Flavonoids, Flavonoid Subclasses, and Esophageal Cancer Risk: A Meta-Analysis of Epidemiologic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Inclusion Criteria and Exclusion Criterion
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Included Studies
3.2. Meta-Analysis of Flavonoids Intake and Esophageal Cancer Risk
3.3. Source of Heterogeneity
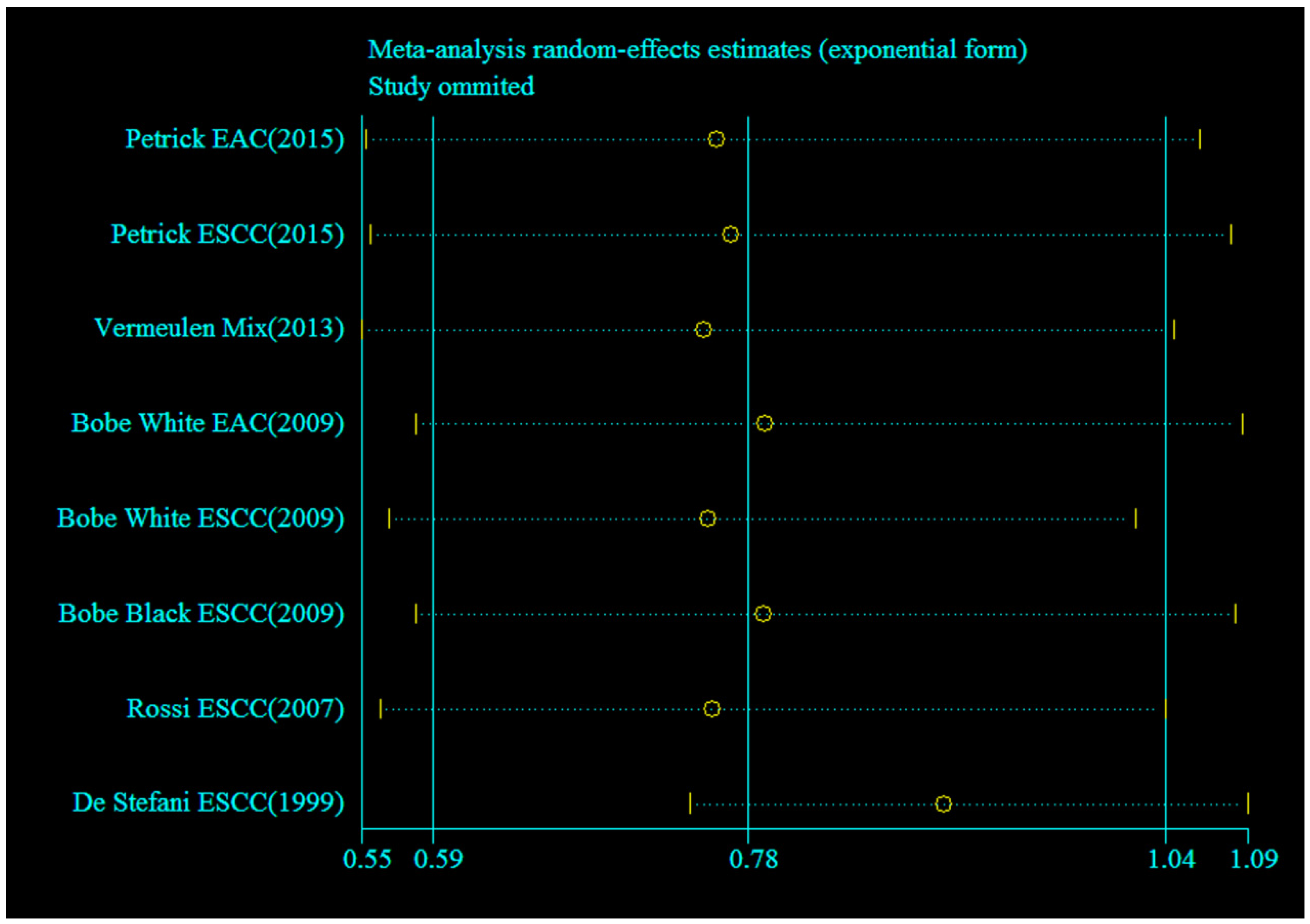
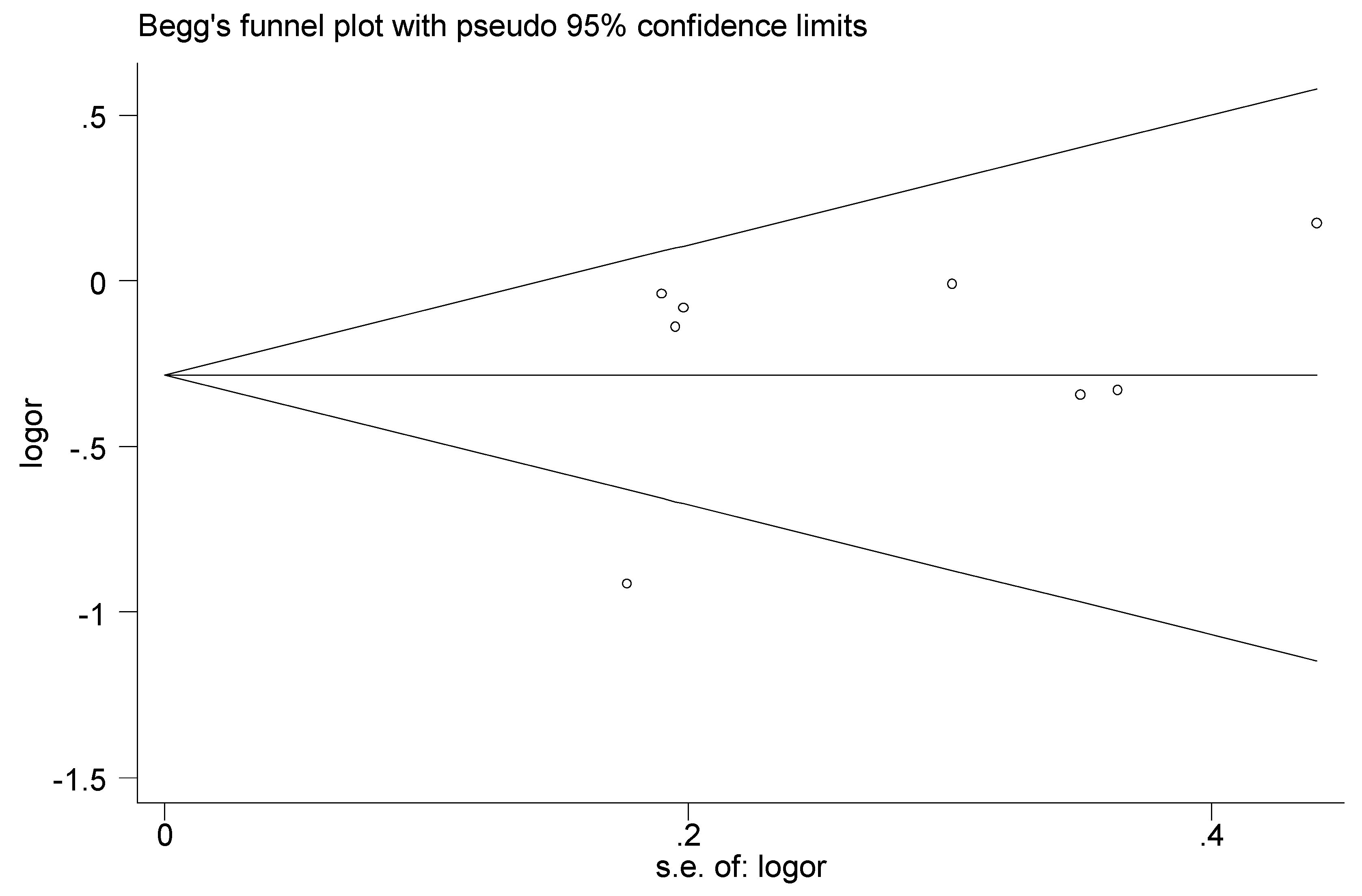
3.4. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Ganai, B.A.; Sameer, A.S.; Masood, A. Esophageal cancer: Associated factors with special reference to the kashmir valley. Tumori 2012, 98, 191–203. [Google Scholar] [PubMed]
- Ibiebele, T.I.; Hughes, M.C.; Nagle, C.M.; Bain, C.J.; Whiteman, D.C.; Webb, P.M. Dietary antioxidants and risk of barrett’s esophagus and adenocarcinoma of the esophagus in an australian population. Int. J. Cancer 2013, 133, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Mlombe, Y.B.; Rosenberg, N.E.; Wolf, L.L.; Dzamalala, C.P.; Chalulu, K.; Chisi, J.; Shaheen, N.J.; Hosseinipour, M.C.; Shores, C.G. Environmental risk factors for oesophageal cancer in malawi: A case-control study. Malawi Med. J. 2015, 27, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Leng, Y.; Lv, C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: A meta-analysis of observational studies. Int. J. Cancer 2013, 133, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jiang, G.; Zhang, G.; Xue, Q.; Zhang, H.; Wang, C.; Zhao, T. Intake of vegetables and fruit and risk of esophageal adenocarcinoma: A meta-analysis of observational studies. Eur. J. Nutr. 2014, 53, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Rosato, V.; Andreano, A.; Ferraroni, M.; Decarli, A.; Edefonti, V.; La Vecchia, C. Dietary patterns and gastric cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2013, 24, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Wakai, K.; Tamakoshi, A.; Tsuji, I.; Tanaka, K.; Matsuo, K.; Nagata, C.; Mizoue, T.; Inoue, M.; Tsugane, S.; et al. Association of vegetable and fruit intake with gastric cancer risk among Japanese: A pooled analysis of four cohort studies. Ann. Oncol. 2014, 25, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Koushik, A.; Hunter, D.J.; Spiegelman, D.; Beeson, W.L.; van den Brandt, P.A.; Buring, J.E.; Calle, E.E.; Cho, E.; Fraser, G.E.; Freudenheim, J.L.; et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J. Natl. Cancer Inst. 2007, 99, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Z.; Yang, X.N.; Coburn, R.A.; Morris, M.E. Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem. Pharmacol. 2005, 70, 627–639. [Google Scholar] [CrossRef] [PubMed]
