Fish Consumption and Age-Related Macular Degeneration Incidence: A Meta-Analysis and Systematic Review of Prospective Cohort Studies
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Inclusion Criteria
2.2. Data Extraction and Assessment of Study Quality
2.3. Statistical Methods for the Meta-Analysis
3. Results
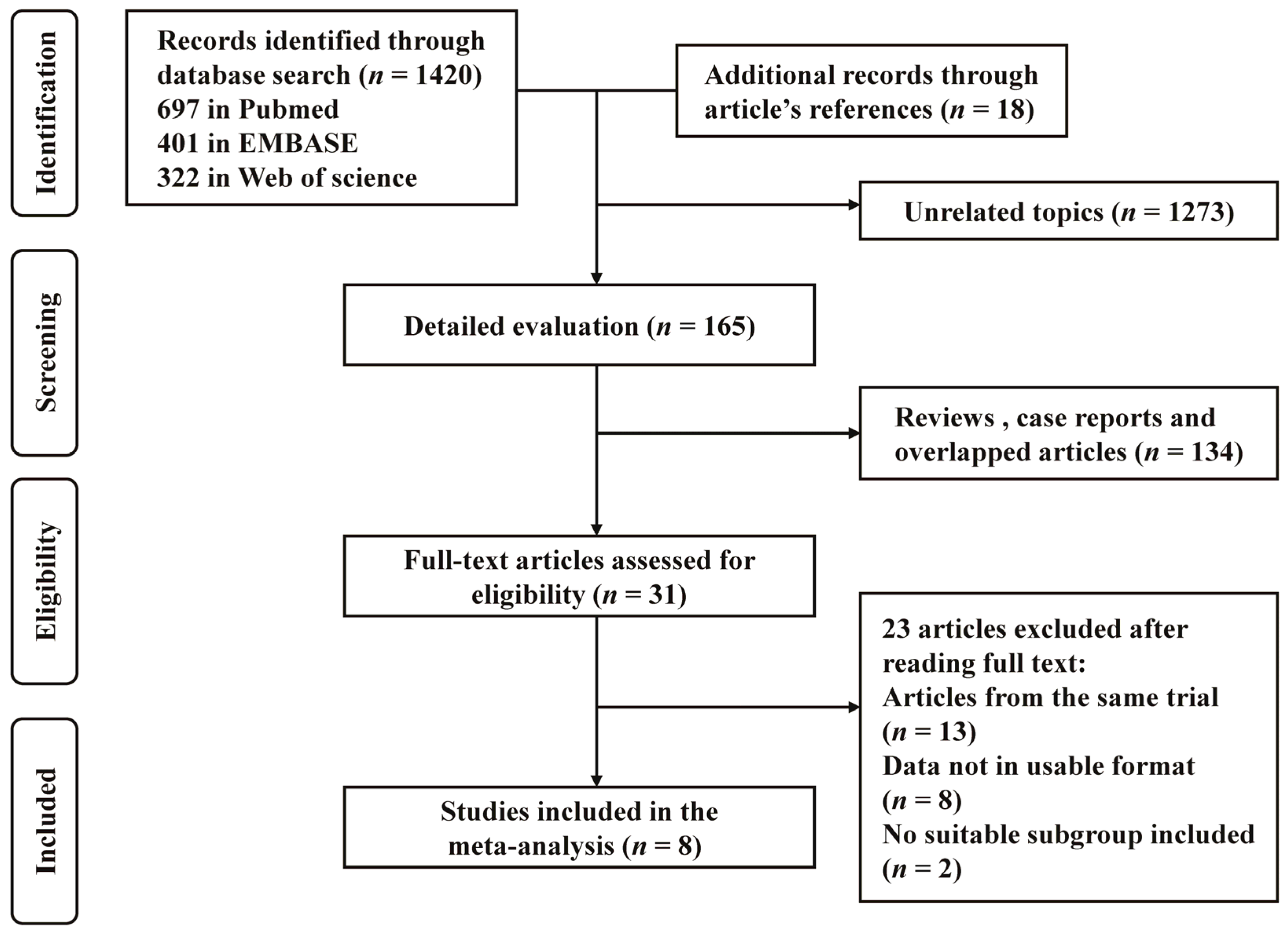
3.1. Identification and Selection of Studies
3.2. Study Characters and Quality Scores
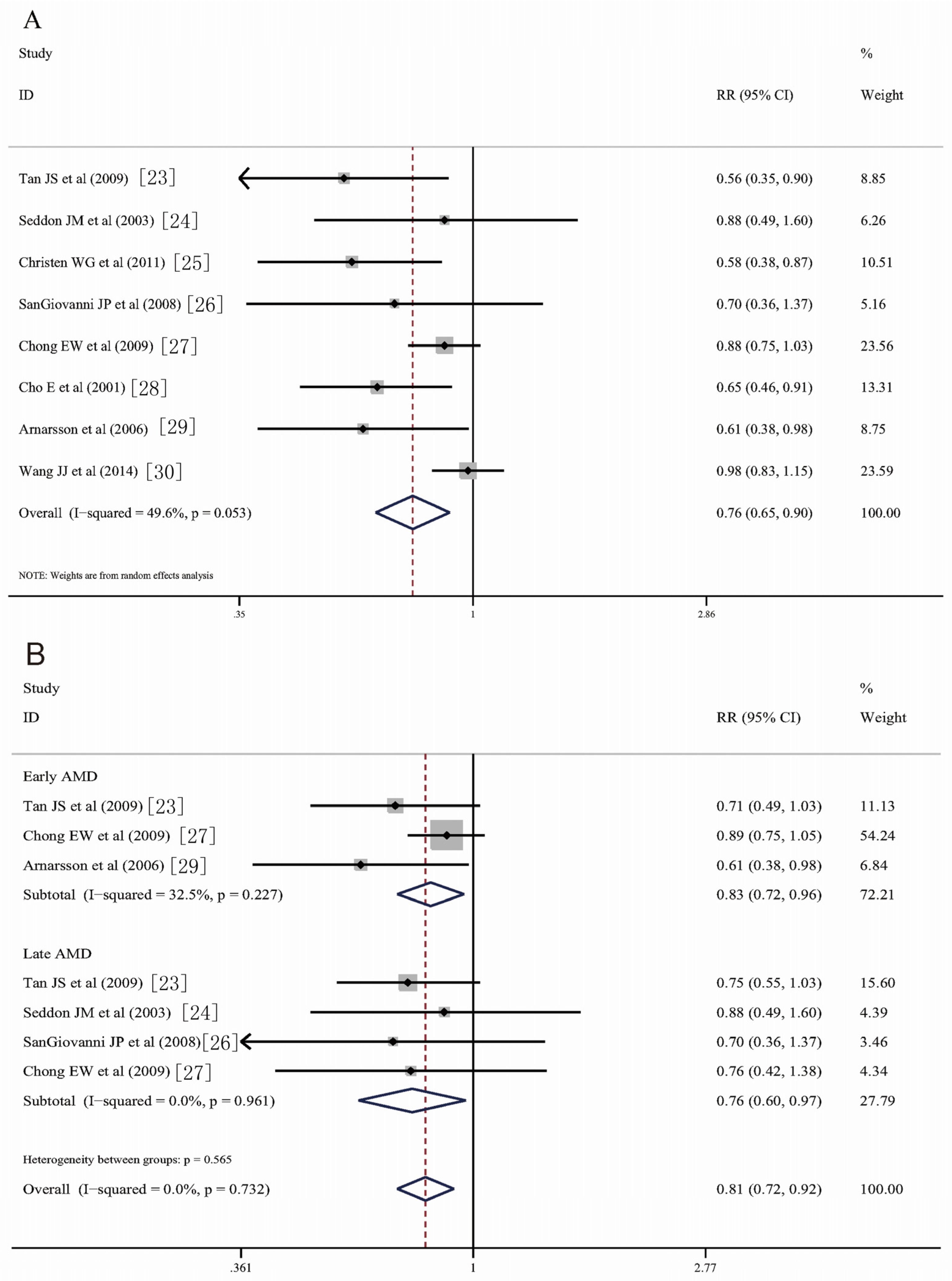
3.3. Fish Consumption and Risk of AMD
3.4. Heterogeneity and Sensitivity Analysis
3.5. Dose-Response Meta-Analysis
3.6. Publication Bias
4. Discussion
5. Conclusions
Conflicts of Interest
References
- Owen, C.G.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.E.; Rudnicka, A.R. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br. J. Ophthalmol. 2012, 96, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.A.; Sprinkhuizen, S.M.; Barthelmes, D.; Blumenkranz, M.; Cheung, G.; Haller, J.; Johnston, R.; Kim, R.; Klaver, C.; McKibbin, M.; et al. Defining a Minimum Set of Standardized Patient-centered Outcome Measures for Macular Degeneration. Am. J. Ophthalmol. 2016, 168, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saksens, N.T.; Lechanteur, Y.T.; Verbakel, S.K.; Groenewoud, J.M.; Daha, M.R.; Schick, T.; Fauser, S.; Boon, C.J.; Hoyng, C.B.; den Hollander, A.I. Analysis of Risk Alleles and Complement Activation Levels in Familial and Non-Familial Age-Related Macular Degeneration. PLoS ONE 2016, 11, e0144367. [Google Scholar] [CrossRef] [PubMed]
- Akuffo, K.O.; Beatty, S.; Stack, J.; Dennison, J.; O’Regan, S.; Meagher, K.A.; Peto, T; Nolan, J. Central Retinal Enrichment Supplementation Trials (CREST): Design and methodology of the CREST randomized controlled trials. Ophthalmic Epidemiol. 2014, 21, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.; Voland, R.P.; Moeller, S.M.; Blodi, B.A.; Ritenbaugh, C.; Chappell, R.J.; Wallace, R.B.; Mares, J.A.; CAREDS Research Study Group. Association between dietary fat intake and age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS): An ancillary study of the Women’s Health Initiative. Arch. Ophthalmol. 2009, 127, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Chua, B.; Flood, V.; Rochtchina, E.; Wang, J.J.; Smith, W.; Mitchell, P. Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch. Ophthalmol. 2006, 124, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.; Delyfer, M.N.; Korobelnik, J.F.; Rougier, M.B.; Malet, F.; Feart, C.; Le Goff, M.; Peuchant, E.; Letenneur, L.; Dartigues, J.F.; et al. High concentrations of plasma n3 fatty acids are associated with decreased risk for late age-related macular degeneration. J. Nutr. 2013, 143, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A. Omega-3 fatty acids: Comparison of plant and seafood sources in human nutrition. J. Am. Diet. Assoc. 