1. Introduction
Iodine deficiency remains a global health problem, but there has been great progress in eliminating iodine deficiency disorders over the last few decades [
1,
2]. Based on median urinary iodine concentration (UIC) among school-aged children, the number of countries with adequate iodine intake has increased from 67 to 112 over the past 10 years. In addition, iodine excess has become more prevalent over the past decade mainly because of salt iodization, and, in 2013, 10 countries were classified with excessive iodine intakes (median UIC ≥ 300 µg/L) [
3]. Fetuses and newborns are high-risk groups for excessive iodine exposure, since their thyroid gland is immature and has less adaptive abilities for high iodine doses than for adults. This is probably because the thyroids of fetuses and newborns are unable to escape from the acute Wolff-Chaikoff effect [
4,
5,
6,
7]. In addition, infants are particularly vulnerable when it comes to iodine excess, since thyroid hormone disturbances may affect their growth and developmental skills [
8,
9]. For breastfed infants, the mother’s iodine intake is of importance, since iodine is excreted through breastmilk [
10,
11,
12].
Although chronical iodine excess in pediatric populations is an area of limited research, studies have shown both increased thyroid volume [
13,
14] and thyroid disturbances [
15,
16] in children exposed to excessive iodine. However, there is limited knowledge concerning the longitudinal effects of chronically excessive iodine exposure in children, particularly at the preschool age.
In the southwest of the Algerian desert near the city of Tindouf, approximately 165,000 refugees are living in the Saharawi refugee camps. People living in these camps are exposed to high iodine from groundwater [
17], which is probably responsible for endemic goiter among women and children [
18,
19] and a high prevalence of thyroid dysfunction among women [
20,
21]. We conducted two studies in the refugee camps to assess the consequences of chronically high iodine exposure in lactating women and their children: a cross-sectional iodine study in 2010 (baseline study) and a second study in 2013 (follow-up study) [
20,
21]. The main objective of the present paper is to describe breast milk iodine concentration (BMIC) and the iodine status among breastfed children aged 0–6 months living in an area of high iodine exposure (at baseline) and to describe serum concentration of thyroid hormones, thyroid function, and iodine status among the children at three years of age (at follow-up). Our hypotheses were as follows: the children have (a) excess iodine intake in early infancy (baseline) and early childhood (follow-up); and (b) thyroid hormone disturbances and elevated serum thyroglobulin (Tg) during early childhood (follow-up).
3. Results
Background characteristics and iodine status from the baseline and follow-up studies are described in
Table 2. The median age of the children was 3.1 months at baseline, while it was 31.4 months at follow-up. At baseline, 16.5%, 13.8%, and 8.2% were underweight, stunted, and wasted, respectively. At follow-up, 11.8% were underweight, 33.2% were stunted, and 3.8% were wasted. All children were breastfed at baseline, and 13.8% were still breastfed at follow-up. The median (p25–p75) UIC were 722 (393–1133) µg/L and 458 (275–1026) µg/L at baseline and follow-up, respectively (
p = 0.003). The median (p25–p75) BMIC at baseline was 479 (330–702) µg/L.