- McPhail, D.B.; Hartley, R.C.; Gardner, P.T.; Duthie, G.G. Kinetic and stoichiometric assessment of the antioxidant activity of flavonoids by electron spin resonance spectroscopy. J. Agric. Food Chem. 2003, 51, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Hong, J.; Yang, G.Y.; Liao, J.; Yang, C.S. Inhibition of carcinogenesis by polyphenols: Evidence from laboratory investigations. Am. J. Clin. Nutr. 2005, 81, 284S–291S. [Google Scholar] [PubMed]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J. Gastroenterol. 2013, 19, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, K.; Baskaran, K.; Ilakkia, A.; Vanitha, K.; Selvaraj, S.; Sakthisekaran, D. Antitumor efficacy of tangeretin by targeting the oxidative stress mediated on 7,12-dimethylbenz(a) anthracene-induced proliferative breast cancer in sprague-dawley rats. Cancer Chemother. Pharmacol. 2015, 75, 263–272. [Google Scholar] [CrossRef] [PubMed]
- LeJeune, T.M.; Tsui, H.Y.; Parsons, L.B.; Miller, G.E.; Whitted, C.; Lynch, K.E.; Ramsauer, R.E.; Patel, J.U.; Wyatt, J.E.; Street, D.S.; et al. Mechanism of action of two flavone isomers targeting cancer cells with varying cell differentiation status. PLoS ONE 2015, 10, e0142928. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, A.; Liu, X.; Sang, M.; Shan, B.; Meng, F.; Cao, Q.; Ji, X. The flavonoid Baohuoside-I inhibits cell growth and downregulates survivin and cyclin D1 expression in esophageal carcinoma via β-catenin-dependent signaling. Oncol. Rep. 2011, 26, 1149–1156. [Google Scholar] [PubMed]
- Shumway, B.S.; Kresty, L.A.; Larsen, P.E.; Zwick, J.C.; Lu, B.; Field, H.W.; Mumper, R.J.; Stoner, G.D.; Mallery, S.R. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin. Cancer Res. 2008, 14, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Mallery, S.R.; Tong, M.; Shumway, B.S.; Curran, A.E.; Larsen, P.E.; Ness, G.M.; Kennedy, K.S.; Blakey, G.H.; Kushner, G.M.; Vickers, A.M.; et al. Topical application of a mucoadhesive freeze-dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: Results from a multicentered, placebo-controlled clinical trial. Clin. Cancer Res. 2014, 20, 1910–1924. [Google Scholar] [PubMed]
