The Role of Nutrition in Periodontal Health: An Update
Abstract
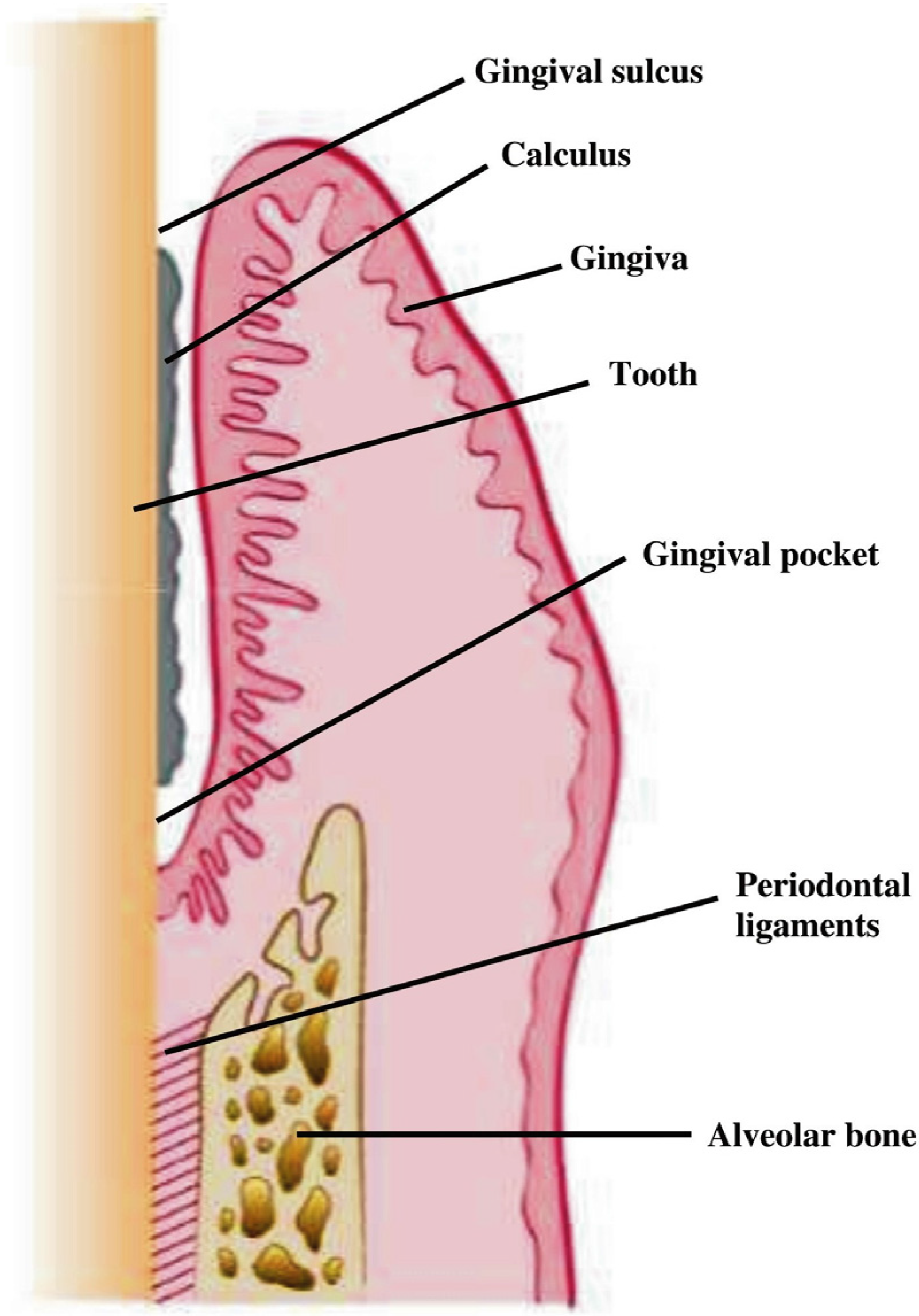
:1. Introduction
2. Role of Key Nutrients in Periodontal Health
2.1. Carbohydrates and Periodontal Health
2.2. Vitamins
2.2.1. Vitamin A
2.2.2. Vitamin B Complex
2.2.3. Vitamin C
2.2.4. Vitamin D
2.2.5. Vitamin E
2.2.6. Vitamin K
3. Antioxidants and Periodontal Health
3.1. Vitamins as Antioxidants
3.2. Lycopene
3.3. Melatonin
4. Dietary Minerals and Trace Elements
4.1. Calcium
4.2. Magnesium
4.3. Iron
4.4. Zinc
4.5. Fluoride
5. Nutrients, Periodontal Health, and Specific Conditions
5.1. Pregnancy
5.2. Ageing Population and Role of Diet Nutrition
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Newman, G.M.; Takei, H.H.; Klokkevold, R.P.; Carranza, A.F. Carranza’s Clinical Periodontology. Classification of Diseases and Conditions Affecting the Periodontium. In Carranza’s Clinical Periodontology, 12th ed.; Michael, G.N., Henry, H.T., Perry, R.K., Fermín, A.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 45–67. [Google Scholar]
- Armitage, G.C.; Robertson, P.B. The Biology, Prevention, Diagnosis and Treatment of Periodontal Diseases: Scientific Advances in the United States. J. Am. Dent. Assoc. 2009, 140, 36S–43S. [Google Scholar] [CrossRef] [PubMed]
- Deas, D.E.; Moritz, A.J.; Sagun, R.S.; Gruwell, S.F.; Powell, C.A. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol. 2000 2016, 71, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S.; Goodson, J.M. Clinical parameters as predictors of destructive periodontal disease activity. J. Clin. Periodontol. 1983, 10, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontol. 2000 2005, 38, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yoneda, M.; Hirofuji, T. Mixed red-complex bacterial infection in periodontitis. Int. J. Dent. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.; Rutherford, R.B. The effects of interleukin 1 on collagenolytic activity and prostaglandin-E secretion by human periodontal-ligament and gingival fibroblast. Arch. Oral Biol. 1988, 33, 237–243. [Google Scholar] [CrossRef]
- Cawson, R.A. Cawson’s Essentials of Oral Pathology and Oral Medicine; Churchill Livingstone: Edinburgh, The Netherlands, 2002; p. 402. [Google Scholar]
- Fahad, K.; Aziz, A.; Shahab, S.; Zafar, M. Laboratorial and clinical impacts of tobacco on periodontal health: A systematic review. Int. Dent. J. Stud. Res. 2015, 3, 72–78. [Google Scholar]
- Ashimoto, A.; Chen, C.; Bakker, I.; Slots, J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol. Immunol. 1996, 11, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Garcia, R.; Heiss, G.; Vokonas, P.S.; Offenbacher, S. Periodontal disease and cardiovascular disease. J. Periodontol. 1996, 67, 1123–1137. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P.P.; Drangsholt, M.; Spiekerman, C.; DeRouen, T.A. Periodontal disease and coronary heart disease risk. JAMA 2000, 284, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Bi-directional relationship between pregnancy and periodontal disease. Periodontol. 2000 2013, 61, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Sharma, P.; Walter, C.; Weston, P.; Beck, J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J. Clin. Periodontol. 2013, 40, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Papapanou, P.N. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes—Systematic review. J. Clin. Periodontol. 2013, 40, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lii, C.; Tai, K.; Chou, M. Adverse effects of arecoline and nicotine on human periodontal ligament fibroblasts in vitro. J. Clin. Periodontol. 2001, 28, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, T.; Musskopf, M.L.; Oppermann, R.V.; Susin, C. Is there a positive effect of smoking cessation on periodontal health? A systematic review. J. Periodontol. 2014, 85, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Zafar, M.; Al-Wasifi, Y.; Fareed, W.; Khurshid, Z. Role of enamel deminerlization and remineralization on microtensile bond strength of resin composite. Eur. J. Dent. 2016, 10, 376–380. [Google Scholar] [PubMed]
