The Stoichiometry of Isoquercitrin Complex with Iron or Copper Is Highly Dependent on Experimental Conditions
Abstract
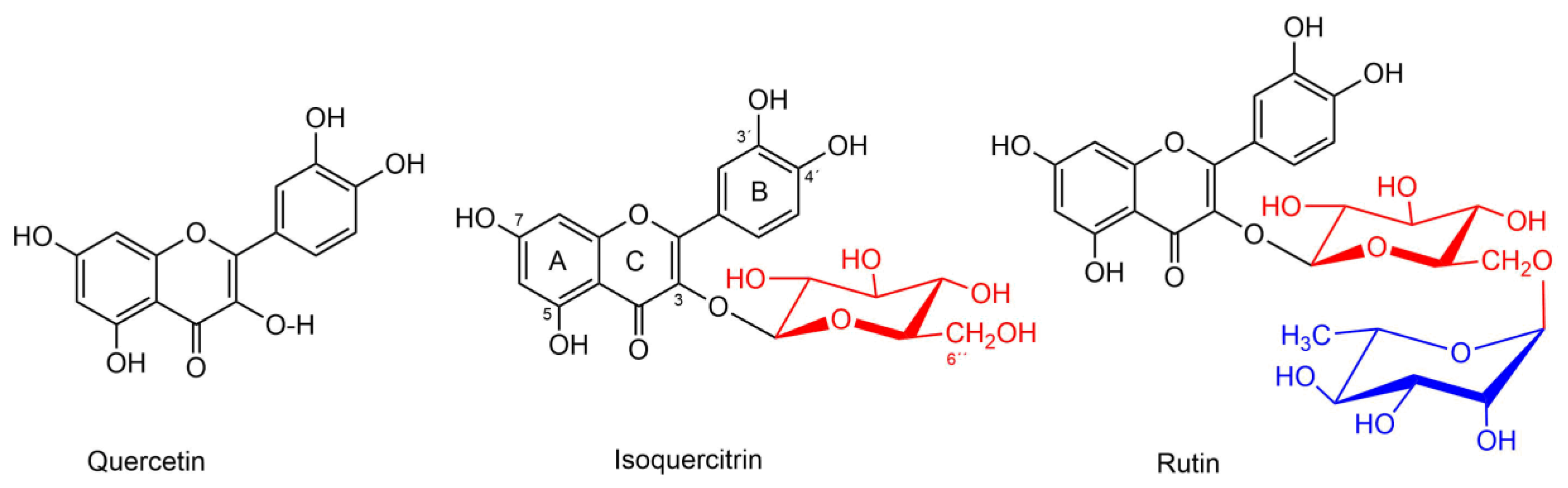
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Solutions, and Equipment
2.2. PH Conditions
2.3. Assessment of Iron/Copper Complex Stoichiometry
2.3.1. Complex Formation
2.3.2. Job’s Method
2.3.3. Complementary Method
2.4. Competitive Measurement of Metal Chelation and Reduction
2.4.1. Ferrozine Method
2.4.2. Hematoxylin Method
2.4.3. BCS Method
2.5. Statistical Analysis
3. Results
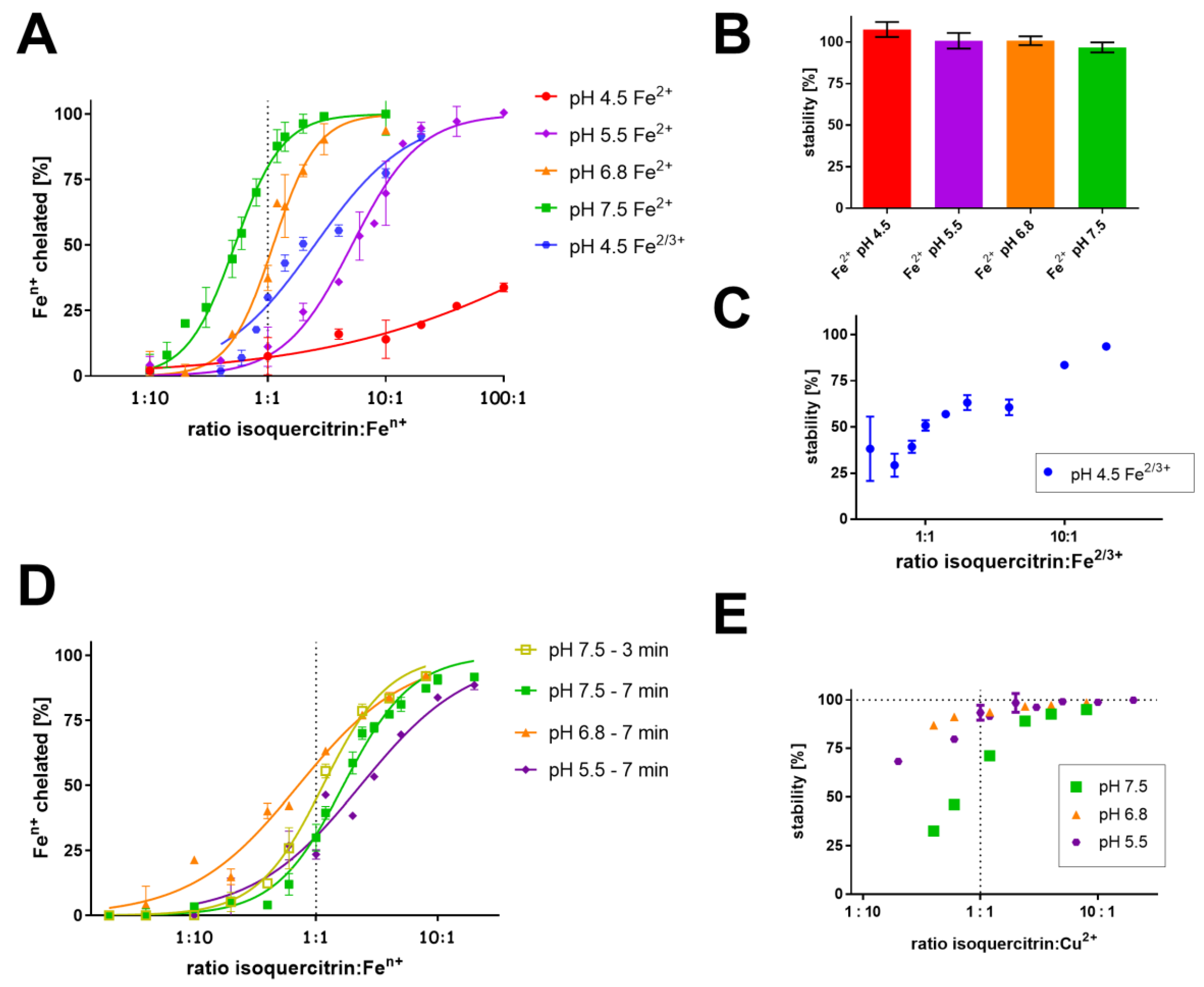
3.1. Determination of Complex Stoichiometry by Job’s and Complementary Methods
3.2. Competitive Methods
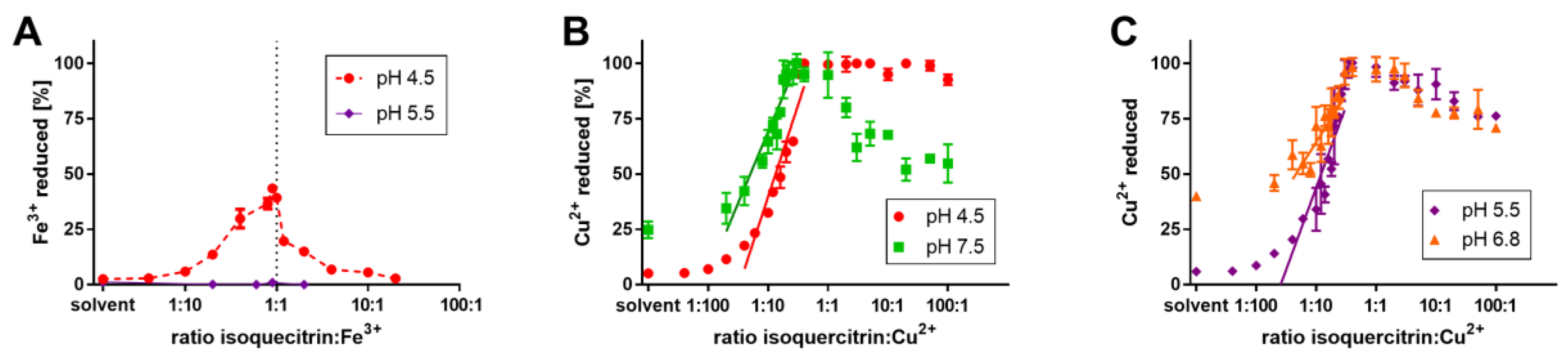
3.3. Iron and Copper Reduction
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BCS | bathocuproinedisulphonic acid disodium salt |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid |
| HA | hydroxylamine hydrochloride |
References
- Valentova, K.; Vrba, J.; Bancirova, M.; Ulrichova, J.; Kren, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Sun, C.X.; Mao, L.K.; Ma, P.H.; Liu, F.G.; Yang, J.; Gao, Y.X. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Li, R.; Yuan, C.; Dong, C.; Shuang, S.; Choi, M.M. In vivo antioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney. Naunyn Schmied. Arch. Pharmacol. 2011, 383, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Nonaka, M.; Narahara, M.; Torii, I.; Kawaguchi, K.; Yoshikawa, T.; Kumazawa, Y.; Morikawa, S. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci. 2003, 74, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kimura, M.; Ishii, Y.; Yamamoto, R.; Morita, R.; Hayashi, S.M.; Suzuki, K.; Shibutani, M. Effect of enzymatically modified isoquercitrin on preneoplastic liver cell lesions induced by thioacetamide promotion in a two-stage hepatocarcinogenesis model using rats. Toxicology 2013, 305, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, A., Jr.; Gasparotto, F.M.; Lourenco, E.L.; Crestani, S.; Stefanello, M.E.; Salvador, M.J.; da Silva-Santos, J.E.; Marques, M.C.; Kassuya, C.A. Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: Evidence for the inhibition of angiotensin converting enzyme. J. Ethnopharmacol. 2011, 134, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside. Biofactors 2007, 31, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; da Silva, E.V.G.