- Mallery, S.R.; Zwick, J.C.; Pei, P.; Tong, M.; Larsen, P.E.; Shumway, B.S.; Lu, B.; Fields, H.W.; Mumper, R.J.; Stoner, G.D. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008, 68, 4945–4957. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Burke, C.A.; Hasson, H.; Kuo, C.T.; Molmenti, C.L.S.; Seguin, C.; Liu, P.Y.; Huang, T.H.M.; Frankel, W.L.; Stoner, G.D. A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev. Res. 2014, 7, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Arnold, M.; Huang, Y.W.; Sardo, C.; Seguin, C.; Martin, E.; Huang, T.H.; Riedl, K.; Schwartz, S.; Frankel, W.; et al. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: A phase I pilot study. Clin. Cancer Res. 2011, 17, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Kresty, L.A.; Frankel, W.L.; Hammond, C.D.; Baird, M.E.; Mele, J.M.; Stoner, G.D.; Fromkes, J.J. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: Interim results show berries modulate markers of oxidative stress in barrett’s esophagus patients. Nutr. Cancer 2006, 54, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yan, F.; Qian, J.; Guo, M.; Zhang, H.; Tang, X.; Chen, F.; Stoner, G.D.; Wang, X. Randomized phase II trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev. Res. (Phila.) 2012, 5, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rathkopf, D.; Dickson, M.A.; Feldman, D.R.; Carvajal, R.D.; Shah, M.A.; Wu, N.; Lefkowitz, R.; Gonen, M.; Cane, L.M.; Dials, H.J.; et al. Phase I study of flavopiridol with oxaliplatin and fluorouracil/leucovorin in advanced solid tumors. Clin. Cancer Res. 2009, 15, 7405–7411. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.K.; Ilson, D.; Saltz, L.; O’Reilly, E.; Tong, W.; Maslak, P.; Werner, J.; Perkins, P.; Stoltz, M.; Kelsen, D. Phase II study of the cyclin-dependent kinase inhibitor flavopiridol administered to patients with advanced gastric carcinoma. J. Clin. Oncol. 2001, 19, 1985–1992. [Google Scholar] [PubMed]
- Morimoto, Y.; Maskarinec, G.; Park, S.Y.; Ettienne, R.; Matsuno, R.K.; Long, C.; Steffen, A.D.; Henderson, B.E.; Kolonel, L.N.; Le Marchand, L.; et al. Dietary isoflavone intake is not statistically significantly associated with breast cancer risk in the multiethnic cohort. Br. J. Nutr. 2014, 112, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.P.; Zhou, B.; Wang, B.; Yu, R.B.; Ma, J. Flavonoids intake and risk of lung cancer: A meta-analysis. Jpn. J. Clin. Oncol. 2009, 39, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Huang, S.; Su, Y. Dietary flavonols intake and risk of esophageal and gastric cancer: A meta-analysis of epidemiological studies. Nutrients 2016, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Lee, A.H.; Xu, F.; Zhang, T.; Lei, J.; Binns, C.W. Soya and isoflavone intakes associated with reduced risk of oesophageal cancer in north-west China. Public Health Nutr. 2015, 18, 130–134. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Ronco, A.; Mendilaharsu, M.; Deneo-Pellegrini, H. Diet and risk of cancer of the upper aerodigestive tract—II. Nutrients. Oral Oncol. 1999, 35, 22–26. [Google Scholar] [CrossRef]
