glucose measurements | 0–49, 50–99, 100+ |
| 4 | Number of booklet entries | month 1–6 | Number of days a participant recorded at least one item in the hunger training booklet 1 | 0–29, 30–59, 60+ |
| 5 | Type of hunger (mouth, heart, stomach) | month 1–6 | Days that the specific type of hunger was selected divided by the total number of days with a type of hunger selected (%) | 0–20%, 20.1%–40%, 40.1%–60%, 60.1%–80%, 80.1%–100% |
| Characteristic | Entire Sample | Completers | p-Value |
|---|---|---|---|
| n | 50 | 34 | |
| Age (years) | 40.8 (10.9) | 42.0 (10.3) | 0.637 |
| Female n (%) | 31 (62%) | 21 (62%) | 0.595 |
| University degree n (%) | 27 (54%) | 18 (53%) | 1.000 |
| Ethnicity (%) | 1.000 | ||
| European | 45 (90%) | 31 (91%) | |
| Other | 5 (10%) | 3 (9%) | |
| Partnered (%) | 43 (86%) | 31 (91.2%) | 0.707 |
| Weight (kg) | 95.9 (17.0) | 95.3 (17.5) | 0.865 |
| Height (cm) | 170.3 (9.5) | 170.2 (9.2) | 0.982 |
| BMI (kg/m2) | 33.0 (4.3) | 32.7 (4.3) | 0.798 |
| Waist (cm) | 100.3 (12.9) | 100.2 (14.6) | 1.000 |
| Fasting glucose (mmol/L) | 5.46 (1.09) | 5.55 (0.74) | 0.613 |
| Variable | Point Estimate | 95% CI 1 | p-Value 1 | |
|---|---|---|---|---|
| Lower | Upper | |||
| (Intercept) | 0.6 | |||
| Sex (male vs. female) | −4.7 | −8.1 | −1.4 | 0.007 |
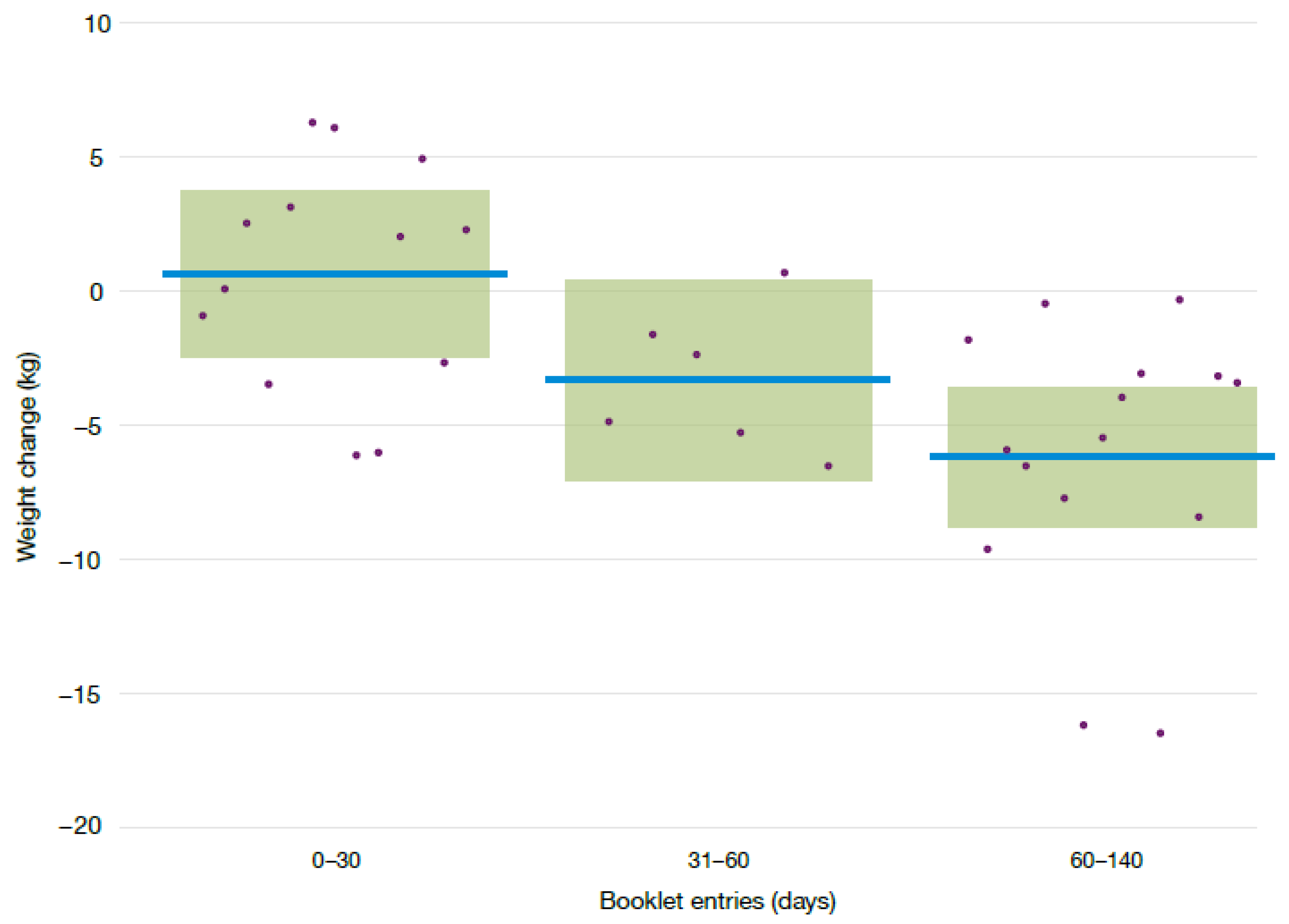
| Frequency of booklet entry: 30–59 days 2 | −4.0 | −9.6 | 1.7 | 0.093 |
| Frequency of booklet entry: 60–140 days 2 | −6.8 | −11.0 | −2.6 | 0.001 |
| Variable | n | Frequency Days | Month 0 Mean (SD) | Month 6 Mean (SD) | Difference 1 Mean (95% CI) |
|---|---|---|---|---|---|
| Weight (kg) | 13 | 0–29 | 96.3 (16.2) | 94.4 (16.0) | |
| 6 | 30–59 | 89.5 (14.7) | 85.4 (11.5) | −4.0 (−9.6, 1.7) | |
