Early Life Fructose Exposure and Its Implications for Long-Term Cardiometabolic Health in Offspring
Abstract
:1. Introduction
2. An Overview of Fructose
2.1. Consumption of Fructose Is Increasing
2.2. Adverse Metabolic Effects of Fructose
3. Early Life Fructose Exposure and Long-Term Cardiometabolic Health
3.1. Implications of Human Studies
3.2. Implications of Rodent Experiments
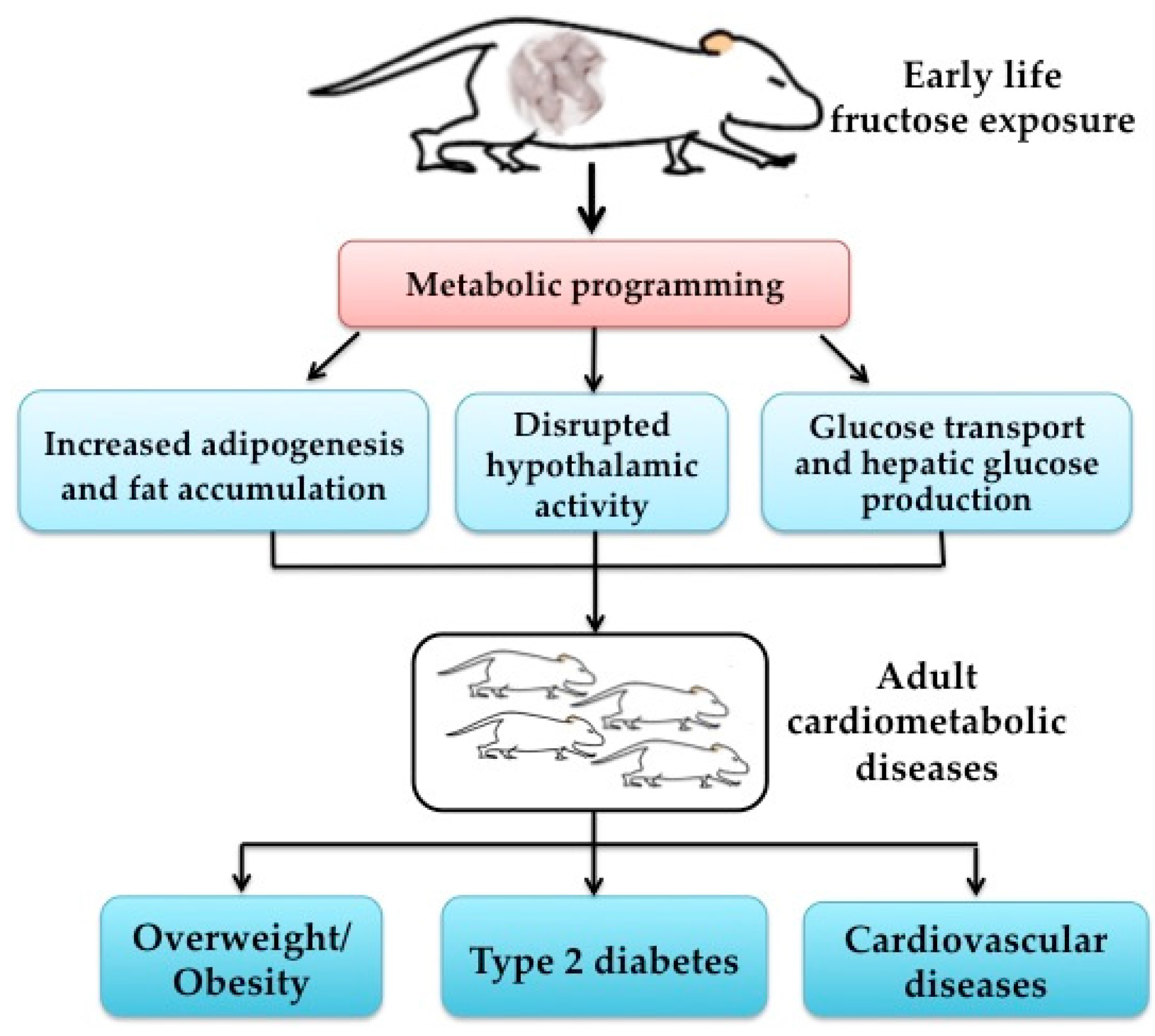
3.3. Potential Mechanisms of Early Life Fructose Exposure and Cardiometabolic Health
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Diabetes Federation (IDF). IDF Diabetes Atlas, 7th ed.; IDF: Brussels, Belgium, 2015. [Google Scholar]
- Pinhas-Hamiel, O.; Zeitler, P. The global spread of type 2 diabetes mellitus in children and adolescents. J. Pediatr. 2005, 146, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Rando, O.J.; Simmons, R.A. I’m eating for two: Parental dietary effects on offspring metabolism. Cell 2015, 161, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Pasupathy, D.; Poston, L. Determining the consequences of maternal obesity for offspring health. Exp. Physiol. 2015, 100, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. Fructose and cardiometabolic health: What the evidence from sugar-sweetened beverages tells us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228s–1235s. [Google Scholar] [CrossRef] [PubMed]
- Stephan, B.C.; Wells, J.C.; Brayne, C.; Albanese, E.; Siervo, M. Increased fructose intake as a risk factor for dementia. J. Gerontol. Ser. A Biol. Sci. Méd. Sci. 2010, 65, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Malik, V.S. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: Epidemiologic evidence. Physiol. Behav. 2010, 100, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Kit, B.K.; Carroll, M.D.; Park, S. Consumption of sugar drinks in the United States, 2005–2008. NCHS Data Brief 2011, 71, 1–8. [Google Scholar]
- Rosset, R.; Surowska, A.; Tappy, L. Pathogenesis of cardiovascular and metabolic diseases: Are fructose-containing sugars more involved than other dietary calories? Curr. Hypertens. Rep. 2016, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Dumke, K.; Bouret, S.G.; Kayser, B.; Walker, R.W.; Blumberg, B. The obesogenic effect of high fructose exposure during early development. Nat. Rev. Endocrinol. 2013, 9, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [PubMed]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [PubMed]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippe, J.M.; Angelopoulos, T.J. Fructose-containing sugars and cardiovascular disease. Adv. Nutr. 2015, 6, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Manson, J.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004, 292, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Malik, V.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Sweetened beverage consumption and risk of coronary heart disease in women. Am. J. Clin. Nutr. 2009, 89, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Narain, A.; Kwok, C.S.; Mamas, M.A. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: A systematic review and meta-analysis. Int. J. Clin. Pract. 2016, 70, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Brantsaeter, A.L.; Haugen, M.; Samuelsen, S.O.; Torjusen, H.; Trogstad, L.; Alexander, J.; Magnus, P.; Meltzer, H.M. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J. Nutr. 2009, 139, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Borgen, I.; Aamodt, G.; Harsem, N.; Haugen, M.; Meltzer, H.M.; Brantsaeter, A.L. Maternal sugar consumption and risk of preeclampsia in nulliparous Norwegian women. Eur. J. Clin. Nutr. 2012, 66, 920–925. [Google Scholar] [CrossRef] [PubMed]