- PUBMED. Available online: http://www.pubmed.gov (accessed on 2 June 2016).
- EMBASE. Available online: http://store.elsevier.com/embase (accessed on 2 June 2016).
- Web of Science. Available online: http://www.isiknowledge.com (accessed on 2 June 2016).
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 1987, 9, 1–30. [Google Scholar] [PubMed]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yngve, A.; Lagergren, J.; Lu, Y. Dietary intake of lignans and risk of adenocarcinoma of the esophagus and gastroesophageal junction. Cancer Causes Control 2012, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Bosetti, C.; Negri, E.; Lagiou, P.; La Vecchia, C. Flavonoids, proanthocyanidins, and cancer risk: A network of case-control studies from Italy. Nutr. Cancer 2010, 62, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Yuan, J.M.; Lee, M.J.; Yang, C.S.; Gao, Y.T.; Ross, R.K.; Yu, M.C. Urinary tea polyphenols in relation to gastric and esophageal cancers: A prospective study of men in Shanghai, China. Carcinogenesis 2002, 23, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; Chow, W.H.; Engel, L.S.; He, K.; Risch, H.A.; Vaughan, T.L.; Gammon, M.D. Dietary flavonoid intake and barrett’s esophagus in western Washington state. Ann. Epidemiol. 2015, 25, 730.e2–735.e2. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Kramer, J.R.; Rugge, M.; Parente, P.; Verstovsek, G.; Alsarraj, A.; El-Serag, H.B. Dietary intake of vegetables, folate, and antioxidants and the risk of barrett’s esophagus. Cancer Causes Control 2013, 24, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; Trivers, K.F.; Abrahamson, P.E.; Engel, L.S.; He, K.; Chow, W.H.; Mayne, S.T.; Risch, H.A.; et al. Dietary intake of flavonoids and oesophageal and gastric cancer: Incidence and survival in the United States of America (USA). Br. J. Cancer 2015, 112, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yngve, A.; Lagergren, J.; Lu, Y. A dietary pattern rich in lignans, quercetin and resveratrol decreases the risk of oesophageal cancer. Br. J. Nutr. 2014, 112, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.; Zamora-Ros, R.; Duell, E.J.; Lujan-Barroso, L.; Boeing, H.; Aleksandrova, K.; Bueno-de-Mesquita, H.B.; Scalbert, A.; Romieu, I.; Fedirko, V.; et al. Dietary flavonoid intake and esophageal cancer risk in the european prospective investigation into cancer and nutrition cohort. Am. J. Epidemiol. 