| 15 | ≥60 | 96.7 (20.1) | 89.1 (17.3) | −6.8 (−11.0, −2.6) | |
| BMI (kg/m2) | 13 | 0–29 | 32.3 (4.4) | 31.7 (4.4) | |
| 6 | 30–59 | 32.1 (2.2) | 30.7 (1.4) | −1.2 (−3.0, 0.5) | |
| 15 | ≥60 | 33.3 (4.8) | 30.8 (4.6) | −2.2 (−3.5, −0.8) | |
| Waist (cm) | 13 | 0–29 | 100.2 (11.7) | 97.6 (12.0) | |
| 6 | 30–59 | 96.2 (11.4) | 93.2 (7.0) | −2.0 (−9.0, 5.0) | |
| 15 | ≥60 | 101.9 (18.0) | 95.5 (14.5) | −4.7 (−10.0, 0.5) |
| Variable | n | Frequency Days | Month 0 Mean (SD) | Month 6 Mean (SD) | Difference 1 Mean (95% CI) |
|---|---|---|---|---|---|
| Overall Score | 13 | 0–29 | 3.03 (0.48) | 2.93 (0.55) | |
| 6 | 30–59 | 3.02 (0.52) | 3.06 (0.33) | 0.09 (−0.50, 0.67) | |
| 15 | ≥60 | 3.06 (0.49) | 3.37 (0.41) | 0.37 (−0.08, 0.82) | |
| Body-food choice congruence | 13 | 0–29 | 3.38 (0.78) | 3.19 (0.88) | |
| 6 | 30–59 | 3.22 (0.34) | 3.17 (0.94) | 0.29 (−0.52, 1.11) | |
| 15 | ≥60 | 3.40 (0.71) | 3.89 (0.80) | 0.73 (0.12, 1.35) | |
| Unconditional permission to eat | 13 | 0–29 | 3.24 (0.69) | 3.38 (0.61) | |
| 6 | 30–59 | 3.42 (0.38) | 2.94 (0.61) | −0.75 (−1.53, 0.04) | |
| 15 | ≥60 | 3.37 (0.54) | 2.87 (0.65) | −0.68 (−1.28, −0.09) | |
| Reliance on hunger and satiety cues | 13 | 0–29 | 2.78 (0.81) | 2.69 (0.87) | |
| 6 | 30–59 | 2.98 (0.75) | 2.92 (0.35) | −0.06 (−1.13, 1.00) | |
| 15 | ≥60 | 2.78 (0.67) | 3.44 (0.78) | 0.68 (−0.13, 1.49) | |
| Eating for physical rather than emotional reasons | 13 | 0–29 | 2.92 (0.89) | 2.69 (1.02) | |
| 6 | 30–59 | 2.67 (0.97) | 3.21 (0.40) | 0.74 (−0.22, 1.70) | |
| 15 | ≥60 | 2.91 (0.84) | 3.50 (0.75) | 0.79 (0.06, 1.51) |
| Variable | n | Frequency Days | Month 0 Mean (SD) | Month 6 Mean (SD) | Difference 1 Mean (95% CI) |
|---|---|---|---|---|---|