- Keestra, J.A.J.; Grosjean, I.; Coucke, W.; Quirynen, M.; Teughels, W. Non-surgical periodontal therapy with systemic antibiotics in patients with untreated chronic periodontitis: A systematic review and meta-analysis. J. Periodontol. Res. 2015, 50, 294–314. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Zafar, M.; Khurshid, Z.; Almas, K. Oral tissue engineering progress and challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Matinlinna, J.P.; Siddiqui, F.; Nassani, M.Z.; Baroudi, K. Nanomodified peek dental implants: Bioactive composites and surface modification—A Review. Int. J. Dent. 2015. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.A.; Khurshid, Z.; Zafar, M.S.; Najeeb, S. Immediate implants: Clinical guidelines for esthetic outcomes. Dent. J. 2016, 4, 21. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 2015. [Google Scholar] [CrossRef]
- Chapple, I. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.; Schaeublin, M.; Jeker, H.; Fuller, K.; Chambers, T. The role of reactive oxygen intermediates in osteoclastic bone resorption. Biochem. Biophys. Res. Commun. 1995, 207, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Kamal, R.; Luthra, R.; Mishra, R.; Saini, G. Miswak: A periodontist’s perspective. J. Ayurveda Integr. Med. 2012, 3, 184. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Kamal, R.; Gupta, R.; Bhardwaj, R.; Chaudhary, K.; Kaur, S. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Dodington, D.W.; Fritz, P.C.; Sullivan, P.J.; Ward, W.E. Higher intakes of fruits and vegetables, beta-carotene, Vitamin C, α-tocopherol, EPA, and DHA are positively associated with periodontal healing after nonsurgical periodontal therapy in nonsmokers but not in smokers. J. Nutr. 2015, 145, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Antonoglou, G.; Knuuttila, M.; Niemelä, O.; Raunio, T.; Karttunen, R.; Vainio, O.; Hedberg, P.; Ylöstalo, P.; Tervonen, T. Low serum level of 1,25(OH)2D is associated with chronic periodontitis. J. Periodont. Res. 2015, 50, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.S.; Kinane, D.F. Nutrition, inflammation, and periodontal disease. Nutrition 2003, 19, 475. [Google Scholar] [CrossRef]
- Lieberman, S.; Bruning, N.P.; Bruning, N. The Real Vitamin and Mineral Book: A Definitive Guide to Designing Your Personal Supplement Program; Penguin: New York, NY, USA, 2007. [Google Scholar]
- Liebler, D.C.; Stratton, S.P.; Kaysen, K.L. Antioxidant actions of β-carotene in liposomal and microsomal membranes: Role of carotenoid-membrane incorporation and α-tocopherol. Arch. Biochem. Biophys. 1997, 338, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Rekha, C.; Poornima, G.; Manasa, M.; Abhipsa, V.; Devi, J.P.; Kumar, H.T.V.; Kekuda, T.R.P. Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem. Sci. Trans. 2012, 1, 303–310. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thévenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jingers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996, 21, 845–853. [Google Scholar] [CrossRef]
- García, J.J.; Reiter, R.J.; Guerrero, J.M.; Escames, G.; Yu, B.P.; Oh, C.S.; Muñoz-Hoyos, A. Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 1997, 408, 297–300. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zohaib, S.; Zafar, M.S. Therapeutic potential of melatonin in oral medicine and periodontology. Kaohsiung J. Med. Sci. 2016, 32, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Manz, M.C.; Taylor, G.W.; Yoshihara, A.; Miyazaki, H. Relations of serum ascorbic acid and α-tocopherol to periodontal disease. J. Dent. Res. 2012, 91, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Muniz, F.W.; Nogueira, S.B.; Mendes, F.L.V.; Rösing, C.K.; Moreira, M.M.S.M.; de Andrade, G.M.; de Sousa, R.C. The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: A systematic review. Arch. Oral Biol. 2015, 60, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P. Dietary carbohydrates and dental-systemic diseases. J. Dent. Res. 2009, 88, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.A.; Pamer, C.A. Diet and Nutrition in Oral Health; Prentice Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Palmer, C.A.; Burnett, D.J.; Dean, B. It’s more than just candy: Important relationships between nutrition and oral health. Nutr. Today 2010, 45, 154–164. [Google Scholar] [CrossRef]
- Edgar, W. Extrinsic and intrinsic sugars: A review of recent UK recommendations on diet and caries. Caries Res. 1993, 27, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Shimazaki, Y.; Koga, T.; Tsuzuki, M.; Ohshima, A. Relationship between upper body obesity and periodontitis. J. Dent. Res. 2001, 80, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.; Joshipura, K.; Douglass, C. Periodontal Disease and Body Composition in Older Adults: Preliminary Results. In Procceedings of the AAP-NIDCR Symposium, Bethesda, ML, USA, 18–20 April 2001.
