Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments
Abstract
:1. Introduction
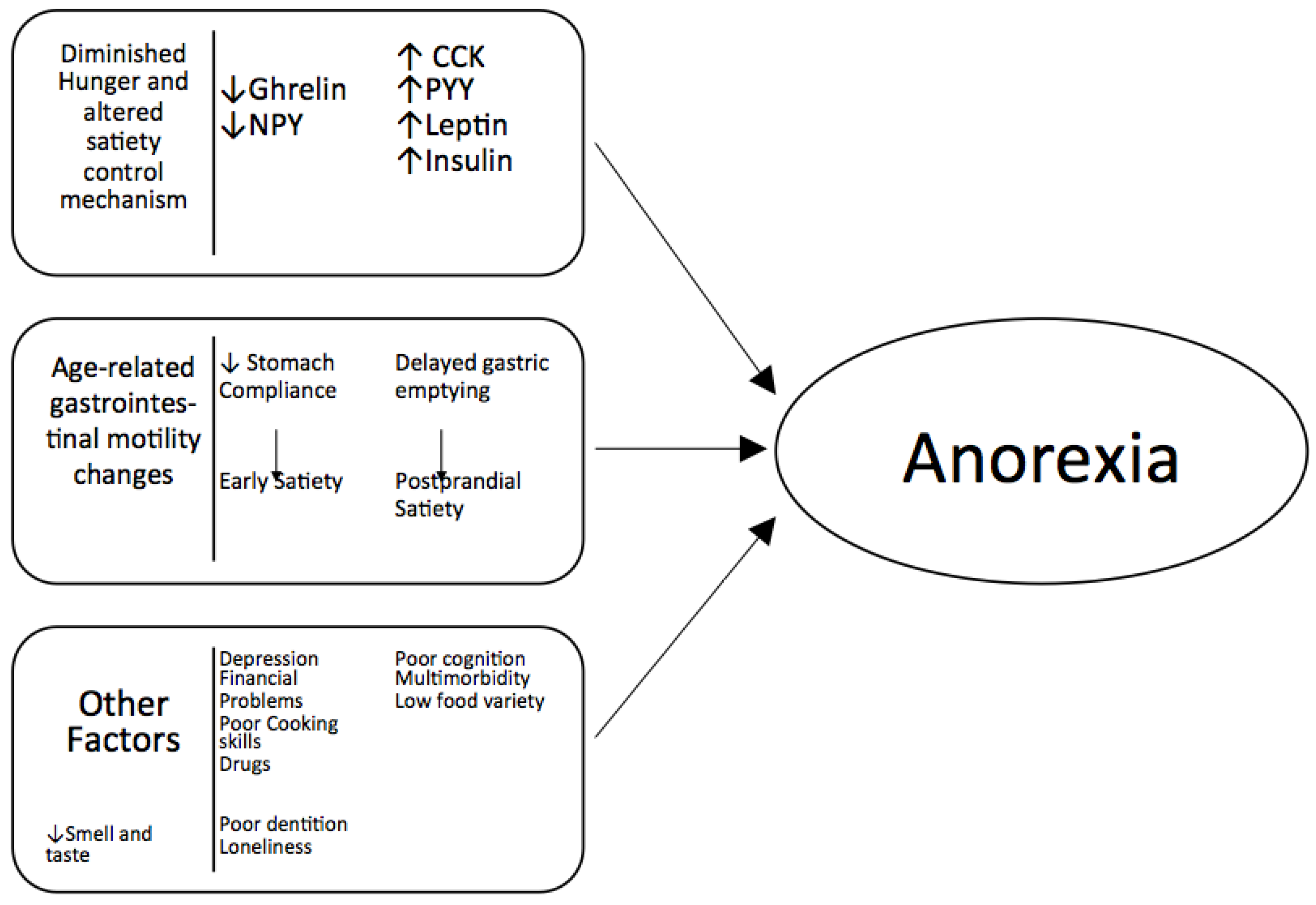
2. Mechanisms of Anorexia of Aging
2.1. Smell and Taste
2.2. Hormones
2.3. Gastrointestinal Function
2.4. Inflammation
3. Risk Factors for Anorexia of Aging
3.1. Physical Factors

3.2. Medical Factors
3.3. Medications
3.4. Social Factors
4. Assessment of Anorexia of Aging
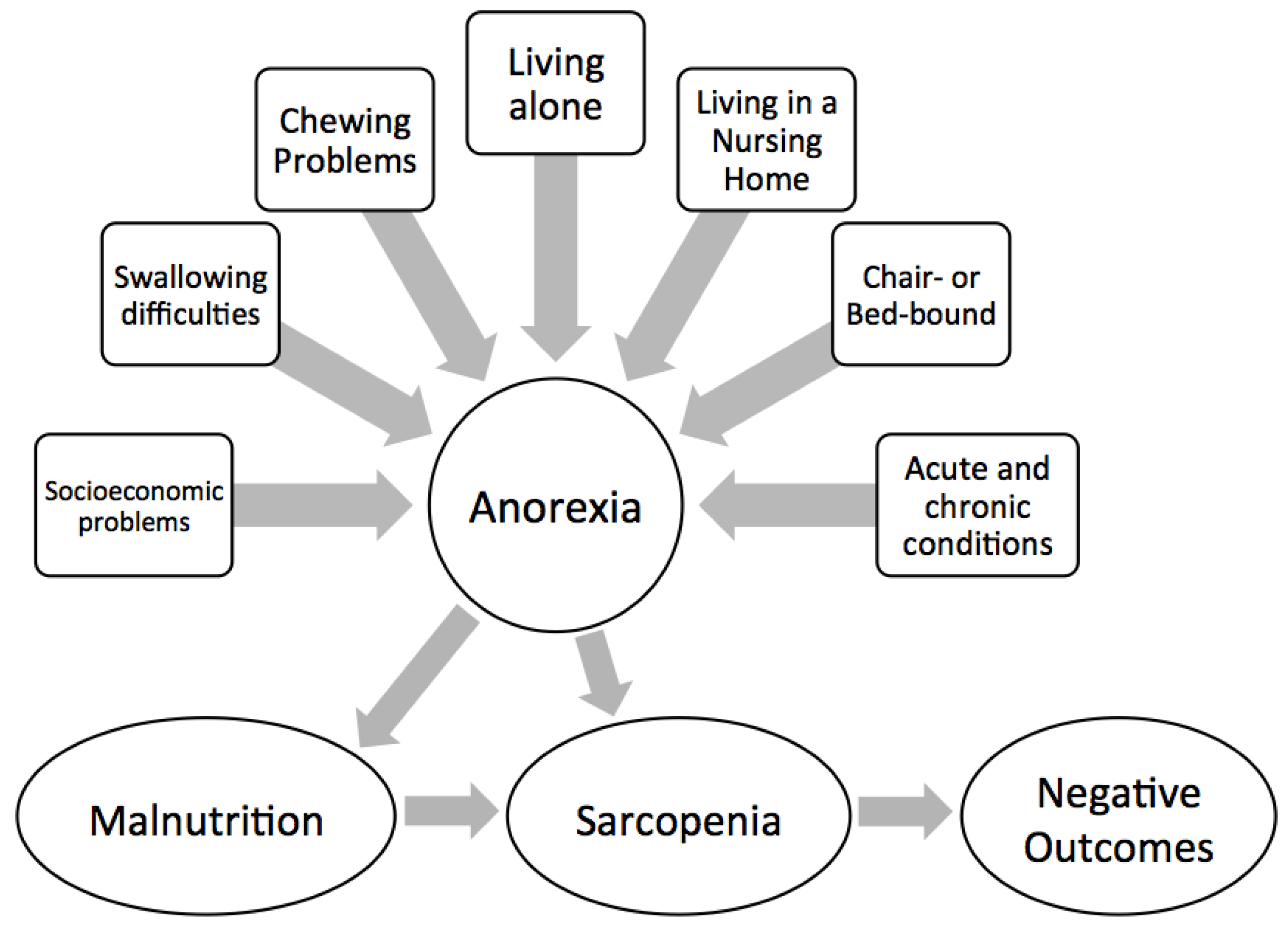

5. Consequences of Anorexia of Aging
5.1. Malnutrition
5.2. Frailty and Sarcopenia
5.3. Mortality
6. Treatment of Anorexia of Aging
6.1. Food Manipulation
6.2. Environmental Adaptation
6.3. Medication
6.4. Medical Diagnoses
6.5. Specific Treatments
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Di Francesco, V.; Barazzoni, R.; Bissoli, L.; Fantin, F.; Rizzotti, P.; Residori, L.; Antonioli, A.; Graziani, M.S.; Zanetti, M.; Bosello, O.; et al. The quantity of meal fat influences the profile of postprandial hormones as well as hunger sensation in healthy elderly people. J. Am. Med. Dir. Assoc. 2010, 11, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, B.A.; Blanton, C.A.; McDonald, R.B. Physiologic determinants of the anorexia of aging: Insights from animal studies. Annu. Rev. Nutr. 2002, 22, 417–438. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Cascino, A.; Bartali, B.; Donini, L.; Rossi-Fanelli, F.; Laviano, A. Anorexia of Aging. Curr. Nutr. Food Sci. 2009, 5, 9–12. [Google Scholar] [CrossRef]
- MacIntosh, C.; Morley, J.E.; Chapman, I.M. The anorexia of aging. Nutrition 2000, 16, 983–995. [Google Scholar] [CrossRef]
- Di Francesco, V.; Fantin, F.; Omizzolo, F.; Residori, L.; Bissoli, L.; Bosello, O.; Zamboni, M. The anorexia of aging. Dig. Dis. 2007, 25, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Chapman, I.M. The anorexia of aging. Clin. Geriatr. Med. 2007, 23, 735–756. [Google Scholar] [CrossRef] [PubMed]