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, L.M.; Wasowski, C.; Marder, M. Neuroactive flavonoid glycosides from Tilia petiolaris DC. extracts. Phytother. Res. 2009, 23, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Paulke, A.; Eckert, G.P.; Schubert-Zsilavecz, M.; Wurglics, M. Isoquercitrin provides better bioavailability than quercetin: Comparison of quercetin metabolites in body tissue and brain sections after six days administration of isoquercitrin and quercetin. Pharmazie 2012, 67, 991–996. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Feng, Y.; Ouyang, H.; Yu, B.; Chang, Y.; Pan, G.; Dong, G.; Wang, T.; Gao, X. A sensitive LC-MS/MS method for simultaneous determination of six flavonoids in rat plasma: Application to a pharmacokinetic study of total flavonoids from mulberry leaves. J. Pharm. Biomed. Anal. 2013, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, Y.; Su, D.; Gao, G.; Zhou, X.; Sun, L.; Ba, X.; Chen, X.; Bi, K. A sensitive LC-MS-MS method for simultaneous quantification of two structural isomers, hyperoside and isoquercitrin: Application to pharmacokinetic studies. Chromatographia 2011, 73, 353–359. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.W.; Zhang, L.; Liu, X.; Chen, X.H.; Bi, K.S. HPLC analysis and pharmacokinetic study of quercitrin and isoquercitrin in rat plasma after administration of Hypericum japonicum Thunb. extract. Biomed. Chromatogr. 2008, 22, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Guo, J.; Qian, D.; Duan, J.A.; Shang, E.; Shu, Y.; Lu, Y. Identification of the potential active components of Abelmoschus manihot in rat blood and kidney tissue by microdialysis combined with ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xue, C.; Duan, J.A.; Qian, D.; Tang, Y.; You, Y. Anticonvulsant, antidepressant-like activity of Abelmoschus manihot ethanol extract and its potential active components in vivo. Phytomedicine 2011, 18, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Gerstorferova, D.; Fliedrova, B.; Halada, P.; Marhol, P.; Kren, V.; Weignerova, L. Recombinant a-l-rhamnosidase from Aspergillus terreus in selective trimming of rutin. Process Biochem. 2012, 47, 828–835. [Google Scholar] [CrossRef]
- Monti, D.; Pisvejcova, A.; Kren, V.; Lama, M.; Riva, S. Generation of an α-l-rhamnosidase library and its application for the selective derhamnosylation of natural products. Biotechnol. Bioeng. 