Anti-Diabetic Activities of Gastrodia elata Blume Water Extracts Are Mediated Mainly by Potentiating Glucose-Stimulated Insulin Secretion and Increasing β-Cell Mass in Non-Obese Type 2 Diabetic Animals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Extract of GEB and Its Gastrodin and P-Hydroxybenzyl Alcohol Contents
2.2. Animals and Ethics
2.3 Experimental Design
2.4. Energy Expenditure by Indirect Calorimetry
2.5. Euglycemic Hyperinsulinemic Clamp
2.6. Hyperglycemic Clamp
2.7. Immunoblot Analysis
2.8. Immunohistochemistry
2.9. Insulin Secretion in Mouse Insulinoma Cells (Min6 Cells)
2.10. Statistical Analyses
3. Results
3.1. Bioactive Compounds in GEB
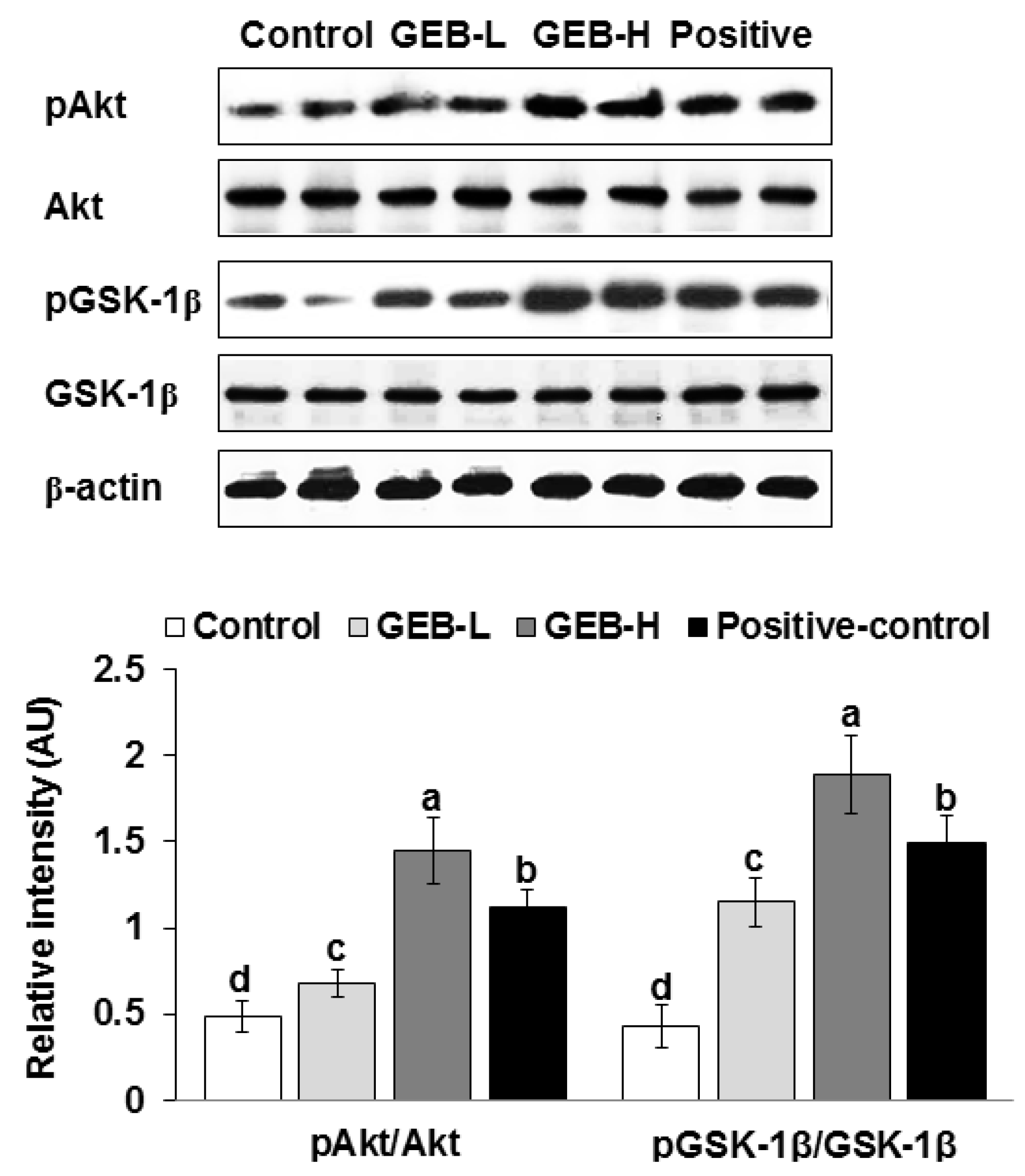
3.2. Hypothalamic Insulin Signaling
3.3. Energy Intake or Expenditure
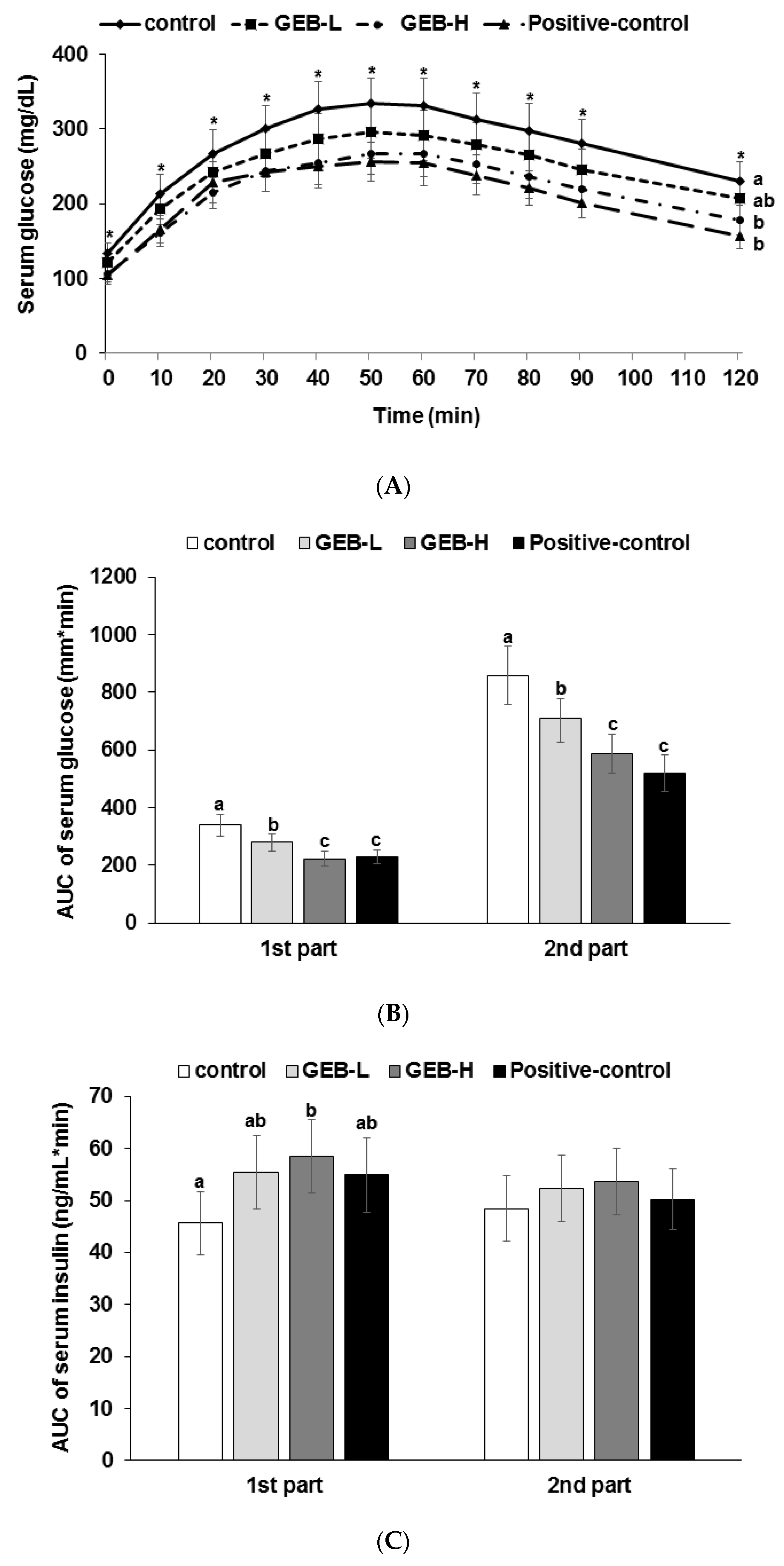
3.4. Glucose Tolerance
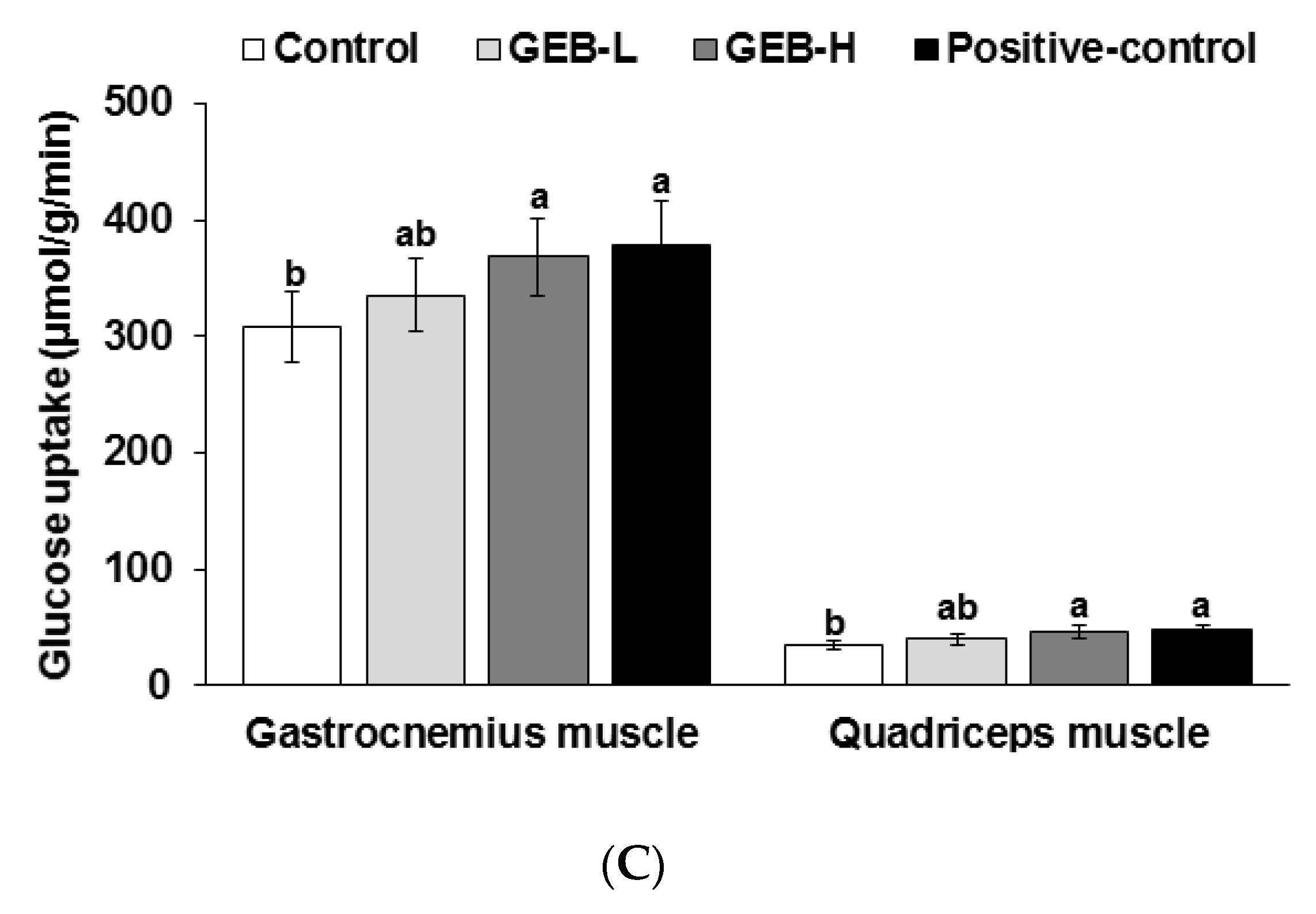
3.5. Insulin Sensitivity at a Hyperinsulinemic State
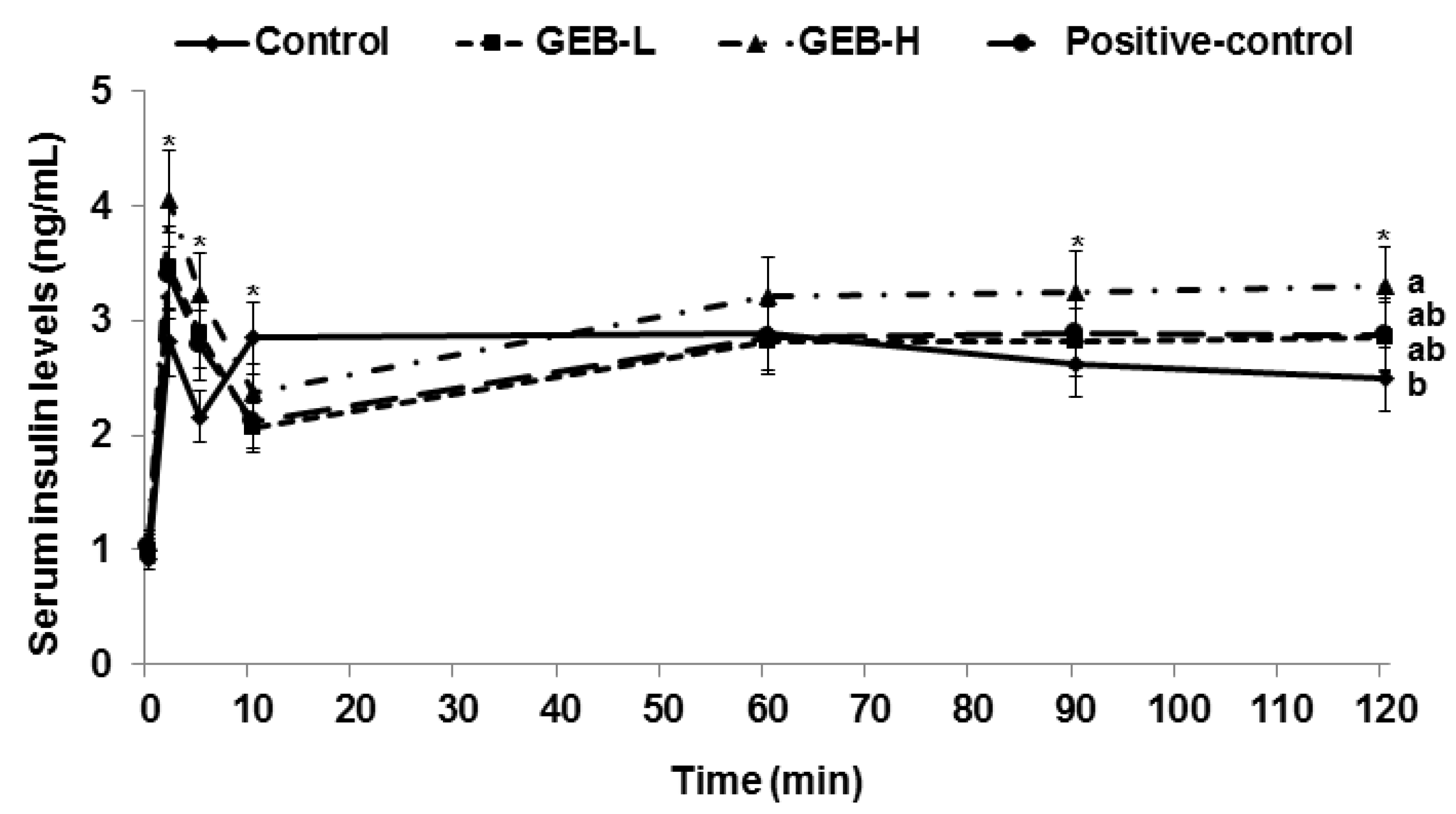
3.6. Glucose-Stimulated Insulin Secretion
3.7. Pancreatic β-Cell Mass, Proliferation, and Apoptosis
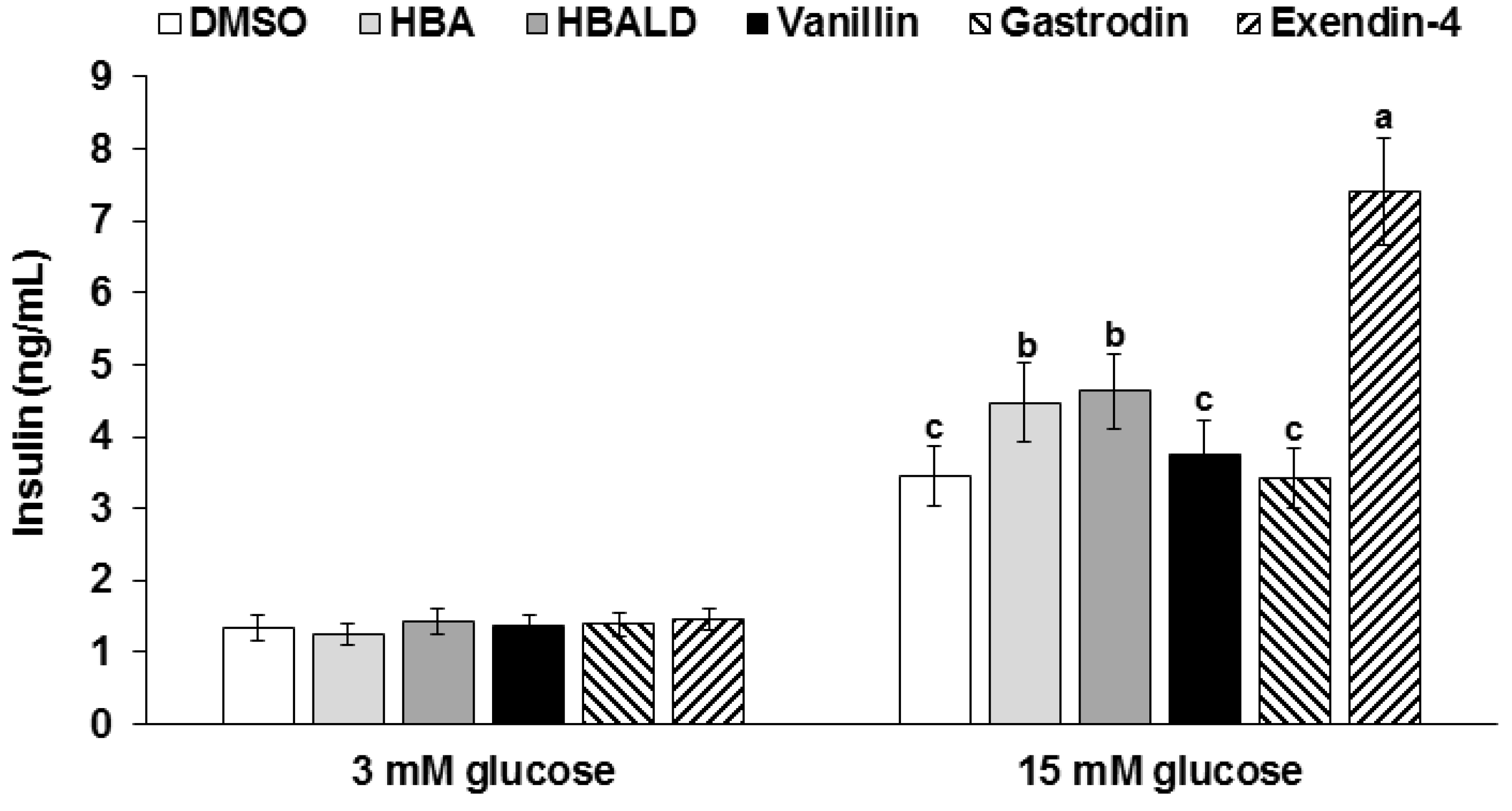
3.8. Glucose-Stimulated Insulin Secretion with Gastrodin, 4-Hydroxybenzyl Alcohol, and 4-Hydroxybenzaldehyde
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Malik, V.; Jia, W.; Kadowaki, T.; Yajnik, C.S.; Yoon, K.H.; Hu, F.B. Diabetes in asia: Epidemiology, risk factors, and pathophysiology. JAMA 2009, 301, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Chik, S.C.; Or, T.C.; Luo, D.; Yang, C.L.; Lau, A.S. Pharmacological effects of active compounds on neurodegenerative disease with gastrodia and uncaria decoction, a commonly used poststroke decoction. Sci. World J. 2013, 2013, 896873. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.B.; Zhao, T.; Muna, S.S.; Jin, H.M.; Park, J.I.; Jo, K.S.; Lee, B.H.; Chae, S.W.; Kim, S.Y.; Park, S.H.; et al. Therapeutic potential of gastrodia elata blume for the treatment of Alzheimer’s disease. Neural. Regen. Res. 2013, 8, 1061–1070. [Google Scholar] [PubMed]
- Jung, T.Y.; Suh, S.I.; Lee, H.; Kim, I.S.; Kim, H.J.; Yoo, H.S.; Lee, S.R. Protective effects of several components of Gastrodia elata on lipid peroxidation in gerbil brain homogenates. Phytother. Res. 2007, 21, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hwang, I.K.; Won, M.H. Vanillin, 4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent hippocampal ca1 cell death following global ischemia. Brain Res. 2007, 1181, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, E.; Velazquez, E.; Hurtado-Carneiro, V.; Ruiz-Albusac, J.M. Insulin in the brain: Its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front. Endocrinol. 2014, 5, 161. [Google Scholar]
- Sebastiao, I.; Candeias, E.; Santos, M.S.; de Oliveira, C.R.; Moreira, P.I.; Duarte, A.I. Insulin as a bridge between type 2 diabetes and alzheimer disease—How anti-diabetics could be a solution for dementia. Front. Endocrinol. 2014, 5, 110. [Google Scholar]