- El-Sharkawy, H.; Aboelsaad, N.; Eliwa, M.; Darweesh, M.; Alshahat, M.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin. J. Periodontol. 2010, 81, 1635–1643. [Google Scholar] [CrossRef]
- Ekuni, D.; Yamamoto, T.; Koyama, R.; Tsuneishi, M.; Naito, K.; Tobe, K. Relationship between body mass index and periodontitis in young Japanese adults. J. Periodont. Res. 2008, 43, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Chawla, T.N.; Glickman, I. Protein deprivation and the periodontal structures of the albino rat. Oral Surg. Oral Med. Oral Pathol. 1951, 4, 578–602. [Google Scholar] [CrossRef]
- Stahl, S.S.; Sandler, H.C.; Cahn, L. The effects of protein deprivation upon the oral tissues of the rat and particularly upon periodontal structures under irritation. Oral Surg. Oral Med. Oral Pathol. 1955, 8, 760–768. [Google Scholar] [CrossRef]
- Adegboye, A.R.; Boucher, B.J.; Kongstad, J.; Fiehn, N.; Christensen, L.B.; Heitmann, B.L. Calcium, vitamin D, casein and whey protein intakes and periodontitis among Danish adults. Public Health Nutr. 2016, 19, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Neiva, R.F.; Al-Shammari, K.; Nociti, F.H., Jr.; Soehren, S.; Wang, H. Effects of vitamin-B complex supplementation on periodontal wound healing. J. Periodontol. 2005, 76, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Bashutski, J.D.; Eber, R.M.; Kinney, J.S.; Benavides, E.; Maitra, S.; Braun, T.M.; Giannobile, W.V.; McCauley, L.K. The impact of vitamin D status on periodontal surgery outcomes. J. Dent. Res. 2011, 90, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, S.; Zhao, D.; Zou, H.; Sun, N.; Liang, X.; Dard, M.; Lanske, B.; Yuan, Q. Vitamin D supplementation enhances the fixation of titanium implants in chronic kidney disease mice. PLoS ONE 2014, 9, e95689. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function; Mosby: St. Louis, MO, USA; London, UK, 2012; p. 411. [Google Scholar]
- Frostell, G.; Birkhed, D.; Edwardsson, S.; Goldberg, P.; Petersson, L.; Priwe, C.; Winholt, A. Effect of Partial substitution of invert sugar for sucrose in combination with Duraphat® treatment on caries development in preschool children: The Malmö study. Caries Res. 1991, 25, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Saido, M.; Asakura, K.; Masayasu, S.; Sasaki, S. Relationship between dietary sugar intake and dental caries among Japanese preschool children with relatively low sugar intake (Japan Nursery School SHOKUIKU Study): A nationwide cross-sectional study. Matern. Child Health J. 2016, 20, 556. [Google Scholar] [CrossRef]
- Keukenmeester, R.; Slot, D.; Rosema, N.; Van Loveren, C.; Van der Weijden, G. Effects of sugar-free chewing gum sweetened with xylitol or maltitol on the development of gingivitis and plaque: A randomized clinical trial. Int. J. Dent. Hyg. 2014, 12, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Na, H.S.; Kim, S.M.; Wallet, S.; Cha, S.; Chung, J. Xylitol, an anticaries agent, exhibits potent inhibition of inflammatory responses in human THP-1-derived macrophages infected with Porphyromonas gingivalis. J. Periodontol. 2014, 85, e212–e223. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, M.H.; Song, Y.R.; Na, H.S.; Chung, J. Aggregatibacter actinomycetemcomitans-induced AIM2 inflammasome activation is suppressed by xylitol in differentiated THP-1 macrophages. J. Periodontol. 2016, 87, e116–e126. [Google Scholar] [CrossRef] [PubMed]
- Gunther, S. Vitamin A acid: Clinical investigations with 405 patients. Cutis 1976, 17, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Abou Sulaiman, A.E.; Shehadeh, R.M. Assessment of total antioxidant capacity and the use of vitamin C in the treatment of non-smokers with chronic periodontitis. J. Periodontol. 2010, 81, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.H.; Tipton, D.A. Vitamin D and its impact on oral health—An update. J. Tenn. Dent. Assoc. 2011, 91, 30. [Google Scholar] [PubMed]