- George, G.C.; Hanss-Nuss, H.; Milani, T.J.; Freeland-Graves, J.H. Food choices of low-income women during pregnancy and postpartum. J. Am. Diet. Assoc. 2005, 105, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Englund-Ogge, L.; Brantsaeter, A.L.; Haugen, M.; Sengpiel, V.; Khatibi, A.; Myhre, R.; Myking, S.; Meltzer, H.M.; Kacerovsky, M.; Nilsen, R.M.; et al. Association between intake of artificially sweetened and sugar-sweetened beverages and preterm delivery: A large prospective cohort study. Am. J. Clin. Nutr. 2012, 96, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Alzamendi, A.; Castrogiovanni, D.; Gaillard, R.C.; Spinedi, E.; Giovambattista, A. Increased male offspring’s risk of metabolic-neuroendocrine dysfunction and overweight after fructose-rich diet intake by the lactating mother. Endocrinology 2010, 151, 4214–4223. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Clayton, Z.E.; Yap, C.; Sloboda, D.M. Maternal fructose intake during pregnancy and lactation alters placental growth and leads to sex-specific changes in fetal and neonatal endocrine function. Endocrinology 2011, 152, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Rawana, S.; Clark, K.; Zhong, S.; Buison, A.; Chackunkal, S.; Jen, K.L. Low dose fructose ingestion during gestation and lactation affects carbohydrate metabolism in rat dams and their offspring. J. Nutr. 1993, 123, 2158–2165. [Google Scholar] [PubMed]
- Ching, R.H.; Yeung, L.O.; Tse, I.M.; Sit, W.H.; Li, E.T. Supplementation of bitter melon to rats fed a high-fructose diet during gestation and lactation ameliorates fructose-induced dyslipidemia and hepatic oxidative stress in male offspring. J. Nutr. 2011, 141, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.F.; Dickerson, J.; Kechichian, T.B.; Yin, H.; Gamble, P.; Salazar, A.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Costantine, M.M. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am. J. Obstet. Gynecol. 2016, 215, 378. [Google Scholar] [CrossRef] [PubMed]
- Alzamendi, A.; Zubiria, G.; Moreno, G.; Portales, A.; Spinedi, E.; Giovambattista, A. High risk of metabolic and adipose tissue dysfunctions in adult male progeny, due to prenatal and adulthood malnutrition induced by fructose rich diet. Nutrients 2016, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.; Long, S.; Green, C.; Gardiner, S.M.; Craigon, J.; Gardner, D.S. Maternal fructose and/or salt intake and reproductive outcome in the rat: Effects on growth, fertility, sex ratio, and birth order. Biol. Reprod. 2013, 89, 51. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.; Gardiner, S.M.; Elmes, M.; Gardner, D.S. Excess maternal salt or fructose intake programmes sex-specific, stress- and fructose-sensitive hypertension in the offspring. Br. J. Nutr. 2016, 115, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y.; Tain, Y.L. Aliskiren administration during early postnatal life sex-specifically alleviates hypertension programmed by maternal high fructose consumption. Front. Physiol. 2016, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Wallack, L.; Thornburg, K. Developmental origins, epigenetics, and equity: Moving upstream. Matern. Child Health J. 2016, 20, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef] [PubMed]