- Park, S.; Kim da, S.; Kang, S. Gastrodia elata blume water extracts improve insulin resistance by decreasing body fat in diet-induced obese rats: Vanillin and 4-hydroxybenzaldehyde are the bioactive candidates. Eur. J. Nutr. 2011, 50, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Lim, J.H.; Park, K.J.; Kang, S.; Kim, D.S.; Park, S. Methyl jasmolate treated buckwheat sprout powder enhances glucose metabolism by potentiating hepatic insulin signaling in estrogen-deficient rats. Nutrition 2016, 32, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, Y.A.; Hosokawa, H.; Chen, C.; Leahy, J.L. Mechanism of impaired glucose-potentiated insulin secretion in diabetic 90% pancreatectomy rats. Study using glucagonlike peptide-1 (7–37). J. Clin. Investig. 1996, 97, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Wilson, R.D. Experimentally induced rodent models of type 2 diabetes. Methods Mol. Biol. 2012, 933, 161–174. [Google Scholar] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. Ain-93 purified diets for laboratory rodents: Final report of the American institute of nutrition ad hoc writing committee on the reformulation of the ain-76a rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Niwa, H.; Ogawa, Y.; Kido, Y.; Abe, Y.; Kobayashi, M.; Mori, T.; Tanaka, T. The rate of lipid oxidation in septic rat models. Jpn. J. Surg. 1989, 19, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Labayen, I.; Forga, L.; Martinez, J.A. Nutrient oxidation and metabolic rate as affected by meals containing different proportions of carbohydrate and fat, in healthy young women. Eur. J. Nutr. 1999, 38, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Cha, M.R.; Yon, G.H.; Yang, H.J.; Kim, M.J.; Kang, S.; Park, S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Biochem. 