1991, 91, 331–337. [Google Scholar] [PubMed]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, Z.; Liao, Y.; Mai, C.; Chen, T.; Tang, H.; Tang, Y. Effectiveness of omega-3 Polyunsaturated Fatty Acids Based Lipid Emulsions for Treatment of Patients after Hepatectomy: A Prospective Clinical Trial. Nutrients 2016, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.; Chappell, R.J.; Millen, A.E.; Albert, D.M.; Mares, J.A. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch. Ophthalmol. 2007, 125, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Mitchell, P.; Leeder, S.R. Dietary fat and fish intake and age-related maculopathy. Arch. Ophthalmol. 2000, 118, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Rosner, B.; Sperduto, R.D.; Yannuzzi, L.; Haller, J.A.; Blair, N.P.; Willett, W. Dietary fat and risk for advanced age-related macular degeneration. Arch. Ophthalmol. 2001, 119, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Mitchell, P.; Kifley, A.; Flood, V.; Smith, W.; Wang, J.J. Smoking and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye Study. Arch. Ophthalmol. 2007, 125, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994, 272, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Mares-Perlman, J.A.; Brady, W.E.; Klein, R.; VandenLangenberg, G.M.; Klein, B.E.; Palta, M. Dietary fat and age-related maculopathy. Arch. Ophthalmol. 1995, 113, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.W.; Kreis, A.J.; Wong, T.Y.; Simpson, J.A.; Guymer, R.H. Alcohol consumption and the risk of age-related macular degeneration: A systematic review and meta-analysis. Am. J. Ophthalmol. 2008, 145, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Vitamin B6 and risk of colorectal cancer: A meta-analysis of prospective studies. JAMA 2010, 303, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Lee, K.L.; Pollock, B.G. Regression models in clinical studies: Determining relationships between predictors and response. J. Natl. Cancer Inst. 1988, 80, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Wang, J.J.; Flood, V.; Mitchell, P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: The Blue Mountains Eye Study. Arch. Ophthalmol. 2009, 127, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Cote, J.; Rosner, B. Progression of age-related macular degeneration: Association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch. Ophthalmol. 2003, 121, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.G.; Schaumberg, D.A.; Glynn, R.J.; Buring, J.E. Dietary omega-3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch. Ophthalmol. 2011, 129, 921–929. [Google Scholar] [CrossRef] [PubMed]
