Alternate-Day High-Fat Diet Induces an Increase in Mitochondrial Enzyme Activities and Protein Content in Rat Skeletal Muscle
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Treatment of Animals
2.3. Measurement of Mitochondrial Enzyme Activities
2.4. Western Blot Analysis
2.5. Analytical Procedure
2.6. Succinate Dehydrogenase (SDH) Staining
2.7. Muscle Glycogen Concentration
2.8. Statistical Analysis
3. Results
3.1. Body Weight, Epididymal Fat Weight, and Plasma Parameters
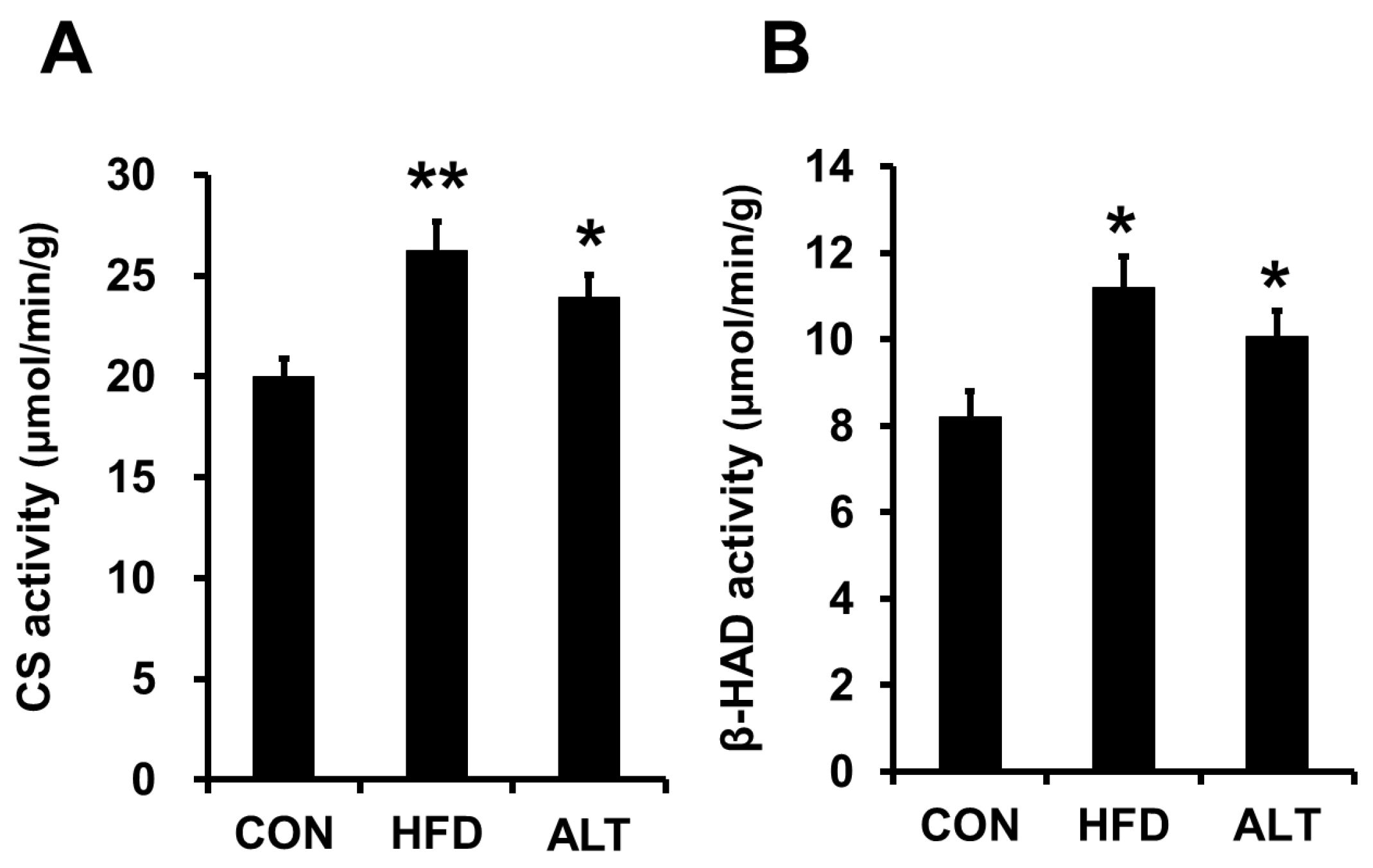
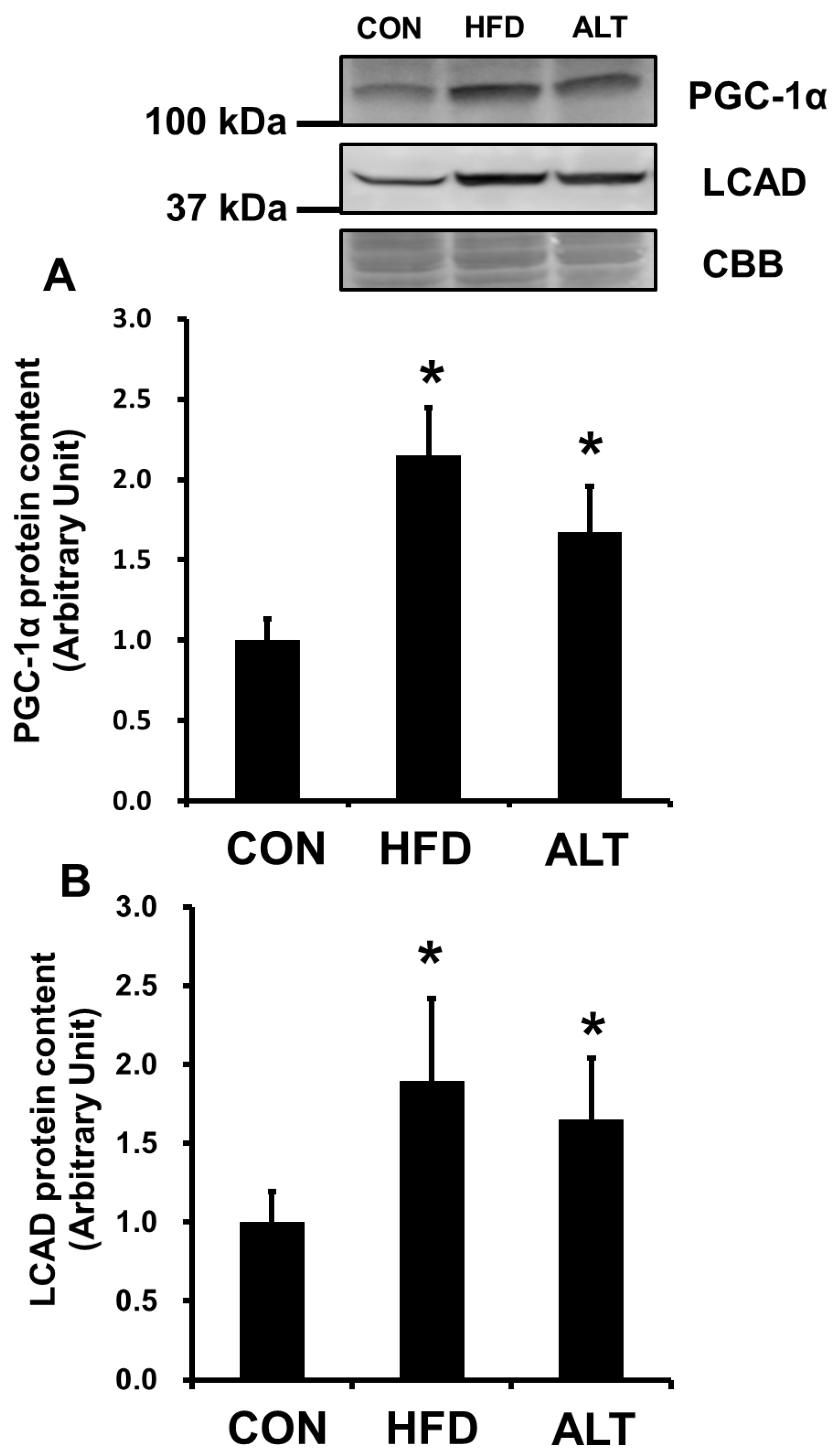
3.2. Mitochondrial Enzymes Activities and PGC-1α Protein Content
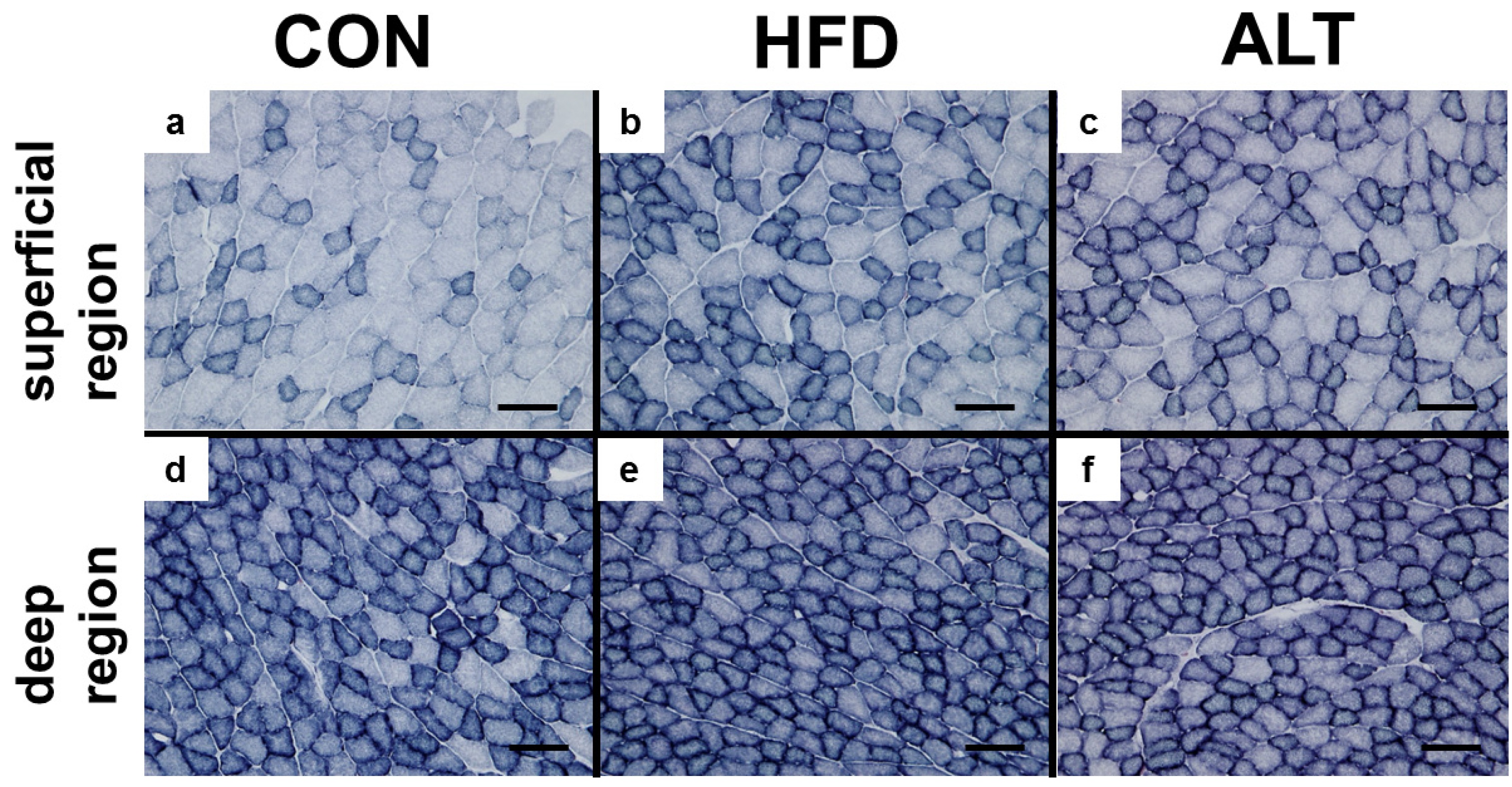
3.3. SDH Activity
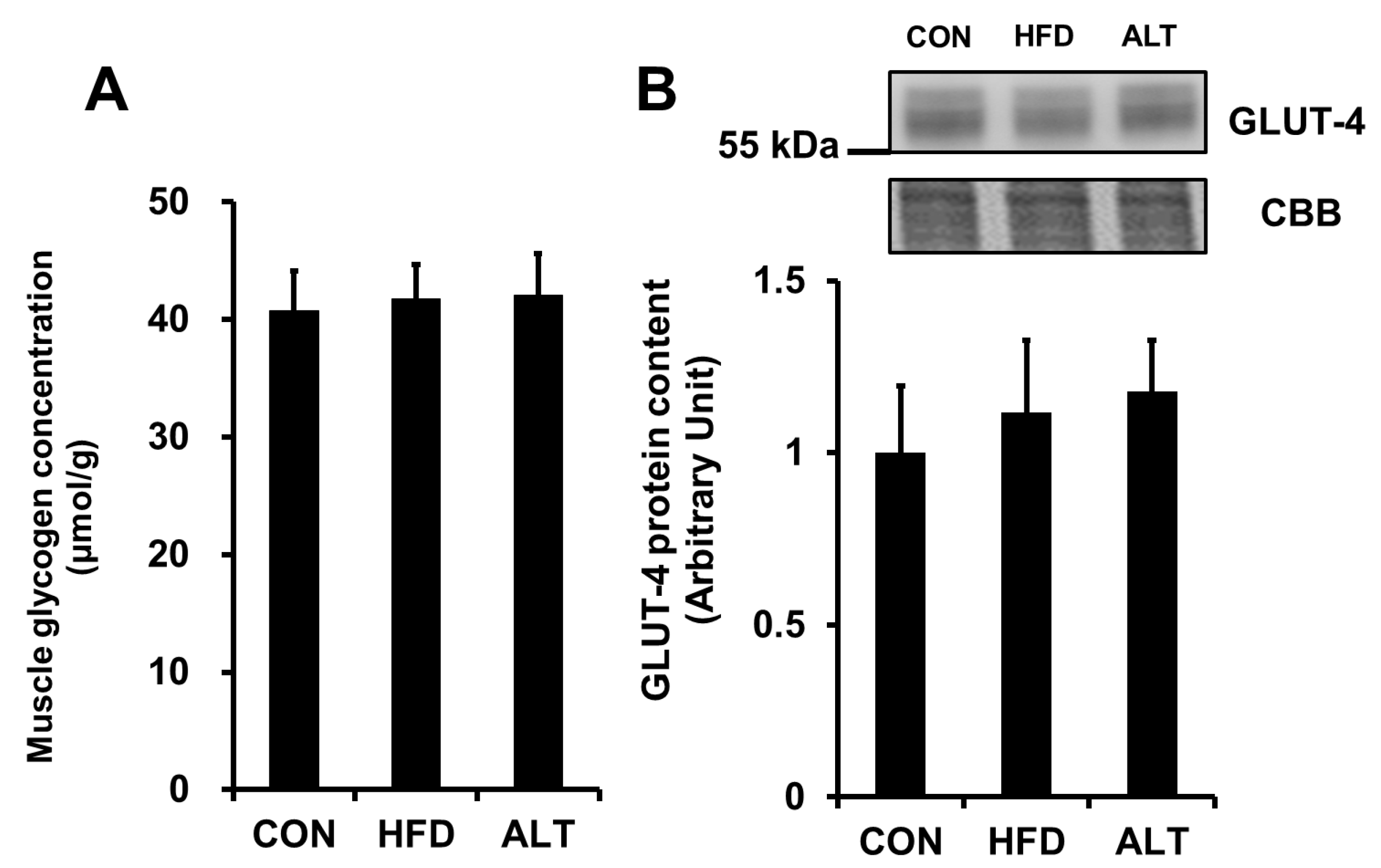
3.4. Muscle Glycogen Concentration and Glucose Transporter-4 Protein Content
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Holloszy, J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967, 242, 2278–2282. [Google Scholar] [PubMed]
- Constable, S.H.; Favier, R.J.; McLane, J.A.; Fell, R.D.; Chen, M.; Holloszy, J.O. Energy metabolism in contracting rat skeletal muscle: Adaptation to exercise training. Am. J. Physiol. 1987, 253, C316–C322. [Google Scholar] [PubMed]