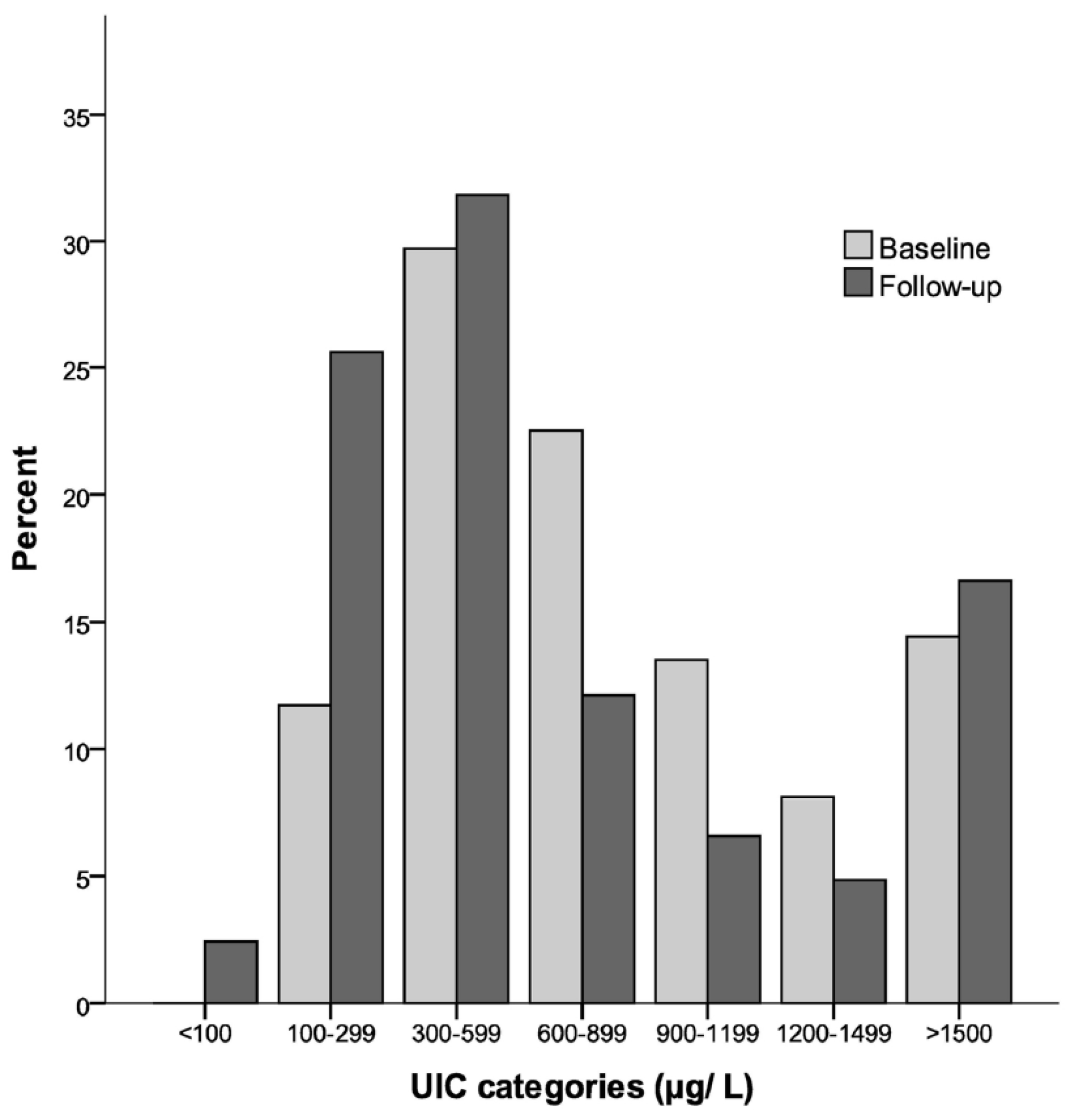
Figure 2 shows the percentage distributions of children in different categories of iodine nutrition based on the UIC from baseline and follow-up. At baseline, none had a UIC < 100 µg/L, 11.7% had a UIC of 100–299 µg/L, and 88.3% had a UIC ≥ 300 µg/L. At follow-up, 2.4% had a UIC < 100 µg/L, 25.6% had a UIC of 100–299 µg/L, and 71.9% had a UIC ≥ 300 µg/L.
Table 3 shows the daily estimated iodine intake among breastfed children from baseline. Estimated iodine intake from breastmilk increases up to five months of age. Across age groups, the average estimated iodine intake from breast milk is 299 (206–438) µg/day for predominantly breastfed children.
Table 4 describes the serum concentrations of thyroid hormones and Tg, as well as the biochemically assessed thyroid function tests among the children. The median/mean values of thyroid hormones and Tg are within the middle of the reference ranges, except for fT3, which is found in the upper area. No children were found to be TgAb or TPOAb positive (data not shown for thyroid antibodies). We found that 9.3% had subclinical hypothyroidism, and among these, 37.0% had elevated Tg and 3.7% had elevated fT3. None had overt hypothyroidism; however, three children had TSH between 10 and 15 mIU/L with fT4 values above the lower quartile. One child (0.4%) had subclinical hyperthyroidism and one (0.4%) had overt hyperthyroidism. We also found three children (1%) with marginally low fT4 and eight children (2.8%) with elevated fT3 together with TSH within the reference ranges. Two of the children with fT3 and fT4 outside the reference ranges had elevated Tg. In total, thyroid function tests were abnormal among 13.8% of the children. We found that 9.7% had elevated Tg without any thyroid disturbances. In total, 13.2% had elevated Tg, counting those with and without abnormal thyroid function tests. The total prevalence with abnormal thyroid function tests and/or elevated Tg was 23.5%.