- SanGiovanni, J.P.; Chew, E.Y.; Agron, E.; Clemons, T.E.; Ferris, F.L., III; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Klein, R.; et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report No. 23. Arch. Ophthalmol. 2008, 126, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.W.; Robman, L.D.; Simpson, J.A.; Hodge, A.M.; Aung, K.Z.; Dolphin, T.K.; English, D.R.; Giles, G.G.; Guymer, R.H. Fat consumption and its association with age-related macular degeneration. Arch. Ophthalmol. 2009, 127, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Hung, S.; Willett, W.C.; Spiegelman, D.; Rimm, E.B.; Seddon, J.M.; Colditz, G.A.; Hankinson, S.E. Prospective study of dietary fat and the risk of age-related macular degeneration. Am. J. Clin. Nutr. 2001, 73, 209–218. [Google Scholar] [PubMed]
- Arnarsson, A.; Sverrisson, T.; Stefansson, E.; Sigurdsson, H.; Sasaki, H.; Sasaki, K.; Jonasson, F. Risk factors for five-year incident age-related macular degeneration: The Reykjavik Eye Study. Am. J. Ophthalmol. 2006, 142, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Buitendijk, G.H.; Rochtchina, E.; Lee, K.E.; Klein, B.E.; van Duijn, C.M.; Flood, V.M.; Meuer, S.M.; Attia, J.; Myers, C.; et al. Genetic susceptibility, dietary antioxidants, and long-term incidence of age-related macular degeneration in two populations. Ophthalmology 2014, 121, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, A.; Giorgianni, F.; New, D.D.; Hollingsworth, T.J.; Umfress, A.; Alhatem, A.H.; Neeli, I.; Lenchik, N.I.; Jennings, B.J.; Calzada, J.I.; et al. Circulating Autoantibodies in Age-Related Macular Degeneration Recognize Human Macular Tissue Antigens Implicated in Autophagy, Immunomodulation, and Protection from Oxidative Stress and Apoptosis. PLoS ONE 2015, 10, e0145323. [Google Scholar] [CrossRef] [PubMed]
- Cialdella-Kam, L.; Nieman, D.C.; Knab, A.M.; Shanely, R.A.; Meaney, M.P.; Jin, F.; Sha, W.; Ghosh, S. A Mixed Flavonoid-Fish Oil Supplement Induces Immune-Enhancing and Anti-Inflammatory Transcriptomic Changes in Adult Obese and Overweight Women—A Randomized Controlled Trial. Nutrients 2016, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Querques, G.; Merle, B.M.; Pumariega, N.M.; Benlian, P.; Delcourt, C.; Zourdani, A.; Leisy, H.B.; Lee, M.D.; Smith, R.T.; Souied, E.H. Dynamic Drusen Remodelling in Participants of the Nutritional AMD Treatment-2 (NAT-2) Randomized Trial. PLoS ONE 2016, 11, e0149219. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, J.; Jentsch, S.; Schweitzer, D.; Hammer, M.; Lang, G.E.; Strobel, J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: the LUTEGA study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Robman, L.; Vu, H.; Hodge, A.; Tikellis, G.; Dimitrov, P.; McCarty, C.; Guymer, R. Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration. Can. J. Ophthalmol. 2007, 42, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.W.; Kreis, A.J.; Wong, T.Y.; Simpson, J.A.; Guymer, R.H. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: A systematic review and meta-analysis. Arch. Ophthalmol. 2008, 126, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Kabasawa, S.; Mori, K.; Horie-Inoue, K.; Gehlbach, P.L.; Inoue, S.; Awata, T.; Katayama, S.; Yoneya, S. Associations of cigarette smoking but not serum fatty acids with age-related macular degeneration in a Japanese population. Ophthalmology 2011, 118, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; George, S.; Rosner, B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: The US Twin Study of Age-Related Macular Degeneration. Arch. Ophthalmol. 2006, 124, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.P.; Kamel, F.; Pericak-Vance, M.A.; Haines, J.L.; Postel, E.A.; Agarwal, A.; Richards, M.; Scott, W.K.; Schmidt, S. Overall diet quality and age-related macular degeneration. Ophthalmic Epidemiol 2010, 17, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Augood, C.; Chakravarthy, U.; Young, I.; Vioque, J.; de Jong, P.T.; Bentham, G.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am. J. Clin. Nutr. 2008, 88, 398–406. [Google Scholar] [PubMed]
- Souied, E.H.; Delcourt, C.; Querques, G.; Bassols, A.; Merle, B.; Zourdani, A.; Smith, T.; Benlian, P.; Nutritional AMD Treatment 2 Study Group. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology 2013, 120, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, Zeaxanthin and Meso-zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Akuffo, K.O.; Nolan, J.M.; Howard, A.N.; Moran, R.; Stack, J.; Klein, R.; Klein, B.E.; Meuer, S.M.; Sabour-Pickett, S.; Thurnham, D.I.; et al. Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration. Eye 2015, 29, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Lawrenson, J.G.; Evans, J.R. Omega 3 fatty acids for preventing or slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2015, 11, CD010015. [Google Scholar]
- Brasky, T.M.; Darke, A.K.; Song, X.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L., Jr.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl. Cancer Inst. 2013, 105, 1132–1141. [Google Scholar] [CrossRef] [PubMed]