- García-Closas, R.; Berenguer, A.; Tormo, M.J.; Sánchez, M.J.; Quiros, J.R.; Navarro, C.; Arnaud, R.; Dorronsoro, M.; Chirlaque, M.D.; Barricarte, A. Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. Br. J. Nutr. 2004, 91, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Shklar, G. The effect of vitamin E on the healing of gingival wounds in rats. J. Periodontol. 1983, 54, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Mason, J.B.; Meydani, S.N.; Cornwall, S.; Grand, R.J. Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption. Gastroenterology 1992, 102, 2139–2142. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Metabolism and cell biology of vitamin K. Thromb. Haemost. 2008, 100, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Coutu, D.L.; Wu, J.H.; Monette, A.; Rivard, G.E.; Blostein, M.D.; Galipeau, J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J. Biol. Chem. 2008, 283, 17991–18001. [Google Scholar] [CrossRef] [PubMed]
- Aral, K.; Alkan, B.A.; Saraymen, R.; Yay, A.; Şen, A.; Önder, G.Ö. Therapeutic effects of systemic vitamin K2 and vitamin D3 on gingival inflammation and alveolar bone in rats with experimentally induced periodontitis. J. Periodontol. 2015, 86, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Holtfreter, B.; Scott, A.E.; Völzke, H.; Petersmann, A.; Dietrich, T.; Newson, R.S.; Kocher, T. Serum vitamin B12 is inversely associated with periodontal progression and risk of tooth loss: A prospective cohort study. J. Clin. Periodontol. 2016, 43, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Warad, S.; Kalburgi, N.B.; Manak, M.; Kalburgi, V.C.; Koregol, A.C.; Patanashetti, J.; Rao, S.; Kokatnur, M.V. Determining the Effect of Gutkha on Serum Levels of Vitamin B12 and Folic Acid as Compared to Smoking among Chronic Periodontitis Subjects : A Cross-Sectional Study. J. Clin. Diagn. Res. 2014, 8, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, E.O.; Bergstrom, J. Effect of smoking on folic acid and vitamin B12 after nonsurgical periodontal intervention. J. Clin. Periodontol. 2007, 34, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, E.O.; Bergstrom, J. Relationship between smoking and folic acid, vitamin B12 and some haematological variables in patients with chronic periodontal disease. J. Clin. Periodontol. 2006, 33, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Barlow, T. On Cases Described as “Acute Rickets”, which are probably a combination of Scurvy and Rickets, the Scurvy being an essential, and the rickets a variable, element. Med. Chir. Trans. 1883, 66, 159–220. [Google Scholar] [CrossRef] [PubMed]
- Camarena, V.; Wang, G. The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. 2016, 73, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, K.; Fujikawa, H.; Kajikawa, T.; Takedachi, M.; Yamamoto, T.; Murakami, S. Effects of l-ascorbic acid 2-phosphate magnesium salt on the properties of human gingival fibroblasts. J. Periodont. Res. 2012, 47, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, Y.; Nakayama, Y.; Ogata, Y.; Tamazawa, K.; Shimauchi, H.; Nishida, T.; Ito, K.; Chikazawa, T.; Kataoka, S.; Murakami, S. Effects of an ascorbic acid—Derivative dentifrice in patients with gingivitis: A double-masked, randomized, controlled clinical trial. J. Periodontol. 2015, 86, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Grossi, S.G.; Dunford, R.G.; Ho, A.W.; Trevisan, M.; Genco, R.J. Dietary vitamin C and the risk for periodontal disease. J. Periodontol. 2000, 71, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Je, D.; Won, S.; Paik, D.; Bae, K. Association between vitamin D deficiency and periodontal status in current smokers. Community Dent. Oral Epidemiol. 2015, 43, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Antonoglou, G.N.; Suominen, A.L.; Knuuttila, M.; Ylöstalo, P.; Ojala, M.; Männistö, S.; Marniemi, J.; Lundqvist, A.; Tervonen, T. Associations between serum 25-hydroxyvitamin D and periodontal pocketing and gingival bleeding: Results of a study in a non-smoking population in Finland. J. Periodontol. 2015, 86, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Alshouibi, E.N.; Kaye, E.K.; Cabral, H.J.; Leone, C.W.; Garcia, R.I. Vitamin D and periodontal health in older men. J. Dent. Res. 2013, 92, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Malmstrom, H.; Kellesarian, S.V.; Al-Kheraif, A.A.; Vohra, F.; Romanos, G.E. Efficacy of Vitamin D3 Supplementation on Osseointegration of Implants. Implant. Dent. 2016, 25, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Takano-Yamamoto, T. Local injection of 1,25-dihydroxyvitamin D3 enhanced bone formation for tooth stabilization after experimental tooth movement in rats. J. Bone Miner. Metab. 2004, 22, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.; Kocadereli, I.; Atilla, P.; Aşan, E. Comparison of the effects of 1,25 dihydroxycholecalciferol and prostaglandin E 2 on orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 607–614. [Google Scholar] [CrossRef]