- Chapman, I.M. Endocrinology of anorexia of ageing. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Chapman, I.M.; MacIntosh, C.G.; Morley, J.E.; Horowitz, M. The anorexia of ageing. Biogerontology 2002, 3, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, V.; Zamboni, M.; Zoico, E.; Mazzali, G.; Dioli, A.; Omizzolo, F.; Bissoli, L.; Fantin, F.; Rizzotti, P.; Solerte, S.B.; et al. Unbalanced serum leptin and ghrelin dynamics prolong postprandial satiety and inhibit hunger in healthy elderly: Another reason for the “anorexia of aging”. Am. J. Clin. Nutr. 2006, 83, 1149–1152. [Google Scholar] [PubMed]
- Laviano, A.; Meguid, M.M.; Inui, A.; Muscaritoli, M.; Rossi-Fanelli, F. Therapy insight: Cancer anorexia-cachexia syndrome—When all you can eat is yourself. Nat. Clin. Pract. Oncol. 2005, 2, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-S.; Blackwood, K.; Schuster, M.W. The cytokine basis of cachexia and its treatment: Are they ready for prime time? J. Am. Med. Dir. Assoc. 2008, 9, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Thomas, D.R. Anorexia and aging: Pathophysiology. Nutrition 1999, 15, 499–503. [Google Scholar] [CrossRef]
- Landi, F.; Lattanzio, F.; Dell’Aquila, G.; Eusebi, P.; Gasperini, B.; Liperoti, R.; Belluigi, A.; Bernabei, R.; Cherubini, A. Prevalence and potentially reversible factors associated with anorexia among older nursing home residents: Results from the ULISSE project. J. Am. Med. Dir. Assoc. 2013, 14, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Mir, F.; Zafar, F.; Morley, J.E. Anorexia of aging: Can we decrease protein energy undernutrition in the nursing home? J. Am. Med. Dir. Assoc. 2013, 14, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Pathophysiology of the anorexia of aging. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Anorexia of aging: Physiologic and pathologic. Am. J. Clin. Nutr. 1997, 66, 760–773. [Google Scholar] [PubMed]
- Onder, G.; Landi, F.; Fusco, D.; Corsonello, A.; Tosato, M.; Battaglia, M.; Mastropaolo, S.; Settanni, S.; Antocicco, M.; Lattanzio, F. Recommendations to prescribe in complex older adults: Results of the CRIteria to assess appropriate Medication use among Elderly complex patients (CRIME) project. Drugs Aging 2014, 31, 33–45. [Google Scholar] [CrossRef] [PubMed]
- De Castro, J.M. Age-related changes in spontaneous food intake and hunger in humans. Appetite 1993, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.-M.G.; Thomas, D.R.; Rubenstein, L.Z.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Diebold, M.R.; Morley, J.E. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [PubMed]