2013, 178, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Bobe, G.; Peterson, J.J.; Gridley, G.; Hyer, M.; Dwyer, J.T.; Brown, L.M. Flavonoid consumption and esophageal cancer among black and white men in the United States. Int. J. Cancer 2009, 125, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Garavello, W.; Talamini, R.; La Vecchia, C.; Franceschi, S.; Lagiou, P.; Zambon, P.; Dal Maso, L.; Bosetti, C.; Negri, E. Flavonoids and risk of squamous cell esophageal cancer. Int. J. Cancer 2007, 120, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Naringenin inhibits UVB irradiation-induced inflammation and oxidative stress in the skin of hairless mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Q.; Chen, X.G.; Guo, B.L.; Huang, W.H.; Shen, T.; Sun, X.G.; Xiao, P.G.; Zhou, Q. Induction of apoptosis by icariside II through extrinsic and intrinsic signaling pathways in human breast cancer MCF7 cells. Biosci. Biotechnol. Biochem. 2012, 76, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Xie, Q.; Zhang, Q.Y.; Peng, X.L.; Zhu, J.D.; Mi, M.T. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar]
- Chen, M.N.; Rao, Y.H.; Zheng, Y.; Wei, S.Q.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zikri, N.N.; Riedl, K.M.; Wang, L.S.; Lechner, J.; Schwartz, S.J.; Stoner, G.D. Black raspberry components inhibit proliferation, induce apoptosis, and modulate gene expression in rat esophageal epithelial cells. Nutr. Cancer 2009, 61, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Hecht, S.S.; Carmella, S.G.; Yu, N.; Larue, B.; Henry, C.; McIntyre, C.; Rocha, C.; Lechner, J.F.; Stoner, G.D. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev. Res. (Phila.) 2009, 2, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, D.S.; Zimmerman, N.P.; Wang, L.S.; Ransom, B.W.S.; Carmella, S.G.; Kuo, C.T.; Siddiqui, J.; Chen, J.H.; Oshima, K.; Huang, Y.W.; et al. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev. Res. (Phila.) 2014, 7, 574–584. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Country | Study-Design | Source of Control | Dietary Assessment | Participants (Cases) | Total/Subclasses of Flavonoid | Comparison | HR or OR (95% CI) | Adjustment for Covariates |
|---|---|---|---|---|---|---|---|---|---|
| Petrick, 2015 [46] | USA | Case-control | PB | Validated FFQ-104 items | 1127 (465) | Total flavonoids | ≥217.36 vs. 0–63.8 mg/day | 0.92 (0.63, 1.37) for EAC | Age, sex, race, geographic centre, cigarette smoking, and dietary energy intake |