- Schulze-Späte, U.; Dietrich, T.; Wu, C.; Wang, K.; Hasturk, H.; Dibart, S. Systemic vitamin D supplementation and local bone formation after maxillary sinus augmentation—A randomized, double-blind, placebo-controlled clinical investigation. Clin. Oral Implants Res. 2016, 27, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, I.E. Trace Elements, Micronutrients, and Free Radicals; Humana Press: New York, NY, USA, 2012. [Google Scholar]
- Slade, E.W., Jr.; Bartuska, D.; Rose, L.F.; Cohen, D.W. Vitamin E and periodontal disease. J. Periodontol. 1976, 47, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Åsman, B.; Wijkander, P.; Hjerpe, A. Reduction of collagen degradation in experimental granulation tissue by vitamin E and selenium. J. Clin. Periodontol. 1994, 21, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Meyer, D. Effect of dietary vitamin E supplementation and rotational stress on alveolar bone loss in rice rats. Arch. Oral Biol. 1993, 38, 601–606. [Google Scholar] [CrossRef]
- Pacht, E.R.; Kaseki, H.; Mohammed, J.R.; Cornwell, D.G.; Davis, W.B. Deficiency of vitamin E in the alveolar fluid of cigarette smokers. Influence on alveolar macrophage cytotoxicity. J. Clin. Investig. 1986, 77, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Conly, J.; Stein, K. Reduction of vitamin K2 concentrations in human liver associated with the use of broad spectrum antimicrobials. Clin. Investig. Med. 1994, 17, 531. [Google Scholar]
- Elliott, M.J.; Zimmerman, D.; Holden, R.M. Warfarin anticoagulation in hemodialysis patients: A systematic review of bleeding rates. Am. J. Kidney Dis. 2007, 50, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, W.E. Vitamin K deficiency. Southeast Asian J. Trop. Med. Public Health 1993, 24, 5–9. [Google Scholar] [PubMed]
- Bayr, H. Reactive oxygen species. Crit. Care Med. 2005, 33, S498–S501. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W.; Xu, J.; Min, F.; Wang, L.; Wang, X.; Cao, S.; Tan, D.; Qi, W.; Reiter, R.J. Inhibitory effect of melatonin on diquat-induced lipid peroxidation in vivo as assessed by the measurement of F2-isoprostanes. J. Pineal Res. 2006, 40, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Liu, Z.; Wang, Y.; Xiao, H.; Wu, W.; Xiao, C.; Liu, X. Carnosic acid attenuates lipopolysaccharide-induced liver injury in rats via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2013, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chander Narula, S.; Kumar Sharma, R.; Tewari, S.; Kumar Sehgal, P. Vitamin E supplementation, superoxide dismutase status, and outcome of scaling and root planing in patients with chronic periodontitis: A randomized clinical trial. J. Periodontol. 2014, 85, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Agarwal, S. Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.V.; Prabhuji, M.; Roopa, D.A.; Ravirajan, S.; Kishore, H.C. Efficacy of lycopene in the treatment of gingivitis: A randomised, placebo-controlled clinical trial. Oral Health Prev. Dent. 2007, 5, 327. [Google Scholar] [PubMed]
- Arora, N.; Avula, H.; Kumar Avula, J. The adjunctive use of systemic antioxidant therapy (lycopene) in nonsurgical treatment of chronic periodontitis: A short-term evaluation. Quintessence Int. 2013, 44, 395–405. [Google Scholar] [PubMed]
- Belludi, S.A.; Verma, S.; Banthia, R.; Bhusari, P.; Parwani, S.; Kedia, S.; Saiprasad, S. Effect of lycopene in the treatment of periodontal disease: A clinical study. J. Contemp. Dent. Pract. 2013, 14, 1054. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M. Pharmacology and function of melatonin receptors in the mammalian central nervous. In Serotonin; Mylecharane, E., Angus, J.A., de la Lande, I.S., Humphrey, P.A.P., Eds.; MacMillan Press: London, UK, 1989; p. 265. [Google Scholar]
- Pieri, C.; Marra, M.; Moroni, F.; Recchioni, R.; Marcheselli, F. Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994, 55, PL271–PL276. [Google Scholar] [CrossRef]