- Martin, W.H.; Dalsky, G.P.; Hurley, B.F.; Matthews, D.E.; Bier, D.M.; Hagberg, J.M.; Rogers, M.A.; King, D.S.; Holloszy, J.O. Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am. J. Physiol. 1993, 265, E708–E714. [Google Scholar] [PubMed]
- Miller, W.C.; Bryce, G.R.; Conlee, R.K. Adaptations to a high-fat diet that increase exercise endurance in male rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 78–83. [Google Scholar] [PubMed]
- Phinney, S.D.; Bistrian, B.R.; Evans, W.J.; Gervino, E.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 2, 769–776. [Google Scholar] [CrossRef]
- Simi, B.; Sempore, B.; Mayet, M.H.; Favier, R.J. Additive effects of training and high-fat diet on energy metabolism during exercise. J. Appl. Physiol. 1991, 71, 197–203. [Google Scholar] [PubMed]
- Helge, J.W.; Kiens, B. Muscle enzyme activity in humans: Role of substrate availability and training. Am. J. Physiol. 1997, 272, R1620–R1624. [Google Scholar] [PubMed]
- Lee, J.S.; Bruce, C.R.; Spriet, L.L.; Hawley, J.A. Interaction of diet and training on endurance performance in rats. Exp. Physiol. 2001, 86, 499–508. [Google Scholar] [PubMed]