| Author, Year | Study; Follow-up | Duration | Study Design | Site | Age (Year) | Gender, Percent | No. of Case/Cohort | Adjustments of Confounding Factors | Question | Exposure Definition | Study Quality * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tan et al., 2009 [23] | Melbourne Collaborative Cohort Study > 10 years | 1992–2004 | Population-based | Australia | ≥49 | F: 57% | 232/2684 | Age, sex, and smoking | 145-item FFQ | <1/M (Q1) vs. ≥3/W (Q3) | 8 |
| Seddon et al., 2003 [24] | AREDS, 4.6 years | 1989–1998 | Hospital-based | USA | ≥65 | F: 61% | 51/312 | Age-sex group, education, body mass index, systolic blood pressure, cardiovascular disease, log energy, protein intake, energy-adjusted log beta carotene intake, alcohol intake, physical activity, and initial age-related macular degeneration grade, total intake of energy-adjusted log zinc, vitamin C, and vitamin E. | 61-item FFQ | <1/W (Q1) vs. ≥2/W (Q3) | 8 |
| Christen et al., 2011 [25] | Women’s Health Study, 10 years | 1993–2004 | Population-based | USA | ≥45 | F:100% | 235/38257 | Age, randomized treatment assignment, smoking, alcohol use, BMI, menopausal status and use of HT, history of hypertension, history of high cholesterol, history of diabetes multivitamin use, history of eye exam in the last 2 years | 131-item FFQ | <1/M (Q1) vs. >1/M (Q3) | 7 |
| SanGiovanni et al., 2008 [26] | Massachusetts Eye and Ear Infirmary, 6.3 years | 1992–1998 | Population-based | USA | 55–80 | F: 56.1% | 311/2623 | Age, sex, AREDS therapy group, education, race, BMI, smoking, antacid use, iris colour, DHA intake, EPA intake, combined DHA-EPA intake | 90-item FFQ | <1/M (Q1) vs. >2/M (Q5) | 9 |
| Chong et al., 2009 [27] | Nurses’ Health Study, 13 years | 1990–2006 | Population-based | Australia | 66–85 | F: 61% | 1099/7098 | Age, sex, smoking (current, past, or never), energy, vitamin C, vitamin E, carotene, zinc, lutein, zeaxanthin, and supplements (vitamin C, vitamin E, cod liver oil and fish oil (yes/no)) | 121-item FFQ | 0–0.5/W (Q1) vs. ≥2/W (Q3) | 9 |
| Cho et al., 2001 [28] | Blue Mountains Eye Study, 12 years | 1984–1996 | Population-based | USA | 56 | F: 59.0% | 567/73056 | 2-year period, age , smoking, energy and lutein and zeaxanthin intakes, BMI, profession, physical activity (metabolic equivalent quintiles), and alcohol intake | 130-item FFQ | ≤1/M (Q1) vs. ≥4/W (Q5) | 9 |
| Arnarsson et al., 2006 [29] | Reykjavik Eye Study, 5 years | 1996–2001 | Population-based | Iceland | ≥50 | F: 55.8% | 134/1379 | Age, smoking, and sex | 16-item FFQ | ≤1/M (Q1) vs. ≥4/W(Q4) | 7 |