- Reiter, R.; Rosales-Corral, S.; Liu, X.; Acuna-Castroviejo, D.; Escames, G.; Tan, D. Melatonin in the oral cavity: Physiological and pathological implications. J. Periodont. Res. 2015, 50, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Arabacı, T.; Kermen, E.; Özkanlar, S.; Köse, O.; Kara, A.; Kızıldağ, A.; Duman, Ş.B.; Ibişoğlu, E. Therapeutic effects of melatonin on alveolar bone resorption after experimental periodontitis in rats: A biochemical and immunohistochemical study. J. Periodontol. 2015, 86, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Balci Yuce, H.; Karatas, O.; Aydemir Turkal, H.; Pirim Gorgun, E.; Ocakli, S.; Benli, I.; Cayli, S. The effect of melatonin on bone loss, diabetic control and apoptosis in diabetic rats with ligature-induced periodontitis. J. Periodontol. 2016, 87, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Gómez-Moreno, G.; Villalba, J.; Ferrera, M.J.; Escames, G.; Acuña-Castroviejo, D. Relationship between salivary melatonin levels and periodontal status in diabetic patients. J. Pineal Res. 2003, 35, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Lopez-Valverde, A.; Gomez-de-Diego, R.; Arias-Santiago, S.; de Vicente-Jimenez, J. Effect of gingival application of melatonin on alkaline and acid phosphatase, osteopontin and osteocalcin in patients with diabetes and periodontal disease. Med. Oral Patol. Oral Cir. Bucal 2013, 18, 657–663. [Google Scholar] [CrossRef]
- Elgammal, M.Y.A.; Salem, A.S.; Anees, M.M.; Tawfik, M.A. Clinical and radiographic evaluation of immediate loaded dental implants with local application of melatonin: A preliminary randomized controlled clinical trial. J. Oral Implantol. 2016, 42, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Shigeishi, H.; Sasaki, K.; Ota, A.; Ohta, K.; Takechi, M. Combined effects of melatonin and FGF-2 on mouse preosteoblast behavior within interconnected porous hydroxyapatite ceramics—In vitro analysis. J. Appl. Oral Sci. 2016, 24, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Gómez-Moreno, G.; Arana, C.; Muñoz, F.; Lopez-Peña, M.; Stephenson, J.; Reiter, R.J. Melatonin stimulates osteointegration of dental implants. J. Pineal Res. 2008, 45, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Ramírez-Fernández, M.P.; Gómez-Moreno, G.; Maté-Sánchez, J.E.; Delgado-Ruiz, R.; Guardia, J.; López-Marí, L.; Barone, A.; Ortiz-Ruiz, A.J.; Martínez-González, J.M. Melatonin stimulates the growth of new bone around implants in the tibia of rabbits. J. Pineal Res. 2010, 49, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Aguilar Salvatierra, A.; Gargallo-Albiol, J.; Delgado-Ruiz, R.A.; Maté Sanchez, J.E.; Satorres-Nieto, M. Zirconia with laser-modified microgrooved surface vs. titanium implants covered with melatonin stimulates bone formation. Experimental study in tibia rabbits. Clin. Oral Implants Res. 2015, 26, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Arana, C.; Gomez-Moreno, G.; Escames, G.; Lopez, A.; Ferrera, M.J.; Reiter, R.J.; Acuña-Castroviejo, D. Local application of melatonin into alveolar sockets of beagle dogs reduces tooth removal-induced oxidative stress. J. Periodontol. 2007, 78, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, U.; Kuzmanova, D.; Chapple, I. Micronutritional approaches to periodontal therapy. J. Clin. Periodontol. 2011, 38, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.N.; Hildebolt, C.F.; Miley, D.D.; Dixon, D.A.; Couture, R.A.; Anderson Spearie, C.L.; Langenwalter, E.M.; Shannon, W.D.; Deych, E.; Mueller, C. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J. Periodontol. 2011, 82, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, A.R.; Christensen, L.B.; Holm-Pedersen, P.; Avlund, K.; Boucher, B.J.; Heitmann, B.L. Intake of dairy products in relation to periodontitis in older Danish adults. Nutrients 2012, 4, 1219–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Staudte, H.; Kranz, S.; Völpel, A.; Schütze, J.; Sigusch, B.W. Comparison of nutrient intake between patients with periodontitis and healthy subjects. Quintessence Int. 2012, 43, 907–916. [Google Scholar] [PubMed]