- David, E.S.; Cingari, D.S.; Ferraris, R.P. Dietary induction of intestinal fructose absorption in weaning rats. Pediatr. Res. 1995, 37, 777–782. [Google Scholar] [CrossRef] [PubMed]
| Fructose Exposure | Species | Age | Metabolic Disorders | Potential Mechanism | Reference |
|---|---|---|---|---|---|
| Maternal iso-caloric 10% fructose rich diet during lactation | Sprague Dawley rats | Between 49–60 days | Increased body weight and food intake, enhanced leptinemia, and impaired insulin sensitivity | Disrupted hypothalamic activity: decreased hypothalamic ob-Rb gene expression and STAT-3 phosphorylation | Alzamendi et al. [23] |
| Maternal 20% of caloric intake from fructose from day 1 of pregnancy until postnatal day 10 | Wistar rats | Embryonic day 21 and postnatal day 10 | Elevated circulating plasma fructose, insulin, and leptin levels | Placental fructose sensitivity and transfer: glucose transporter 5 and IGF-1 | Vickers et al. [24] |
| Maternal 100 g/L fructose water during pregnancy | Sprague Dawley rats | Postweaning day 5 | Hyperglycemia and hyperinsulinemia | Elevated phosphoenolpyruvate carboxykinase | Rawana et al. [25] |
| Maternal 60% fructose throughout pregnancy and lactation | Sprague Dawley rats | 14–23 weeks old | Increased serum triglycerides, free fatty acids, and insulin | Increased expression of ACC2 and CPT1α, and decreased expression of PPARα and PGC1-α | Ching et al. [26] |
| Maternal 10% fructose during pregnancy | C57BL/6J mouse | 1 year old | Hypertension, insulin resistance, and obesity | Increased expression of PTP1B and JNK | Saad et al. [27] |
| Maternal 10% fructose during pregnancy | Sprague Dawley rats | 60 days | Hyperglycemia, hypertriglyceridemia, and hyperleptinemia | Reduced adipocyte precursor cells number | Alzamendi et al. [28] |
| Maternal 10% fructose during before conception and during the mating period | Sprague Dawley rats | At day 20 gestation | Growth, fertility, sex ratio, and birth order | Glycolyzable monosaccharide on the maternal ovary and/or ovulated oocyte | Gray et al. [29] |
| Maternal 10% fructose before and during gestation and through lactation | Sprague Dawley rats | 9 to 14 weeks of age | Hypertension | Vasoconstrictor, anti-natriuretic, or diminished vasodilatory pathways | Gray et al. [30] |
| 60% fructose throughout pregnancy and lactation | Sprague Dawley rats | 12 weeks of age | Hypertension | Nitric oxide and arachidonic acid metabolites | Tain et al. [31] |
| 60% fructose throughout pregnancy and lactation | Sprague Dawley rats | 12 weeks of age | Hypertension | ACE and MAS | Hsu et al. [32] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Feng, Q.; Zhang, Q.; Wang, T.; Xiao, X. Early Life Fructose Exposure and Its Implications for Long-Term Cardiometabolic Health in Offspring. Nutrients 2016, 8, 685. https://doi.org/10.3390/nu8110685
Zheng J, Feng Q, Zhang Q, Wang T, Xiao X. Early Life Fructose Exposure and Its Implications for Long-Term Cardiometabolic Health in Offspring. Nutrients. 2016; 8(11):685. https://doi.org/10.3390/nu8110685
Chicago/Turabian StyleZheng, Jia, Qianyun Feng, Qian Zhang, Tong Wang, and Xinhua Xiao. 2016. "Early Life Fructose Exposure and Its Implications for Long-Term Cardiometabolic Health in Offspring" Nutrients 8, no. 11: 685. https://doi.org/10.3390/nu8110685