- Rolland, Y.; Perrin, A.; Gardette, V.; Filhol, N.; Vellas, B. Screening older people at risk of malnutrition or malnourished using the Simplified Nutritional Appetite Questionnaire (SNAQ): A comparison with the Mini-Nutritional Assessment (MNA) tool. J. Am. Med. Dir. Assoc. 2012, 13, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, J.M.; Cella, D.; Hahn, E.A.; Lloyd, S.R.; Tchekmedyian, N.S.; von Roenn, J.; Leslie, W.T. Re-validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual. Life Res. 2000, 9, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Bernabei, R.; Landi, F.; Onder, G.; Liperoti, R.; Gambassi, G. Second and third generation assessment instruments: The birth of standardization in geriatric care. J. Gerontol. A. Biol. Sci. Med. Sci. 2008, 63, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Laviano, A.; Cruz-Jentoft, A.J. The anorexia of aging: Is it a geriatric syndrome? J. Am. Med. Dir. Assoc. 2010, 11, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Onder, G.; Vetrano, D.L.; Ortolani, E.; Tosato, M.; Marzetti, E.; Landi, F. Anorexia of aging: A modifiable risk factor for frailty. Nutrients 2013, 5, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Anorexia of aging: A true geriatric syndrome. J. Nutr. Health Aging 2012, 16, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Martone, A.M.; Marzetti, E.; Onder, G.; Savera, G.; Lorenzi, M.; Serafini, E.; Bernabei, R.; Landi, F. Pre-hospital dietary intake correlates with muscle mass at the time of fracture in older hip-fractured patients. Front. Aging Neurosci. 2014, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Russo, A.; Liperoti, R.; Tosato, M.; Barillaro, C.; Pahor, M.; Bernabei, R.; Onder, G. Anorexia, physical function, and incident disability among the frail elderly population: Results from the ilSIRENTE study. J. Am. Med. Dir. Assoc. 2010, 11, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Barillaro, C.; Capoluongo, E.; Bernabei, R.; Onder, G. Association of anorexia with sarcopenia in a community-dwelling elderly population: Results from the ilSIRENTE study. Eur. J. Nutr. 2013, 52, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.C.; Drummond, M.J.; Pennings, B.; Fujita, S.; Glynn, E.L.; Chinkes, D.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E392–E400. [Google Scholar] [CrossRef] [PubMed]