- Sparks, L.M.; Xie, H.; Koza, R.A.; Mynatt, R.; Hulver, M.W.; Bray, G.A.; Smith, S.R. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 2005, 54, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.J.; Barakat, H.A.; Dohm, G.L.; Pories, W.J.; MacDonald, K.G.; Cunningham, P.R.; Swanson, M.S.; Houmard, J.A. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1191–E1196. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.S.; Carey, J.O.; Azevedo, J.L.; Houmard, J.A.; Pories, W.J.; Israel, R.G.; Dohm, G.L. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am. J. Physiol. 1995, 268, E453–E457. [Google Scholar] [PubMed]
- Higashida, K.; Kim, S.H.; Higuchi, M.; Holloszy, J.O.; Han, D.H. Normal adaptations to exercise despite protection against oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E779–E784. [Google Scholar] [CrossRef] [PubMed]
- Hancock, C.R.; Han, D.H.; Chen, M.; Terada, S.; Yasuda, T.; Wright, D.C.; Holloszy, J.O. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl. Acad. Sci. USA 2008, 105, 7815–7820. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Roves, P.; Huss, J.M.; Han, D.H.; Hancock, C.R.; Iglesias-Gutierrez, E.; Chen, M.; Holloszy, J.O. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc. Natl. Acad. Sci. USA 2007, 104, 10709–10713. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Bruce, C.R.; Beale, S.M.; Hoehn, K.L.; So, T.; Rolph, M.S.; Cooney, G.J. Excess Lipid Availability Increases Mitochondrial Fatty Acid Oxidative Capacity in Muscle. Evidence against a Role for Reduced Fatty Acid Oxidation in Lipid-Induced Insulin Resistance in Rodents. Diabetes 2007, 56, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, N.; Jacobs, D.L.; Mills, D.B.; Winder, W.W.; Hancock, C.R. Chronic AMP-activated protein kinase activation and a high-fat diet have an additive effect on mitochondria in rat skeletal muscle. J. Appl. Physiol. 2010, 109, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Srere, P.A. Citrate synthase. Methods Enzymol. 1969, 13, 3–5. [Google Scholar]
- Bass, A.; Brdiczka, D.; Eyer, P.; Hofer, S.; Pette, D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur. J. Biochem. 1968, 10, 198–206. [Google Scholar] [CrossRef]
- Welinder, C.; Ekblad, L. Coomassie staining as loading control in Western blot analysis. J. Proteome Res. 2011, 10, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Passonneau, J.V. A Flexible System of Enzymatic Analysis; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Fisher, J.S.; Nolte, L.A.; Kawanaka, K.; Han, D.H.; Jones, T.E.; Holloszy, J.O. Glucose transport rate and glycogen synthase activity both limit skeletal muscle glycogen accumulation. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1214–E1221. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.E.; Cobb, L.A.; Short, F.A.; Ross, R.; Gunn, D.R. Effects of Long-Term Exercise on Human Muscle Mitochondria. Adv. Exp. Med. Biol. 1971, 11, 87–95. [Google Scholar]
- Favier, R.J.; Constable, S.H.; Chen, M.; Holloszy, J.O. Endurance exercise training reduces lactate production. J. Appl. Physiol. 1986, 61, 885–889. [Google Scholar] [PubMed]
- Kim, J.Y.; Nolte, L.A.; Hansen, P.A.; Han, D.H.; Ferguson, K.; Thompson, P.A.; Holloszy, J.O. High-fat diet-induced muscle insulin resistance: Relationship to visceral fat mass. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R2057–R2065. [Google Scholar] [PubMed]
- Racette, S.B.; Evans, E.M.; Weiss, E.P.; Hagberg, J.M.; Holloszy, J.O. Abdominal adiposity is a stronger predictor of insulin resistance than fitness among 50–95 years old. Diabetes Care 2006, 29, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Spiegelman, B.M. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 2006, 27, 728–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helge, J.W.; Wulff, B.; Kiens, B. Impact of a fat-rich diet on endurance in man: Role of the dietary period. Med. Sci. Sports Exerc. 1998, 30, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2013.