2004, 87, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Kim, Y.A.; Kim, M.M.; Park, J.S.; Kim, J.A.; Kim, S.K.; Lee, B.J.; Nam, T.J.; Seo, Y. Flavonoid glycosides isolated from Salicornia herbacea inhibit matrix metalloproteinase in HT1080 cells. Toxicol. In Vitro 2008, 22, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Salucci, M.; Stivala, L.A.; Maiani, G.; Bugianesi, R.; Vannini, V. Flavonoids uptake and their effect on cell cycle of human colon adenocarcinoma cells (Caco2). Br. J. Cancer 2002, 86, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.; Kim, E.J.; Choi, J.S.; Park, H.J. Simultaneous quantification and peroxynitrite-scavenging activities of flavonoids in Polygonum aviculare L. herb. J. Pharm. Biomed. Anal. 2014, 89, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Cornard, J.P.; Merlin, J.C. Complexes of aluminium(III) with isoquercitrin: Spectroscopic characterization and quantum chemical calculations. Polyhedron 2002, 21, 2801–2810. [Google Scholar] [CrossRef]
- Li, X.C.; Jiang, Q.; Wang, T.T.; Liu, J.J.; Chen, D.F. Comparison of the antioxidant effects of quercitrin and isoquercitrin: Understanding the role of the 6′′-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Mitsukuni, Y.; Ichikawa, M.; Tsushida, T.; Miyamoto, S.; Terao, J. Quercetin-4′-glucoside is more potent than quercetin-3-glucoside in protection of rat intestinal mucosa homogenates against iron ion-induced lipid peroxidation. J. Agric. Food. Chem. 2004, 52, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Macakova, K.; Mladenka, P.; Filipsky, T.; Riha, M.; Jahodar, L.; Trejtnar, F.; Bovicelli, P.; Proietti Silvestri, I.; Hrdina, R.; Saso, L. Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chem. 2012, 135, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.M.; Leavitt, P.S.; Lin, C.P. Dietary flavonoids interact with trace metals and affect metallothionein level in human intestinal cells. Biol. Trace Elem. Res. 1998, 62, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Riha, M.; Karlickova, J.; Filipsky, T.; Macakova, K.; Rocha, L.; Bovicelli, P.; Silvestri, I.P.; Saso, L.; Jahodar, L.; Hrdina, R.; et al. In vitro evaluation of copper-chelating properties of flavonoids. RSC Adv. 2014, 4, 32628–32638. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Fazal, F.; Rahman, A.; Hadi, S.M.; Parish, J.H. Activities of flavonoids for the cleavage of DNA in the presence of Cu(II)—Correlation with generation of active oxygen species. Carcinogenesis 1992, 13, 605–608. [Google Scholar] [CrossRef]
- Rahman, A.; Shahabuddin; Hadi, S.M.; Parish, J.H. Complexes involving quercetin, DNA and Cu(II). Carcinogenesis 1990, 11, 2001–2003. [Google Scholar] [CrossRef] [PubMed]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florencio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Filipsky, T.; Riha, M.; Hrdina, R.; Vavrova, K.; Mladenka, P. Mathematical calculations of iron complex stoichiometry by direct UV-Vis spectrophotometry. Bioorg. Chem. 2013, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Markovic, J.M.D.; Markovic, Z.S.; Brdaric, T.P.; Pavelkic, V.M.; Jadranin, M.B. Iron complexes of dietary flavonoids: Combined spectroscopic and mechanistic study of their free radical scavenging activity. Food Chem. 2011, 129, 1567–1577. [Google Scholar] [CrossRef]