2013, 24, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.J.; Fillmore, J.J.; Chen, Y.; Moore, I.; Lee, J.; Yuan, M.; Li, Z.W.; Karin, M.; Perret, P.; et al. Prevention of fat-induced insulin resistance by salicylate. J. Clin. Investig. 2001, 108, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Jang, J.S.; Park, S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology 2005, 146, 4786–4794. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, R.L.; Szczepaniak, L.S.; Myhill, J.; Tamura, Y.; Uchino, H.; Giacca, A.; McGarry, J.D. The composition of dietary fat directly influences glucose-stimulated insulin secretion in rats. Diabetes 2002, 51, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hong, S.M.; Lee, J.E.; Sung, S.R. Exercise improves glucose homeostasis that has been impaired by a high-fat diet by potentiating pancreatic beta-cell function and mass through irs2 in diabetic rats. J. Appl. Physiol. 2007, 103, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahn, I.S.; Kim, J.H.; Lee, M.R.; Kim, J.S.; Kim, H.J. Glyceollins, one of the phytoalexins derived from soybeans under fungal stress, enhance insulin sensitivity and exert insulinotropic actions. J. Agric. Food Chem. 2010, 58, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E. Metabolic sensing neurons and the control of energy homeostasis. Physiol. Behav. 2006, 89, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Hwang, J.T.; Kwon, D.Y.; Kim, M.J.; Kang, S.; Moon, N.R.; Park, S. Yuzu extract prevents cognitive decline and impaired glucose homeostasis in beta-amyloid-infused rats. J. Nutr. 2013, 143, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.H.; Lee, J.H.; Kim, J.W.; Cho, J.H.; Choi, Y.H.; Ko, S.H.; Zimmet, P.; Son, H.Y. Epidemic obesity and type 2 diabetes in asia. Lancet 2006, 368, 1681–1688. [Google Scholar] [CrossRef]
- Rossetti, L.; Shulman, G.I.; Zawalich, W.; DeFronzo, R.A. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J. Clin. Invest. 1987, 80, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Laybutt, D.R.; Kaneto, H.; Bonner-Weir, S.; Sharma, A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes 2001, 50, S154–S159. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, D.; Cota, D.; Seeley, R.J. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu. Rev. Physiol. 2008, 70, 513–535. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hong, S.M.; Ahn, I.S. Long-term intracerebroventricular infusion of insulin, but not glucose, modulates body weight and hepatic insulin sensitivity by modifying the hypothalamic insulin signaling pathway in type 2 diabetic rats. Neuroendocrinology 2009, 89, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Kho, M.C.; Lee, Y.J.; Cha, J.D.; Choi, K.M.; Kang, D.G.; Lee, H.S. Gastrodia elata ameliorates high-fructose diet-induced lipid metabolism and endothelial dysfunction. Evid. Based. Complement. Alternat. Med. 2014, 2014, 101624. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.J.; Je, J.H.; Kim, S.H.; Ahn, S.M.; Kim, H.N.; Kim, Y.R.; Choi, Y.W.; Shin, H.K.; Choi, B.T. Gastrodia elata shows neuroprotective effects via activation of pi3k signaling against oxidative glutamate toxicity in ht22 cells. Am. J. Chin. Med. 2014, 42, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Lee, Y.W.; Park, S.J.; Jeon, S.J.; Kim, E.; Lee, S.; Han, A.R.; Seo, E.K.; Ryu, J.H. 4-hydroxybenzyl methyl ether improves learning and memory in mice via the activation of dopamine d1 receptor signaling. Neurobiol. Learn. Mem. 2015, 121, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Henquin, J.C. The k+-atp channel-independent pathway of regulation of insulin secretion by glucose: In search of the underlying mechanism. Diabetes 1998, 47, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.; Singh, J.; Rashed, H.; Tariq, S.; Zilahi, E.; Adeghate, E. Mechanism of the beneficial and protective effects of exenatide in diabetic rats. J. Endocrinol. 2014, 220, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J. Glp-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef] [PubMed]

| Water Extracts (n = 3) | 70% Ethanol Extracts (n = 3) | |
|---|---|---|
| Total phenols | 755 ± 17.3 | 784 ± 19.1 * |
| Total flavonoids | 68.3 ± 2.3 | 69.2 ± 3.5 |
| Gastrodin | 3.78 ± 0.016 | 3.69 ± 0.01 |
| p-hydroxybenzyl alcohol | 0.45 ± 0.0002 | 0.43 ± 0.0001 |