| Emotional Eating | 13 | 0–29 | 2.54 (0.82) | 2.75 (1.01) | |
| 6 | 30–59 | 2.66 (1.00) | 2.36 (0.44) | −0.65 (−1.22, -0.09) | |
| 15 | ≥60 | 2.43 (0.77) | 2.01 (1.01) | −0.70 (−1.13, −0.27) | |
| Restraint | 13 | 0–29 | 2.58 (0.62) | 2.55 (0.60) | |
| 6 | 30–59 | 2.55 (0.39) | 3.17 (0.32) | 0.85 (0.13, 1.57) | |
| 15 | ≥60 | 2.68 (0.57) | 2.87 (0.65) | 0.33 (−0.22, 0.88) | |
| External Eating | 13 | 0–29 | 3.43 (0.75) | 3.50 (0.77) | |
| 6 | 30–59 | 3.28 (0.35) | 3.18 (0.76) | −0.25 (−0.89, 0.39) | |
| 15 | ≥60 | 2.97 (0.71) | 2.65 (0.60) | −0.43 (-0.92, 0.06) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jospe, M.R.; Taylor, R.W.; Athens, J.; Roy, M.; Brown, R.C. Adherence to Hunger Training over 6 Months and the Effect on Weight and Eating Behaviour: Secondary Analysis of a Randomised Controlled Trial. Nutrients 2017, 9, 1260. https://doi.org/10.3390/nu9111260
Jospe MR, Taylor RW, Athens J, Roy M, Brown RC. Adherence to Hunger Training over 6 Months and the Effect on Weight and Eating Behaviour: Secondary Analysis of a Randomised Controlled Trial. Nutrients. 2017; 9(11):1260. https://doi.org/10.3390/nu9111260
Chicago/Turabian StyleJospe, Michelle R., Rachael W. Taylor, Josie Athens, Melyssa Roy, and Rachel C. Brown. 2017. "Adherence to Hunger Training over 6 Months and the Effect on Weight and Eating Behaviour: Secondary Analysis of a Randomised Controlled Trial" Nutrients 9, no. 11: 1260. https://doi.org/10.3390/nu9111260
APA StyleJospe, M. R., Taylor, R. W., Athens, J., Roy, M., & Brown, R. C. (2017). Adherence to Hunger Training over 6 Months and the Effect on Weight and Eating Behaviour: Secondary Analysis of a Randomised Controlled Trial. Nutrients, 9(11), 1260. https://doi.org/10.3390/nu9111260