- Job, P. Recherches sur la formation des complexes mineraux en solution, et sur leur stabilité. Ann. Chim. 1928, 9, 113–134. [Google Scholar]
- Mladenka, P.; Macakova, K.; Zatloukalova, L.; Rehakova, Z.; Singh, B.K.; Prasad, A.K.; Parmar, V.S.; Jahodar, L.; Hrdina, R.; Saso, L. In vitro interactions of coumarins with iron. Biochimie 2010, 92, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Riha, M.; Karlickova, J.; Filipsky, T.; Macakova, K.; Hrdina, R.; Mladenka, P. Novel method for rapid copper chelation assessment confirmed low affinity of D-penicillamine for copper in comparison with trientine and 8-hydroxyquinolines. J. Inorg. Biochem. 2013, 123, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, J.A.; Stainer, A.; Hobbs, D.A. Role of flavonoids and nitrates in cardiovascular health. Proc. Nutr. Soc. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mladenka, P.; Macakova, K.; Filipsky, T.; Zatloukalova, L.; Jahodar, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.F.V.; Sussuchi, E.M.; De Giovani, W.F. Synthesis, electrochemical, spectral, and antioxidant properties of complexes of flavonoids with metal ions. Synth. React. Inorg. Met. 2003, 33, 1125–1144. [Google Scholar] [CrossRef]
- Guo, M.L.; Perez, C.; Wei, Y.B.; Rapoza, E.; Su, G.; Bou-Abdallah, F.; Chasteen, N.D. Iron-binding properties of plant phenolics and cranberry’s bio-effects. Dalton Trans. 2007, 4951–4961. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C.; Aisen, P. Facilitation of Fe(II) Autoxidation by Fe(III) complexing agents. Biochim. Biophys. Acta 1973, 329, 156–158. [Google Scholar] [CrossRef]
- Hasumura, M.; Yasuhara, K.; Tamura, T.; Imai, T.; Mitsumori, K.; Hirose, M. Evaluation of the toxicity of enzymatically decomposed rutin with 13-weeks dietary administration to Wistar rats. Food Chem. Toxicol. 2004, 42, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Brune, M.; Rossander, L.; Hallberg, L. Iron absorption and phenolic compounds: Importance of different phenolic structures. Eur. J. Clin. Nutr. 1989, 43, 547–557. [Google Scholar] [PubMed]