Predictors for TSH, fT4, and fT3 at follow-up are shown in
Table 5. In the multiple model, Tg and fT3 were positively associated with TSH, explaining 11.3% of the variance in TSH. There were no significant associations between TSH and UIC, fT4, breastfeeding status, age, or gender. fT4 was positively associated with Tg, explaining 1.4% of the variance in fT4. No significant associations were found between fT4 and UIC, breastfeeding status, age, or gender. For fT3, a negative association with UIC was found, where UIC explained 6.7% of the variance in fT3. No significant associations were found between fT3 and Tg, breastfeeding status, age or gender. We also explored predictors for Tg, UIC baseline, and UIC follow-up (data only presented in the text). Tg was associated with TSH as the only variable, explaining 11.6% of the variance. Iodine status, expressed as UIC, was positively associated with BMIC as the only variable at both follow-up and baseline. At baseline, BMIC explained 23.1% of the variance in UIC, while at follow-up, gender was the only significant predictor of UIC, where UIC seemed to be higher among male children than female, explaining 2.9% of the variance.
Nutritional status according to subclinical hypothyroidism is presented in
Table 6. Children with subclinical hypothyroidism had significantly lower HAZ (
p = 0.004) and WAZ (
p = 0.012) compared to those without hypothyroidism; this result was the same after adjusting for breastfeeding status.
4. Discussion
In this study, we found a high prevalence of thyroid disturbances among young children who reside in an area of chronically high iodine exposure. High iodine intakes were identified both at baseline and follow-up.
As illustrated in
Figure 2, 88% and 72% had UIC ≥ 300 µg/L at baseline and follow-up, respectively. UIC ≥ 300 µg/L is used as a cut-off by the WHO for indicating iodine excess with a risk of adverse health consequences for school-aged children [
1]. However, for younger children, a cut-off indicating excessive iodine intake based on the UIC has not yet been developed. UIC were assessed with spot urine samples, which is the recommended method for assessing iodine status in population groups by the WHO [
1], and also recently reviewed as a reliable biomarker for groups [
28]. Even so, spot sampling has weaknesses because it is affected by day-to-day hydration level, and there may be wide daily variation in UIC [
29,
30,
31], meaning that an individual showing UIC < 100 µg/L does not necessarily need to be iodine deficient. In this study, all of the children were breastfed at baseline and the mean estimated iodine intake from breast milk was 299 µg/day. This is over three times higher than the recommended daily intake from the WHO of 90 µg/day for children under two years of age [
1], and one and a half times as high as the US Institute of Medicine and the European Scientific Committee on Food’s tolerable upper intake level (UL) of 200 µg/day for children of 1–3 years [
32,
33]. This finding is in accordance with the UIC levels, indicating excessive iodine intakes among the children.
Median UIC was significantly higher at baseline than at follow-up. The iodine content in the urine samples was analyzed in two different laboratories, which may explain some of the differences. Both laboratories used a modified Sandell–Kolthoff reaction for iodine measurement; however, we have no data for comparison analysis. All children from baseline were breastfed, while only a small proportion were breastfed at follow-up, since these children were older. The median BMIC was extremely high (479 µg/L), and since the children were receiving more breastmilk at baseline than at follow-up, the difference in UIC can likely be explained by this.
In the final regression model, BMIC was a predictor of the children’s UIC at baseline. This finding is in concordance with other studies [
34,
35,
36]. Even though the explained variance was small (2.9%), gender was the only predictor of UIC follow-up, whereas male children seemed to have higher UIC than female children, at follow-up. This may be due to different dietary habits between male and female children. However, we do not see gender differences in regards to any of the thyroid parameters. We also explored whether TSH could be associated with iodine status, and TSH was tested for associations with UIC follow-up, UIC baseline, and BMIC at baseline. No significant associations were found for any of the mentioned variables. This may be due to the very large intra-individual variability of the UIC and BMIC measurements. Similarly, no association was found between UIC and TSH in two Iranian studies where urinary iodine was not associated with thyroid function or TSH in newborns in an iodine-sufficient area [
35,
37], or in a recent study by Nepal et al., where TSH was associated to neither UIC nor serum Tg in children living under chronic iodine excess [
15].