- Chakraborty, S.; Tewari, S.; Sharma, R.K.; Narula, S.C.; Ghalaut, P.S.; Ghalaut, V. Impact of iron deficiency anemia on chronic periodontitis and superoxide dismutase activity: A cross-sectional study. J. Periodontal Implant Sci. 2014, 44, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pushparani, D. Zinc and type 2 diabetes mellitus with periodontitis—A systematic review. Curr. Diabetes Rev. 2014, 10, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A. ADA clinical recommendations on topical fluoride for caries prevention. Evid. Based Dent. 2014, 15, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Sojka, J.E.; Weaver, C.M. Magnesium supplementation and osteoporosis. Nutr. Rev. 1995, 53, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Toba, Y.; Kajita, Y.; Masuyama, R.; Takada, Y.; Suzuki, K.; Aoe, S. Dietary magnesium supplementation affects bone metabolism and dynamic strength of bone in ovariectomized rats. J. Nutr. 2000, 130, 216–220. [Google Scholar] [PubMed]
- Velazguez, J.; Jimenez, A.; Chomon, B.; Villa, T. Magnesium supplementation and bone turnover. Nutr. Rev. 1999, 57, 227. [Google Scholar]
- Osis, D.; Kramer, L.; Wiatrowski, E.; Spencer, H. Dietary zinc intake in man. Am. J. Clin. Nutr. 1972, 25, 582–588. [Google Scholar] [PubMed]
- Lansdown, A.B.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Ågren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Mirastschijski, U.; Haaksma, C.J.; Tomasek, J.J.; Ågren, M.S. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp. Cell. Res. 2004, 299, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Rostan, E.F.; DeBuys, H.V.; Madey, D.L.; Pinnell, S.R. Evidence supporting zinc as an important antioxidant for skin. Int. J. Dermatol. 2002, 41, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.F.; Knöfler, G.U.; Purschwitz, R.E.; Eick, S. Periodontal Dressing as an Adjunct after Scaling and Root Planing—A Useful Preventive Tool? Oral Health Prev. Dent. 2016, 14, 101–109. [Google Scholar] [PubMed]
- Pushparani, D.S. Serum Zinc and β D Glucuronidase Enzyme Level in Type 2 Diabetes Mellitus with Periodontitis. Curr. Diabetes Rev. 2015, 11. [Google Scholar] [CrossRef]
- Kulkarni, V.; Bhatavadekar, N.B.; Uttamani, J.R. The effect of nutrition on periodontal disease: A systematic review. J. Calif. Dent. Assoc. 2014, 42, 302–311. [Google Scholar] [PubMed]
- Zhang, J.; Liu, L.; Zhao, S.; Wang, H.; Yang, G. Characterization and In vivo evaluation of trace element-loaded implant surfaces in ovariectomized rats. Int. J. Oral Maxillofac. Implants 2015. [Google Scholar] [CrossRef] [PubMed]
- Seppa, L.; Forss, H.; Ogaard, B. The effect of fluoride application on fluoride release and the antibacterial action of glass ionomers. J. Dent. Res. 1993, 72, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Zafar, M.S. Oral and dental delivery of fluoride: A review. Fluoride 2015, 48, 195–204. [Google Scholar]
- Zafar, M.S.; Ahmed, N. Therapeutic roles of fluoride released from restorative dental materials. Fluoride 2015, 48, 184–194. [Google Scholar]
- Zafar, M.S. Effects of Surface Pre-Reacted Glass Particles on Fluoride Release of Dental Restorative Materials. World Appl. Sci. J. 2013, 28, 457–462. [Google Scholar]
- American Academy of Pediatric Dentistry. Guideline on fluoride therapy. Pediatr. Dent. 2013, 35, 165–168. [Google Scholar]
- Karadeniz, E.I.; Gonzales, C.; Nebioglu-Dalci, O.; Dwarte, D.; Turk, T.; Isci, D.; Sahin-Saglam, A.M.; Alkis, H.; Elekdag-Turk, S.; Darendeliler, M.A. Physical properties of root cementum: Part 20. Effect of fluoride on orthodontically induced root resorption with light and heavy orthodontic forces for 4 weeks: A microcomputed tomography study. Am. J. Orthod. Dentofac. Orthop. 2011, 140, e199–e210. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zafar, M.S.; Zohaib, S.; Najeeb, S.; Naseem, M. Green tea (Camellia Sinensis): Chemistry and oral health. Open Dent. J. 2016, 10, 116–173. [Google Scholar] [CrossRef] [PubMed]
- Milward, M.; Chapple, I. The role of diet in periodontal disease. Clin. Dent. Health 2013, 52, 18–21. [Google Scholar]
- Naseem, M.; Khurshid, Z.; Khan, H.A.; Niazi, F.; Zohaib, S.; Zafar, M.S. Oral health challenges in pregnant women: Recommendations for dental care professionals. Saudi J. Dent. Res. 2016, 7, 138–146. [Google Scholar] [CrossRef]
- Hemalatha, V.; Manigandan, T.; Sarumathi, T.; Aarthi Nisha, V.; Amudhan, A. Dental Considerations in Pregnancy—A Critical Review on the Oral Care. J. Clin. Diagn. Res. 2013, 7, 948. [Google Scholar]
- Centers for Disease Control. Ten great public health achievements—United States, 1900–1999. Morb. Mortal. Wkly. Rep. 1999, 48, 241–243. [Google Scholar]
- Ham-Chande, R.; Orlando, F. Shapes and Limits of Longevity in Mexico. In Proceedings of the Living to 100 and Beyond Symposium, Orlando, FL, USA, 12–14 January 2005.