| Wang et al., 2014 [30] | Rotterdam Study, 15 years | 1990–2001 | Population-based | The Netherlands | ≥55 | F: 58.8% | 1573/3579 | Age- and sex-adjusted | 170-item FFQ | <1/W (Q1) vs. ≥1/W(Q2) | 8 |
| Subgroups | No. of Studies | Summary Effect | Study Heterogeneity | ||
|---|---|---|---|---|---|
| RR (95% CI) | p Value | I2, % | p Value | ||
| Data source | |||||
| Population based | 7 | 0.75; (0.63–0.89) | 0.001 | 56.7 | 0.031 |
| Hospital based | 1 | 0.88 (0.49–1.59) | 0.672 | - | - |
| Country | |||||
| USA | 4 | 0.84 (0.72–0.98) | <0.001 | 0 | 0.724 |
| Australia | 2 | 0.74 (0.48–1.14) | 0.174 | 68.50 | 0.075 |
| Iceland | 1 | 0.61 (0.38–0.98) | 0.002 | - | - |
| Netherlands | 1 | 0.98 (0.83–1.15) | 0.787 | - | - |
| Follow-up | |||||
| >10 years | 5 | 0.81 (0.67–0.97) | 0.024 | 53.6 | 0.072 |
| < 10 years | 3 | 0.70 (0.51–0.97) | 0.033 | 0 | 0.638 |
| Subgroups | Summary Effect | Study Heterogeneity | ||||
|---|---|---|---|---|---|---|
| RR | 95% Lower Limiter | 95% Upper Limiter | p Value | I2, % | p Value | |
| Fish types | ||||||
| Dark meat fish | 0.68 | 0.46 | 0.99 | 0.047 | 53.70 | 0.091 |
| Tuna fish | 0.58 | 0.47 | 0.71 | <0.001 | 0 | 0.934 |
| Other dark meat fish | 0.96 | 0.75 | 1.24 | 0.34 | - | - |
| Non-dark meat fish | 0.82 | 0.65 | 1.03 | 0.088 | 0.80 | 0.315 |
| Processing | ||||||
| Baked or broiled | 0.98 | 0.87 | 1.11 | 0.762 | 0 | 0.488 |
| Fried fish | 0.97 | 0.83 | 1.14 | 0.731 | 0 | 0.508 |
| Smoked fish | 0.88 | 0.54 | 1.43 | 0.600 | 0 | 0.974 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Wu, Y.; Meng, Y.-F.; Xing, Q.; Tao, J.-J.; Lu, J. Fish Consumption and Age-Related Macular Degeneration Incidence: A Meta-Analysis and Systematic Review of Prospective Cohort Studies. Nutrients 2016, 8, 743. https://doi.org/10.3390/nu8110743
Zhu W, Wu Y, Meng Y-F, Xing Q, Tao J-J, Lu J. Fish Consumption and Age-Related Macular Degeneration Incidence: A Meta-Analysis and Systematic Review of Prospective Cohort Studies. Nutrients. 2016; 8(11):743. https://doi.org/10.3390/nu8110743
Chicago/Turabian StyleZhu, Wei, Yan Wu, Yi-Fang Meng, Qian Xing, Jian-Jun Tao, and Jiong Lu. 2016. "Fish Consumption and Age-Related Macular Degeneration Incidence: A Meta-Analysis and Systematic Review of Prospective Cohort Studies" Nutrients 8, no. 11: 743. https://doi.org/10.3390/nu8110743
APA StyleZhu, W., Wu, Y., Meng, Y.-F., Xing, Q., Tao, J.-J., & Lu, J. (2016). Fish Consumption and Age-Related Macular Degeneration Incidence: A Meta-Analysis and Systematic Review of Prospective Cohort Studies. Nutrients, 8(11), 743. https://doi.org/10.3390/nu8110743