Even though neither UIC nor BMIC predicted TSH in our study, others have reported an association between UIC and TSH in children. In a Chinese study of children living under long-term iodine excess, subclinical hypothyroidism was significantly higher among children with UIC > 600 µg/L than among children with a lower UIC [
16]. A Korean study among preterm infants found that urinary iodine was significantly higher among children with subclinical hypothyroidism after three weeks, but not after six weeks of life [
11]. In our study, serum Tg was positively associated with TSH. Tg has been found to be a sensitive marker of iodine nutrition, also in areas of iodine excess [
1,
38]. At follow-up, 13.8% of the children were still breastfed. Breastfeeding status was not significantly associated with UIC, TSH, fT4, fT3, or Tg at follow-up.
Evaluating the thyroid hormone status in
Table 4, we see that 9.3% had subclinical hypothyroidism. Studies on thyroid function in healthy populations of infants are limited [
39,
40]. The most cited study on thyroid function in a pediatric population was carried out among US adolescents where 1.7% had subclinical hypothyroidism [
41]. A large study among schoolchildren conducted in India after salt iodization revealed prevalences of subclinical and overt hypothyroidism of 6.1% and 0.4%, respectively [
42]. However, the results of these two studies may not be comparable to our data, since it is known that thyroid disturbances may increase when improving iodine status after long-term deficiency [
43], and because higher age groups were studied than in our material. In a retrospective analysis of TSH from a large database in Israel, elevated TSH was found in 2.9% and highly elevated TSH (>10 mlU/L) in 0.4% of children between six months and 16 years of age [
44]. Compared to this study, our prevalence of 9.3% may be considered high, especially taking into account the vulnerable age group of our population group. Studies from other child populations with long-term iodine excess have found similar results to ours; Nepal et al. reported a prevalence of 7.4% with subclinical hypothyroidism and 15.8% with elevated Tg (using the international Tg reference) among Nepalese infants aged 6–24 months [
15]. Moreover, Sang et al. found about 5% of Chinese children of 7–13 years had subclinical hypothyroidism [
16].
We do not know the optimal reference ranges of thyroid hormones and antibodies in our population. The reference used is of great significance of the reported prevalence of thyroid hormone disturbances. In a population of healthy children aged 2–7 years, the range of TSH was found to be 0.10–5.9 mIU/L, measured by AutoDelfia [
45], which corresponds well to the references we have applied.
Ten children (3.5%) had elevated fT3; of which eight had TSH within the reference, one had low TSH, and one had high TSH. All children had normal fT4 values. To the best of our knowledge, the pattern of elevated fT3 in relation to iodine excess has not been described previously. Furthermore, our data suggested a positive association between TSH and fT3 in the multiple regression analyses. This same association was found in a large pediatric population where normal or slightly above normal TSH levels correlated positively with fT3 [
46] and not with fT4 among children of normal weight. The authors of the paper stated that the mechanism by which TSH levels are associated with higher fT3 levels and not higher fT4 levels remains unknown.
Studies examining possible outcomes of untreated subclinical hypothyroidism in children remain scarce [
39], and there are uncertainties related to the effect of treating the condition among children [
47]. Many studies have not seen any clinical manifestations of subclinical hypothyroidism. In our study, children with subclinical hypothyroidism also had statistically significant lower length/height-for-age and weight-for-age than children without, which may suggest a potential link between subclinical hypothyroidism and linear growth and weight faltering, though the observational design of our study limits our ability to infer causality. Improved growth after treatment of subclinical hypothyroidism has been shown in some studies [
48,
49], and the growth of the children in our study should therefore be monitored.
Strengths and Limitations
In this study, we have explored an under-reported phenomenon; the occurrence of thyroid dysfunction in a population with excessive iodine exposure. In addition, information on thyroglobulin levels and fT3 levels in children with chronically high iodine exposure is a contribution to the scientific literature on the subject, as there are limited normative data on these analytes. However, our ability to explore associations with iodine status and thyroid function was limited due to limitations of the available biomarkers. Data on thyroid parameters was not collected from baseline because of the very young age of the children.