| Control (n = 16) | GEB-L (n = 16) | GEB-H (n = 16) | Positive-Control (n = 16) | |
|---|---|---|---|---|
| Body weight (g) | 324 ± 28 | 336 ± 29 | 345 ± 30 | 333 ± 29 |
| Epididymal fat pads (g) | 4.9 ± 0.9 | 5.0 ± 0.8 | 5.2 ± 0.9 | 5.2 ± 0.7 |
| Caloric intake (kcal/day) | 86.6 ± 9.5 | 83.4 ± 9.6 | 80.5 ± 9.2 | 79.6 ± 8.8 |
| Caloric expenditure (kcal/kg bw0.75/day) | 104 ± 11 | 108 ± 12 | 111 ± 11 | 111 ± 12 |
| Carbohydrate oxidation (kcal/kg bw0.75/day) | 4.4 ± 0.6 c | 5.5 ± 0.6 a,b | 6.1 ± 0.7 a | 5.3 ± 0.6 b |
| Fat oxidation (kcal/kg bw0.75/day) | 6.7 ± 0.7 a | 5.9 ± 0.7 a,b | 5.7 ± 0.7 b | 6.5 ± 0.7 a |
| Control (n = 8) | GEB-L (n = 8) | GEB-H (n = 8) | Positive-Control (n = 8) | |
|---|---|---|---|---|
| Overnight fasted serum glucose (mg/dL) | 132 ± 16 a | 120 ± 17 a,b | 108 ± 14 b | 106 ± 11 c |
| Overnight fasted serum insulin (ng/mL) | 0.97 ± 0.11 | 0.99 ± 0.14 | 1.05 ± 0.12 | 1.04 ± 0.11 |
| Area under the curve of serum insulin at first phase (ng/mL·min) | 20.7 ± 2.1 c | 24.7 ± 3.0 b | 29.4 ± 3.1 a | 20.5 ± 2.7 c |
| Area under the curve of serum insulin at second phase (ng/mL·min) | 258 ± 28 b | 254 ± 27 b | 291 ± 30 a | 240 ± 27 b |
| Glucose infusion rate (mg/kg bw/min) | 8.3 ± 1.0 c | 10.6 ± 1.2 b | 13.4 ± 1.5 a | 12.7 ± 1.5 a |
| Insulin sensitivity (µmol glucose·min−1·100 g−1 per µmol insulin/L) | 12.5 ± 1.4 d | 14.6 ± 1.6 c | 16.4 ± 1.7 b | 18.3 ± 1.7 a |
| Control (n = 8) | GEB-L (n = 8) | GEB-H (n = 8) | Positive-Control (n = 8) | |
|---|---|---|---|---|
| β-cell area (%) | 6.5 ± 0.8 b | 7.0 ± 0.9 a,b | 7.6 ± 0.9 a | 7.0 ± 0.9 a,b |