| 0.87 (0.53, 1.41) for ESCC | |||||||||
| Anthocyanidins | ≥18.48 vs. 0–7.21 mg/day | 0.43 (0.29, 0.66) for EAC | |||||||
| 0.43 (0.26, 0.70) for ESCC | |||||||||
| Flavan-3-ols | ≥130.7 vs. 0–10.29 mg/day | 1.02 (0.69, 1.51) for EAC | |||||||
| 0.98 (0.60, 1.59) for ESCC | |||||||||
| Flavanones | ≥49.53 vs. 0–11.57 mg/day | 0.56 (0.37, 0.85) for EAC | |||||||
| 0.48 (0.29, 0.78) for ESCC | |||||||||
| Flavones | ≥2.63 vs. 0–1.29 mg/day | 0.84 (0.56, 1.25) for EAC | |||||||
| 0.55 (0.34, 0.89) for ESCC | |||||||||
| Flavonols | ≥17.8 vs. 0–8.31 mg/day | 0.80 (0.54, 1.18) for EAC | |||||||
| 0.97 (0.62, 1.53) for ESCC | |||||||||
| Isoflavones | ≥0.60 vs. 0–0.27 mg/day | 1.65 (1.02, 2.65) for EAC | |||||||
| 0.72 (0.40, 1.29) for ESCC | |||||||||
| Lignans | ≥0.083 vs. 0–0.045 mg/day | 0.75 (0.49, 1.13) for EAC | |||||||
| 0.38 (0.23, 0.63) for ESCC | |||||||||
| Tang, 2015 [32] | China | Case-control | HB | Validated FFQ-137 items | 739 (359) | Isoflavones | >26.0 vs. <8.0 mg/day | 0.37 (0.25–0.55) | Age, gender, education level, BMI, total energy intake (kJ/d), tobacco smoking, alcohol drinking, and family history of cancer |
| Lin, 2014 [47] | Sweden | Case-control | PB | Validated FFQ-36 items | 1407 (601) | Resveratrol, quercetin, and lignans | Q5 vs. Q1 | 0.24 (0.12–0.49) for EAC 0.31 (0.15–0.65) for ESCC 0.42 (0.26–0.67) for JAC | Age, sex, energy, educational level, smoking, alcohol consumption, BMI, physical activity, reflux, and Helicobacter pylori infection. |
| Vermeulen, 2013 [48] | 23 centers in 10 European countries. | Cohort | PB | Validated FFQ 1877 items | 477,312 (341) | Total flavonoids | Q4 vs. Q1 | 0.96 (0.66–1.39) | Center, age, sex, energy intake, BMI, smoking intensity, educational level, physical activity, alcohol, red and processed meat intake, fiber, vitamin C, and carotenoids |
| Flavanols | 0.65 (0.66–1.38) | ||||||||
| Flavan-3-ol | 0.86 (0.58–1.27) | ||||||||
| Proanthocyanidins | 1.14 (0.77–1.68) | ||||||||
| Theaflavins | 0.76 (0.53–1.10) | ||||||||
| Anthocyanidins | 0.88 (0.58–1.35) | ||||||||
| Flavonols | 0.90 (0.61–1.34) | ||||||||
| Flavanones | 0.93 (0.62–1.38) | ||||||||
| Flavones | 0.73 (0.48–1.10) | ||||||||
| Isoflavones | 0.71 (0.44–1.16) | ||||||||
| Bobe, 2009 [49] | United States | Case-control | PB | Not validated FFQ-57 items | 1728 (493) | Total Flavonoids | >107 vs. <43.0 mg/1000 kcal | 0.71 (0.36–1.42) for White EAC | Smoking duration and intensity, geographical area, age, BMI, hot tea consumption, hard liquor consumption, beer consumption, “moonshine” consumption (only for black men), red wine consumption, white wine consumption (except for ESCC in white men), caloric intake, education (only for black men), and income. |