- Gaffney-Stomberg, E.; Insogna, K.L.; Rodriguez, N.R.; Kerstetter, J.E. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J. Am. Geriatr. Soc. 2009, 57, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.P.; Doehner, W.; Fearon, K.C.H.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Duque, G. Vitamin D in the aging musculoskeletal system: An authentic strength preserving hormone. Mol. Aspects Med. 2005, 26, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Incalzi, R.A.; Gemma, A.; Capparella, O.; Cipriani, L.; Landi, F.; Carbonin, P. Energy intake and in-hospital starvation. A clinically relevant relationship. Arch. Intern. Med. 1996, 156, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Lattanzio, F.; Russo, A.; Tosato, M.; Barillaro, C.; Bernabei, R.; Onder, G. Effects of anorexia on mortality among older adults receiving home care: An observation study. J. Nutr. Health Aging 2012, 16, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Luca, M.; Calandra, C. Eating Disorders in Late-life. Aging Dis. 2015, 6, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Briefel, R.R.; McDowell, M.A.; Alaimo, K.; Caughman, C.R.; Bischof, A.L.; Carroll, M.D.; Johnson, C.L. Total energy intake of the US population: The third National Health and Nutrition Examination Survey, 1988–1991. Am. J. Clin. Nutr. 1995, 62, 1072S–1080S. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. https://doi.org/10.3390/nu8020069
Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, Sisto A, Marzetti E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients. 2016; 8(2):69. https://doi.org/10.3390/nu8020069
Chicago/Turabian StyleLandi, Francesco, Riccardo Calvani, Matteo Tosato, Anna Maria Martone, Elena Ortolani, Giulia Savera, Alex Sisto, and Emanuele Marzetti. 2016. "Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments" Nutrients 8, no. 2: 69. https://doi.org/10.3390/nu8020069