| Individual β-cell size (μm2) | 227 ± 28 a | 208 ± 26 a,b | 187 ± 25 b | 193 ± 27 b |
| Absolute β-cell mass (mg) | 18.7 ± 2.8 c | 23.3 ± 3.0 b | 28.8 ± 3.5 a | 23.8 ± 2.9 b |
| BrdU+ cells (% BrdU+ cells of islets) | 0.84 ± 0.10 b | 0.92 ± 0.12 b | 1.11 ± 0.12 a | 0.93 ± 0.12 b |
| Apoptosis (% apoptotic bodies of islets) | 0.70 ± 0.09 a | 0.65 ± 0.07 a,b | 0.59 ± 0.08 b | 0.61 ± 0.07 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.J.; Kim, M.J.; Kwon, D.Y.; Kim, D.S.; Lee, Y.H.; Kim, J.E.; Park, S. Anti-Diabetic Activities of Gastrodia elata Blume Water Extracts Are Mediated Mainly by Potentiating Glucose-Stimulated Insulin Secretion and Increasing β-Cell Mass in Non-Obese Type 2 Diabetic Animals. Nutrients 2016, 8, 161. https://doi.org/10.3390/nu8030161
Yang HJ, Kim MJ, Kwon DY, Kim DS, Lee YH, Kim JE, Park S. Anti-Diabetic Activities of Gastrodia elata Blume Water Extracts Are Mediated Mainly by Potentiating Glucose-Stimulated Insulin Secretion and Increasing β-Cell Mass in Non-Obese Type 2 Diabetic Animals. Nutrients. 2016; 8(3):161. https://doi.org/10.3390/nu8030161
Chicago/Turabian StyleYang, Hye Jeong, Min Jung Kim, Dae Young Kwon, Da Sol Kim, Young Hyun Lee, Ji Eun Kim, and Sunmin Park. 2016. "Anti-Diabetic Activities of Gastrodia elata Blume Water Extracts Are Mediated Mainly by Potentiating Glucose-Stimulated Insulin Secretion and Increasing β-Cell Mass in Non-Obese Type 2 Diabetic Animals" Nutrients 8, no. 3: 161. https://doi.org/10.3390/nu8030161