| 1.19 (0.50–2.81) for White ESCC | |||||||||
| 0.72 (0.35–1.46) for Black ESCC | |||||||||
| Anthocyanidins | >4.73 vs. <1.45 mg/1000 kcal | 0.47 (0.24–0.91) for White EAC | |||||||
| 0.73 (0.32–1.67) for White ESCC | |||||||||
| 0.86 (0.42–1.75) for Black ESCC | |||||||||
| Flavan-3-ols | >60.6 vs. <10.3 mg/1000 kcal | 1.22 (0.60–2.49) for White EAC | |||||||
| 0.95 (0.36–2.52) for White ESCC | |||||||||
| 0.78 (0.36–1.68) for Black ESCC | |||||||||
| Flavanones | >26.2 vs. <9.3 mg/1000 kcal | 0.99 (0.56–1.75) for White EAC | |||||||
| 0.94 (0.47–1.90) for White ESCC | |||||||||
| 0.57 (0.30–1.08) for Black ESCC | |||||||||
| Flavones | >4.41 vs. <2.08 mg/1000 kcal | 0.81 (0.43–1.51) for White EAC | |||||||
| 0.79 (0.36–1.73) for White ESCC | |||||||||
| 1.02 (0.52–2.00) for Black ESCC | |||||||||
| Flavonols | >15.9 vs. <6.89 mg/1000 kcal | 0.98 (0.47–2.01) for White EAC | |||||||
| 1.09 (0.41–2.87) for White ESCC | |||||||||
| 1.11 (0.54–2.30) for Black ESCC | |||||||||
| Isoflavonoids | >0.019 vs. <0.005 mg/1000 kcal | 0.65 (0.36–1.18) for White EAC | |||||||
| 0.43 (0.20–0.93) for White ESCC | |||||||||
| 0.91 (0.50–1.64) for Black ESCC | |||||||||
| Proanthocyanidins | >272 vs. 45.5 mg/1000 kcal | 0.89 (0.46–1.70) for White EAC | |||||||
| 1.02 (0.46–2.26) for White ESCC | |||||||||
| 0.58 (0.30–1.13) for Black ESCC | |||||||||
| Rossi, 2007 [50] | Italy | Case-control | HB | Validated FFQ-78 items, | 1047 (304) | Total Flavonoids | Q5 vs. Q1 | 0.99 (0.55–1.79) | Age, sex, study centre, education, alcohol consumption, tobacco smoking, BMI, and energy intake. |
| Anthocyanidins | 0.84 (0.46–1.54) | ||||||||
| Flavan-3-ols | 1.06 (0.58–1.94) | ||||||||
| Flavanones | 0.38 (0.23–0.66) | ||||||||
| Flavones | 0.97 (0.57–1.67) | ||||||||
| Flavonols | 0.68 (0.38–1.64) | ||||||||
| De Stefani, 1999 [33] | Uruguay | Case-control | HB | Not validated FFQ-64 items | 459 (66) | Flavonoids | Q3 vs. Q1 | 0.4 (0.3–0.6) | Age, sex, residence, urban/rural, education, BMI, tobacco smoking, alcohol, and energy |
| Subgroups | No. of Studies | No. of Cases | Pooled ORs (95% CI) | p | Heterogeneity Test | ||
|---|---|---|---|---|---|---|---|
| Chi-Square | I2 (%) | phet | |||||
| All studies | 8 | 1673 | 0.78 (0.59–1.04) | 0.088 | 17.95 | 61.0 | 0.012 |
| Subclass of flavonoids | |||||||
| Anthocyanidins | 7 | 1607 | 0.60 (0.49–0.74) | <0.001 | 10.31 | 41.8 | 0.112 |