- Brewer, G.J.; Yuzbasiyangurkan, V.; Dick, R.; Wang, Y.X.; Johnson, V. Does a vegetarian diet control Wilsons-disease. J. Am. Coll. Nutr. 1993, 12, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Biler, M.; Biedermann, D.; Valentova, K.; Kren, V.; Kubala, M. Quercetin and its analogues: Optical and acido-basic properties. Phys. Chem. Chem. Phys. 2017, 19, 26870–26879. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; Sesink, A.L.; Faassen-Peters, M.; Hollman, P.C. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br. J. Nutr. 2004, 91, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Shimizu, R.; Kanemaru, M.; Suzuki, Y.; Moriwaki, M.; Mizukami, H. Enzymatically modified isoquercitrin, α-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biol. Pharm. Bull. 2009, 32, 2034–2040. [Google Scholar] [CrossRef] [PubMed]


| pH 4.5 | pH 5.5 | pH 6.8 | pH 7.5 | ||
|---|---|---|---|---|---|
| Job’s method | 1:1 | 1:1 | 1:1 | 1:1 | |
| Fe3+ | Complementary approach | 1:1 | 1:1 | 1:1 | 1:1 |
| Competitive method | low affinity | X | X | X | |
| Job’s method | no complex | low affinity | 1:1 | 1:1 | |
| Fe2+ | Complementary approach | no complex | low affinity | 1:1 → 3:2 | 1:1 |
| Competitive method | low affinity | low affinity | 2:1 | 3:2 | |
| Job’s method | no complex | 1:1 or 3:2 | 1:1 | 1:1 | |
| Cu2+ | Complementary approach | no complex | 1:1 | 1:1 | 1:1 |
| Competitive method | X | low affinity | 2:1 | 2:1 * | |
| Job’s method | no complex | ||||
| Cu+ | Complementary approach | no complex | |||
| Competitive method | low affinity | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catapano, M.C.; Tvrdý, V.; Karlíčková, J.; Migkos, T.; Valentová, K.; Křen, V.; Mladěnka, P. The Stoichiometry of Isoquercitrin Complex with Iron or Copper Is Highly Dependent on Experimental Conditions. Nutrients 2017, 9, 1193. https://doi.org/10.3390/nu9111193
Catapano MC, Tvrdý V, Karlíčková J, Migkos T, Valentová K, Křen V, Mladěnka P. The Stoichiometry of Isoquercitrin Complex with Iron or Copper Is Highly Dependent on Experimental Conditions. Nutrients. 2017; 9(11):1193. https://doi.org/10.3390/nu9111193
Chicago/Turabian StyleCatapano, Maria Carmen, Václav Tvrdý, Jana Karlíčková, Thomas Migkos, Kateřina Valentová, Vladimír Křen, and Přemysl Mladěnka. 2017. "The Stoichiometry of Isoquercitrin Complex with Iron or Copper Is Highly Dependent on Experimental Conditions" Nutrients 9, no. 11: 1193. https://doi.org/10.3390/nu9111193
APA StyleCatapano, M. C., Tvrdý, V., Karlíčková, J., Migkos, T., Valentová, K., Křen, V., & Mladěnka, P. (2017). The Stoichiometry of Isoquercitrin Complex with Iron or Copper Is Highly Dependent on Experimental Conditions. Nutrients, 9(11), 1193. https://doi.org/10.3390/nu9111193