- US Department of Agriculture/Agricultural Research Service. Nutrient Intake from Food: Percentages of Energy from Protein, Carbohydrate, Fat, and Alcohol, by Gender and Age, in the United States, 2011–2012; USDA: Wahington, DC, USA, 2014. Available online: http://www.ars.usda.gov/SP2UserFiles/Place/80400530/pdf/1112/Table_5_EIN_GEN_11.pdf (accessed on 30 March 2016).
- Shortreed, K.E.; Krause, M.P.; Huang, J.H.; Dhanani, D.; Moradi, J.; Ceddia, R.B.; Hawke, T.J. Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS ONE 2009, 4, e7293. [Google Scholar] [CrossRef] [PubMed]
- Sadler, N.C.; Angel, T.E.; Lewis, M.P.; Pederson, L.M.; Chauvigné-Hines, L.M.; Wiedner, S.D.; Zink, E.M.; Smith, R.D.; Wright, A.T. Activity-based protein profiling reveals mitochondrial oxidative enzyme impairment and restoration in diet-induced obese mice. PLoS ONE 2012, 7, e47996. [Google Scholar] [CrossRef] [PubMed]
- Umanskaya, A.; Santulli, G.; Xie, W.; Andersson, D.C.; Reiken, S.R.; Marks, A.R. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc. Natl. Acad. Sci. USA 2014, 111, 15250–15255. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef] [PubMed]
| CON | HFD | ALT | |
|---|---|---|---|
| Initial body weight (g) | 87 ± 1 | 86 ± 5 | 87 ± 1 |
| Final body weight (g) | 298 ± 5 | 297 ± 9 | 298 ± 3 |
| Epididymal fat mass (g) | 3.1 ± 0.2 | 5.1 ± 0.3 * | 3.8 ± 0.1 |
| Plasma glucose (mg/mL) | 96.9 ± 2.6 | 96.8 ± 6.3 | 81.1 ± 2.6 # |
| Plasma FFA (mEq/L) | 0.28 ± 0.02 | 0.44 ± 0.05 * | 0.44 ± 0.06 * |
| Plasma insulin (µg/L) | 0.39 ± 0.3 | 0.42 ± 0.4 | 0.39 ± 0.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Higashida, K.; Kawamura, T.; Higuchi, M. Alternate-Day High-Fat Diet Induces an Increase in Mitochondrial Enzyme Activities and Protein Content in Rat Skeletal Muscle. Nutrients 2016, 8, 203. https://doi.org/10.3390/nu8040203
Li X, Higashida K, Kawamura T, Higuchi M. Alternate-Day High-Fat Diet Induces an Increase in Mitochondrial Enzyme Activities and Protein Content in Rat Skeletal Muscle. Nutrients. 2016; 8(4):203. https://doi.org/10.3390/nu8040203
Chicago/Turabian StyleLi, Xi, Kazuhiko Higashida, Takuji Kawamura, and Mitsuru Higuchi. 2016. "Alternate-Day High-Fat Diet Induces an Increase in Mitochondrial Enzyme Activities and Protein Content in Rat Skeletal Muscle" Nutrients 8, no. 4: 203. https://doi.org/10.3390/nu8040203