| Flavan-3-ols | 7 | 1607 | 0.97 (0.79–1.18) | 0.735 | 1.22 | 0 | 0.976 |
| Flavanones | 7 | 1607 | 0.65 (0.49–0.86) | 0.002 | 12.33 | 51.3 | 0.055 |
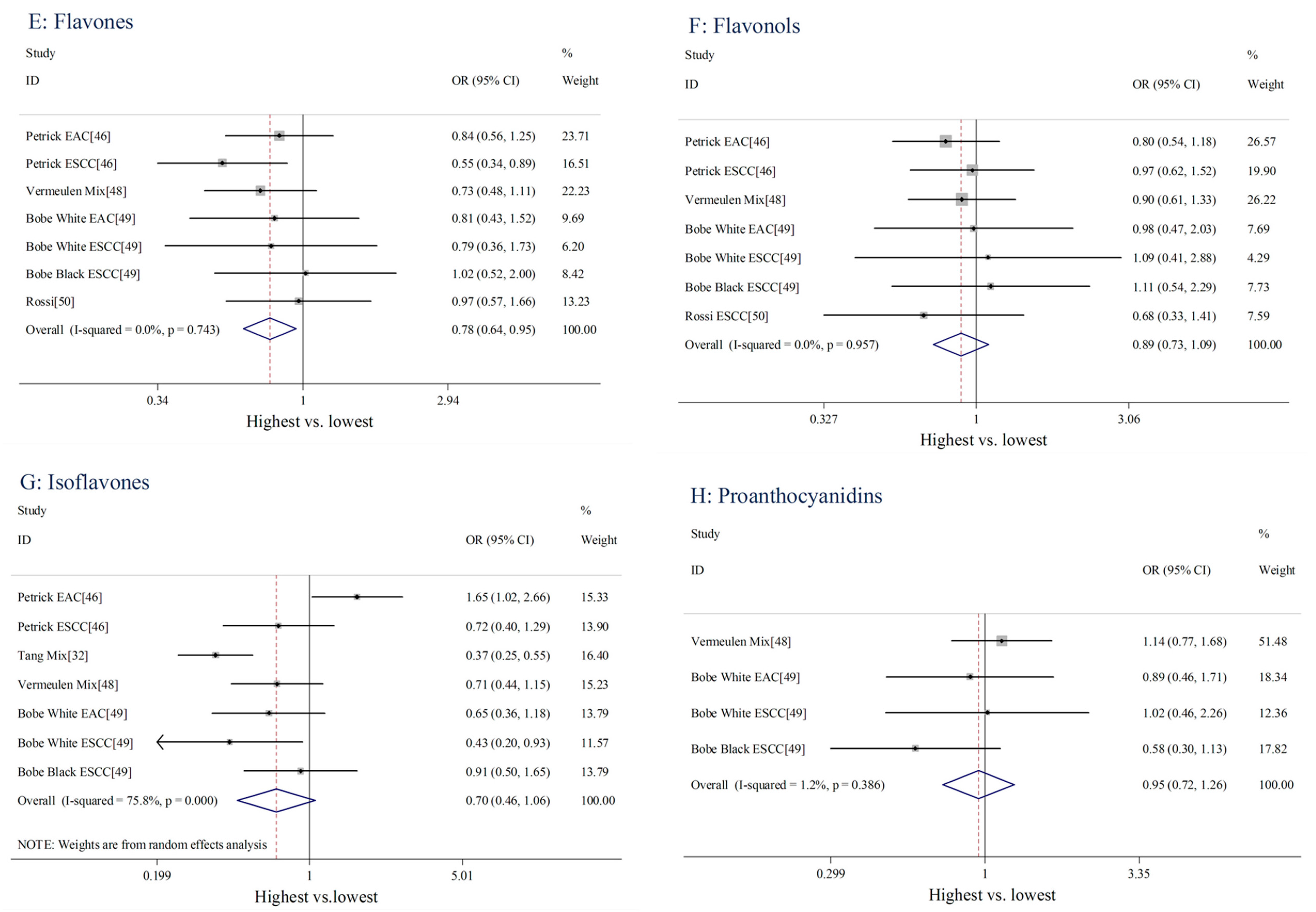
| Flavones | 7 | 1607 | 0.78 (0.64–0.95) | 0.013 | 3.51 | 0 | 0.743 |
| Flavonols | 7 | 1607 | 0.89 (0.73–1.09) | 0.276 | 1.54 | 0 | 0.957 |
| Isoflavones | 7 | 1662 | 0.70 (0.46–1.06) | 0.093 | 24.77 | 75.8 | <0.001 |
| Proanthocyanidins | 4 | 838 | 0.95 (0.72–1.26) | 0.734 | 3.04 | 1.2 | 0.386 |
| Study design | |||||||
| Cohort | 1 | 345 | 0.96 (0.66–1.39) | 0.830 | N/A | N/A | N/A |
| Case-control | 7 | 1328 | 0.76 (0.55–1.04) | 0.088 | 15.92 | 62.3 | 0.014 |
| Pathological type | |||||||
| EAC | 2 | 435 | 0.86 (0.62–1.21) | 0.396 | 0.41 | 0 | 0.520 |
| ESCC | 5 | 893 | 0.74 (0.48–1.15) | 0.051 | 13.68 | 70.8 | 0.008 |
| Mix type | 1 | 345 | 0.96 (0.66–1.39) | 0.830 | N/A | N/A | N/A |
| Source of control | |||||||
| Hospital-based | 2 | 370 | 0.61 (0.25–1.48) | 0.273 | 6.74 | 85.2 | 0.009 |
| Population-based | 6 | 1303 | 0.89 (0.74–1.09) | 0.260 | 1.39 | 0 | 0.926 |
| Geographic locations | |||||||
| Europe | 2 | 649 | 0.97 (0.71–1.33) | 0.842 | 0.01 | 0 | 0.931 |
| America | 6 | 1024 | 0.73 (0.51–1.04) | 0.080 | 14.57 | 65.7 | 0.012 |
| Dietary assessment | |||||||
| Validated FFQ | 4 | 1114 | 0.93 (0.75–1.14) | 0.461 | 0.19 | 0 | 0.979 |
| Not Validated FFQ | 4 | 559 | 0.64 (0.39–1.04) | 0.070 | 7.31 | 59.0 | 0.063 |
| Length of dietary recall | |||||||
| 0–5 years before diagnosis | 5 | 1180 | 0.78 (0.54–1.12) | 0.178 | 16.81 | 76.2 | 0.002 |
| ≥5 years before diagnosis | 3 | 493 | 0.81 (0.53–1.25) | 0.338 | 1.01 | 0 | 0.604 |
| Adjustment for energy intake | |||||||
| Yes | 5 | 1180 | 0.78 (0.54–1.12) | 0.178 | 16.81 | 76.2 | 0.002 |
| No | 3 | 493 | 0.81 (0.53–1.25) | 0.338 | 1.01 | 0 | 0.604 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Liu, X.; Tian, Y.; Xie, C.; Li, Q.; Cui, H.; Sun, C. Flavonoids, Flavonoid Subclasses, and Esophageal Cancer Risk: A Meta-Analysis of Epidemiologic Studies. Nutrients 2016, 8, 350. https://doi.org/10.3390/nu8060350
Cui L, Liu X, Tian Y, Xie C, Li Q, Cui H, Sun C. Flavonoids, Flavonoid Subclasses, and Esophageal Cancer Risk: A Meta-Analysis of Epidemiologic Studies. Nutrients. 2016; 8(6):350. https://doi.org/10.3390/nu8060350
Chicago/Turabian StyleCui, Lingling, Xinxin Liu, Yalan Tian, Chen Xie, Qianwen Li, Han Cui, and Changqing Sun. 2016. "Flavonoids, Flavonoid Subclasses, and Esophageal Cancer Risk: A Meta-Analysis of Epidemiologic Studies" Nutrients 8, no. 6: 350. https://doi.org/10.3390/nu8060350