- Richmond, R.L. The changing face of the Australian population: Growth in centenarians. Med. J. Aust. 2008, 188, 720–723. [Google Scholar] [PubMed]
- White, D.; Tsakos, G.; Pitts, N.; Fuller, E.; Douglas, G.; Murray, J.; Steele, J. Adult Dental Health Survey 2009: Common oral health conditions and their impact on the population. Br. Dent. J. 2000, 213, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Steele, J.; Nuttall, N.; Bradnock, G.; Morris, J.; Nunn, J.; Pine, C.; Pitts, N.; Treasure, E.; White, D. Adult Dental Health Survey—Oral Health in the United Kingdom 1998. Br. Dent. J. 2000, 189, 639–644. [Google Scholar]
- Sheiham, A.; Steele, J. Does the condition of the mouth and teeth affect the ability to eat certain foods, nutrient and dietary intake and nutritional status amongst older people? Public Health Nutr. 2001, 4, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, J.; Laurin, D.; Vallee, R.; Lachapelle, D. Nutrient intake and gastrointestinal disorders related to masticatory performance in the edentulous elderly. J. Prosthet. Dent. 1993, 70, 468–473. [Google Scholar] [CrossRef]
- Papas, A.S.; Palmer, C.A.; Rounds, M.C.; Herman, J.; Mcgandy, R.B.; Hartz, S.C.; Russell, R.M.; Depaola, P. Longitudinal relationships between nutrition and oral health. Ann. NY Acad. Sci. 1989, 561, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Budtz-Jørgensen, E.; Chung, J.; Rapin, C. Nutrition and oral health. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.; Steele, J. The relationship between oral health and nutrition in older people. Mech. Ageing Dev. 2004, 125, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, G.H.; Dominguez, B.L.; Schork, M.A.; Loesche, W.J. Functional units, chewing, swallowing, and food avoidance among the elderly. J. Prosthet. Dent. 1997, 77, 588–595. [Google Scholar] [CrossRef]
- Moynihan, P.; Bradbury, J. Compromised dental function and nutrition. Nutrition 2001, 17, 177–178. [Google Scholar] [CrossRef]
- Shinkai, R.S.; Hatch, J.P.; Sakai, S.; Mobley, C.C.; Saunders, M.J.; Rugh, J.D. Oral function and diet quality in a community-based sample. J. Dent. Res. 2001, 80, 1625–1630. [Google Scholar] [CrossRef] [PubMed]

| Nutrient | Dietary Source(s) | Importance in Periodontal Heath | Reported Improvement in PD and CAL (Mean mm, SD) | References |
|---|---|---|---|---|
| Vitamin A | Cod liver oil, carrots, capsicum, liver, sweet potato, broccoli, leafy vegetables | Not clear. Research indicates insignificant improvement in periodontal health upon supplementation. | PD: 0.52 ± 0.03 CAL: n.d. | [33,65] |
| B-vitamins | B1—Liver, oats, pork, potatoes, eggs B2—Bananas, dairy, green beans B3—Eggs, fish, meat, mushrooms, nuts B5—Avocados, meat, broccoli B6—Meat, vegetables, nuts, banana B7—Raw egg, liver, leafy vegetables, peanuts B9—Cereals, leafy vegetables B12—Animal products | Supplementation may accelerate post-surgical healing. | PD: 1.57 ± 0.34 CAL: 0.41 ± 0.12 | [56] |
| Vitamin C | Citrus fruits, vegetables, liver | Gingival bleeding and inflammation are hallmarks of scurvy. Supplementation may improve outcomes of periodontal therapy. | PD: 0.58 ± 0.14 CAL: n.d. | [66] |
| Vitamin D | Fish eggs, mushrooms, liver, milk | Deficiency may lead to delayed post-surgical healing. Local application may accelerate post-surgical healing/osseointegration | PD: 1.35 (SD n.d.) CAL: 1.4 (SD n.d.) | [34,56,57,67] |
| Vitamin E | poultry, meat, fish, nuts, seeds and cereals | Impaired gingival wound healing | PD: 0.39 ± 0.18 CAL: n.d. | [33,68,69,70] |
| Vitamin K | Green vegetables, egg yolk | Deficiency may lead to gingival bleeding. No known effects on periodontal therapy if supplementation used as an adjunct. | n.d. | [71,72,73] |
| Nutrient | Dietary Source(s) | Importance in Periodontal Heath | Reported Improvement in PD and CAL (Mean mm) | References |
|---|---|---|---|---|
| Calcium | Milk products, eggs, canned bony fish, leafy vegetables, nuts, seeds | Required for formation of teeth and bones. Supplementation improves outcomes of non-surgical periodontal therapy. Local application enhances osseointegration. | n.d. | [121,122,123] |
| Magnesium | Cocoa, soybeans, nuts, spinach, marine vegetables, tomatoes | Required for cell metabolism and bone formation. Supplementation may improve outcomes of non-surgical periodontal therapy. | [124] | |
| Iron | Red meat, tuna, dry beans, spinach | Possible anti-oxidant effect on periodontium. | n.d. | [125] |
| Zinc | Protein-rich foods, spinach, grains | Possible anti-oxidant effect on periodontium. Reduces severity of diabetes-induced periodontitis | n.d. | [126] |
| Fluoride | Grape fruits, cocoa, tea, dried fruits and nuts, fluoridated water | Supplementation and topical application prevents dental caries. | n.d. | [127] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The Role of Nutrition in Periodontal Health: An Update. Nutrients 2016, 8, 530. https://doi.org/10.3390/nu8090530
Najeeb S, Zafar MS, Khurshid Z, Zohaib S, Almas K. The Role of Nutrition in Periodontal Health: An Update. Nutrients. 2016; 8(9):530. https://doi.org/10.3390/nu8090530
Chicago/Turabian StyleNajeeb, Shariq, Muhammad Sohail Zafar, Zohaib Khurshid, Sana Zohaib, and Khalid Almas. 2016. "The Role of Nutrition in Periodontal Health: An Update" Nutrients 8, no. 9: 530. https://doi.org/10.3390/nu8090530







